One-pot synthesis of CexZr1-xO2 solid solution catalysts for the splitting of CO2 to CO via thermochemical cycling
-
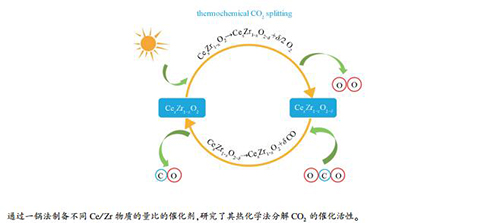
摘要: 采用一锅蒸发诱导自组装法(EISA)制备了一系列不同铈锆物质的量比的铈锆固溶体催化剂,用TGA研究了其热化学循环分解CO2制CO的催化性能,并采用XRD、Raman光谱、H2-TPR、XPS、SEM和N2吸附-脱附等手段对催化剂的物相结构、还原性能和表面化学性质进行了表征分析,用热重分析(TGA)研究了铈锆固溶体对热化学循环分解CO2制CO的催化性能。结果表明,随着Ce/Zr物质的量比增加,铈锆固溶体催化剂的CO2高温分解活性先增大后减小。Ce/Zr物质的量比为1的Ce0.5Zr0.5O2催化剂由于具有较多的晶格缺陷和氧空穴,氧迁移能力强,催化活性高,而Ce/Zr物质的量比为3的Ce0.75Zr0.25O2催化剂具有相对稳定的氧空穴数,循环稳定性好。循环反应后,所有的催化剂均出现了一定程度的烧结,且富锆固溶体发生了相分离,这可能会影响催化剂的性能。
-
关键词:
- CexZr1-xO2 /
- CO2分解 /
- 热化学循环 /
- 氧空穴
Abstract: A series of CeO2-ZrO2 solid solutions with different Ce/Zr molar ratios were synthesized by one-pot evaporation-induced self-assembly (EISA) method and characterized by XRD, Raman spectroscopy, H2-TPR, XPS, SEM and N2 sorption. The catalytic activity of CeO2-ZrO2 solid solutions in the thermochemical splitting of CO2 to CO were investigated by thermogravimetric analysis. The results reveal that with the increase of Ce/Zr molar ratio, the catalytic activity of CeO2-ZrO2 in CO2 splitting increases first and then decreases. The Ce0.5Zr0.5O2 solution with a Ce/Zr molar ratio of 1 exhibits high activity in CO2 splitting, owing to its abundant lattice defects and oxygen vacancies which can promote the oxygen migration. In contrast, the Ce0.75Zr0.25O2 solution with a Ce/Zr molar ratio of 3 shows the best cyclic stability, due to its relatively stable number of oxygen vacancies. Sintering of particles was observed after the cycling reaction, accompanying with the phase separation in the Zr-rich solid solutions, which may influence the catalytic performance of CeO2-ZrO2 solid solutions in the CO2 splitting.-
Key words:
- CexZr1-xO2 /
- CO2 splitting /
- thermochemical cycle /
- oxygen vacancy
-
表 1 新鲜铈锆固溶体的BET比表面积及晶粒粒径
Table 1 BET surface area and crystal size of the CexZr1-xO2 solid solutions
Material Crystallite size
d/nmSurface
area A/(m2·g-1)Ce0.2Zr0.8O2 8.1 51 Ce0.33Zr0.67O2 9.3 33 Ce0.67Zr0.33O2 14.6 17 Ce0.75Zr0.25O2 17.7 14 Ce0.8Zr0.2O2 26.4 12 表 2 循环反应前后铈锆固溶体的表面元素组成及相对含量
Table 2 Surface chemical composition of the fresh and used CexZr1-xO2 solid solutions
Material Chemical composition /% Surface element molar ratio Ce Zr O OⅡ/O Ce3+/Ce4+ Ce0.2Zr0.8O2 4.1(6.8) 21.5(21.2) 74.4(72.0) 0.22(0.23) 0.30(0.31) Ce0.33Zr0.67O2 6.4(9.7) 18.7(17.7) 74.9(72.7) 0.24(0.26) 0.32(0.34) Ce0.67Zr0.33O2 13.9(13.7) 8.3(10.9) 77.8(75.4) 0.26(0.30) 0.33(0.36) Ce0.75Zr0.25O2 15.3(14.9) 6.5(9.4) 78.2(75.7) 0.21(0.22) 0.27(0.28) Ce0.8Zr0.2O2 15.8(15.4) 5.9(8.2) 78.3(76.4) 0.20(0.21) 0.25(0.27) *: the data for the used samples were supplied in parentheses 表 3 TGA实验中产生的O2和CO体积
Table 3 O2 and CO yields over CexZr1-xO2 derived from the TGA results
Sample O2 /(mL·g-1) CO /(mL·g-1) Average
reduction rate /%2nd cycle 3rd cycle 4th cycle average 2nd cycle 3rd cycle 4th cycle average Ce0.2Zr0.8O2 1.18 1.11 1.00 1.10 2.52 2.49 2.46 2.49 13.1 Ce0.33Zr0.67O2 2.77 2.72 2.68 2.72 5.21 5.38 5.33 5.31 20.3 Ce0.67Zr0.33O2 3.23 3.35 3.28 3.29 5.36 5.84 5.99 5.73 13.7 Ce0.75Zr0.25O2 2.56 2.38 2.42 2.45 5.35 5.26 5.43 5.35 9.3 Ce0.8Zr0.2O2 2.42 2.41 2.39 2.41 4.12 4.47 4.44 4.34 8.7 Ce0.5Zr0.5O2* 3.94 3.65 3.89 3.83 6.67 6.76 7.04 6.82 20.2 CeO2[11] 1.28 1.12 1.19 1.20 2.20 2.28 2.35 2.28 3.7 *: from our previous work[19] -
