One-step conversion of syngas to hydrocarbons and ethers over ZIF-8 derived ZnO coupling HZSM-5
-
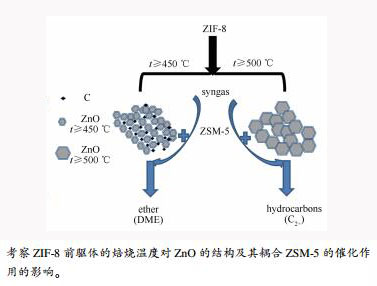
摘要: 通过水热溶剂法合成有机骨架结构材料ZIF-8,以其为前驱体调变焙烧温度制备ZnO纳米粒子。采用XRD、TEM、XPS、Raman等表征研究ZnO的组成结构及晶粒粒径形态变化;将ZnO与HZSM-5耦合形成双功能催化剂,考察其在合成气转化中的催化活性。结果表明,焙烧温度对ZnO的颗粒粒径结构影响较大,温度影响晶粒的形成速率,提高温度会促进ZnO的聚集;ZIF-8衍生ZnO通过调变温度影响ZnO晶粒粒径,起到改变产物分布的作用。当焙烧温度≤ 450℃时,以碳包覆ZnO纳米粒子结构存在,ZnO晶粒粒径小于20 nm,含碳ZnO耦合HZSM-5催化剂的产物以二甲醚