[1] CHUEH W C, STEINFELD A. High-flux solar-driven thermochemical dissociation of CO2 and H2O using nonstoichiometric ceria[J]. Science, 2010, 330(6012):1797-1801. doi: 10.1126/science.1197834 [2] MARXER D, FURLER P, TAKACS M, STEINFELD A. Solar thermochemical splitting of CO2 into separate streams of CO and O2 with high selectivity, stability, conversion, and efficiency[J]. Energy Environ Sci, 2017, 10(5):1142-1149. doi: 10.1039/C6EE03776C [3] CHUEH W C, HAILE S M. A thermochemical study of ceria:Exploiting an old material for new modes of energy conversion and CO2 mitigation[J]. Philos Trans A:Math Phys Eng Sci, 2010, 368(1923):3269-3294. doi: 10.1098/rsta.2010.0114 [4] FURLER P, SCHEFFE J R, STEINFELD A. Syngas production by simultaneous splitting of H2O and CO2 via ceria redox reactions in a high-temperature solar reactor[J]. Energy Environ Sci, 2012, 5(3):6098-6103. doi: 10.1039/C1EE02620H [5] SCHEFFE J R, STEINFELD A. Oxygen exchange materials for solar thermochemical splitting of H2O and CO2:A review[J]. Mater Today, 2014, 17(7):341-348. doi: 10.1016/j.mattod.2014.04.025 [6] KANG M, WU X, ZHANG J, ZHAO N, WEI W, SUN Y. Enhanced thermochemical CO2 splitting over Mg-and Ca-doped ceria/zirconia solid solutions[J]. RSC Adv, 2014, 4(11):5583-5590. doi: 10.1039/c3ra45595e [7] STAMATIOU A, LOUTZENHISER P G, STEINFELD A. Solar syngas production via H2O/CO2-splitting thermochemical cycles with Zn/ZnO and FeO/Fe3O4 redox reactions†[J]. Chem Mater, 2010, 22(3):851-859. doi: 10.1021/cm9016529 [8] ABANADES S, CHAMBON M. CO2 dissociation and upgrading from two-step solar thermochemical processes based on ZnO/Zn and SnO2/SnO redox pairs[J]. Energy Fuels, 2010, 24(12):6667-6674. doi: 10.1021/ef101092u [9] DEY S, NAIDU B S, GOVINDARAJ A, RAO C N R. Noteworthy performance of La1-xCaxMnO3 perovskites in generating H2 and CO by the thermochemical splitting of H2O and CO2[J]. Phys Chem Chem Phys, 2015, 17(1):122-125. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=1cc14340194b06acdfa0730916c654c6 [10] MCDANIEL A H, MILLER E C, ARIFIN D, AMBROSINI A, COKER E N, O'HAYRE R, CHUEH W C, TONG J. Sr- and Mn- doped LaAlO3-δ for solar thermochemical H2 and CO production[J]. Energy Environ Sci, 2013, 6(8):2424-2428. doi: 10.1039/c3ee41372a [11] GAL A L, ABANADES S, FLAMANT G. CO2 and H2O splitting for thermochemical production of solar fuels using nonstoichiometric ceria and ceria/zirconia solid solutions[J]. Energy Fuels, 2011, 25(10):4836-4845. doi: 10.1021/ef200972r [12] ARIFIN D, WEIMER A W. Kinetics and mechanism of solar-thermochemical H2 and CO production by oxidation of reduced CeO2[J]. Sol Energy, 2018, 160:178-185. doi: 10.1016/j.solener.2017.11.075 [13] ZHAO B, HUANG C, RAN R, WU X, DUAN W. Two-step thermochemical looping using modified ceria-based materials for splitting CO2[J]. J Mater Sci, 2016, 51(5):2299-2306. doi: 10.1007/s10853-015-9534-7 [14] ABANADES S, LEGAL A, CORDIER A, PERAUDEAU G, FLAMANT G, JULBE A. Investigation of reactive cerium-based oxides for H2 production by thermochemical two-step water-splitting[J]. J Mater Sci, 2010, 45(15):4163-4173. doi: 10.1007/s10853-010-4506-4 [15] JIANG Q, ZHOU G, JIANG Z, LI C. Thermochemical CO2 splitting reaction with CexM1-xO2-δ (M=Ti4+, Sn4+, Hf4+, Zr4+, La3+, Y3+ and Sm3+) solid solutions[J]. Sol Energy, 2014, 99(99):55-66. doi: 10.1016/j.solener.2013.10.021 [16] JACOT R, MORE R, MICHALSKY R, STEINFELD A, PATZKE G R. Trends in the phase stability and thermochemical oxygen exchange of ceria doped with potentially tetravalent metals[J]. J Mater Chem A, 2017, 5(37):19901-19913. doi: 10.1039/C7TA04063F [17] ABANADES S, GAL A L. CO2 splitting by thermo-chemical looping based on ZrxCe1-xO2 oxygen carriers for synthetic fuel generation[J]. Fuel, 2012, 102(6):180-186. doi: 10.1016/j.fuel.2012.06.068 [18] PETKOVICH N D, RUDISILL S G, VENSTROM L J, BOMAN D B, DAVIDSON J H, STEIN A. Control of heterogeneity in nanostructured Ce1-xZrxO2 binary oxides for enhanced thermal stability and water splitting activity[J]. J Phys Chem C, 2011, 115(43):21022-21033. doi: 10.1021/jp2071315 [19] SHI H, LUO J, WANG F, PU Y, YANG J, XIAO F, ZHAO N, SONG Q, CHEN Z. Synthesis of CeO2-ZrO2 solid solutions for thermochemical CO2 splitting[J]. Energy Technol-Ger, 2019, 7(4):1800890. doi: 10.1002/ente.201800890 [20] ALIFANTI M, BAPS B, BLANGENOIS N, NAUD J, GRANGE P, DELMON B. Characterization of CeO2-ZrO2 mixed oxides. Comparison of the citrate and Sol-Gel preparation methods[J]. Chem Mater, 2003, 15(2):395-403. [21] KUHN M, BISHOP S R, RUPP J L M, TULLER H L. Structural characterization and oxygen nonstoichiometry of ceria-zirconia (Ce1-xZrxO2-δ) solid solutions[J]. Acta Mater, 2013, 61(11):4277-4288. doi: 10.1016/j.actamat.2013.04.001 [22] L PEZ E F, ESCRIBANO V S, PANIZZA M, CARNASCIALI M M, BUSCA G. Vibrational and electronic spectroscopic properties of zirconia powders[J]. J Mater Chem, 2001, 11(11):1891-1897. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fc42945e9134d36e1059f6b64eebc311 [23] REDDY B M, KHAN A. Structural characterization of CeO2-TiO2 And V2O5/CeO2-TiO2 catalysts by raman and XPS techniques[J]. J Phys Chem B, 2003, 107(22):5162-5167. doi: 10.1021/jp0344601 [24] PRUSTY D, PATHAK A, MUKHERJEE M, MUKHERJEE B, CHOWDHURY A. TEM and XPS studies on the faceted nanocrystals of Ce0.8Zr0.2O2[J]. Mater Charact, 2015, 100:31-35. doi: 10.1016/j.matchar.2014.12.009 [25] MOULDER J F, STICKLE W F, SOBOL P E, BOMBEN K D, CHASTAIN J, KING R C. Handbook of X-Ray Photoelectron Spectroscop[M]. Perkin-Elmer Corp:Eden Prairie, MN, 1979. [26] SHINDE S S, RAJPURE K Y. X-ray photoelectron spectroscopic study of catalyst based zinc oxide thin films[J]. J Alloy Compd, 2011, 509(13):4603-4607. doi: 10.1016/j.jallcom.2011.01.117 [27] TROVARELLI A, ZAMAR F, LLORCA J, LEITENBURG C D, DOLCETTI G, KISS J T. Nanophase fluorite-structured CeO2-ZrO2 catalysts prepared by high-energy mechanical milling[J]. J Catal, 1997, 169(2):490-502. doi: 10.1006/jcat.1997.1705 [28] FALLY F, PERRICHON V, VIDAL H, KASPAR J, BLANCO G, PINTADO J M, BERNAL S, COLON G, DATURI M, LAVALLEY J C. Modification of the oxygen storage capacity of CeO2 -ZrO2 mixed oxides after redox cycling aging[J]. Catal Today, 2000, 59(3):373-386. doi: 10.1016-S0920-5861(00)00302-3/ -





 下载:
下载: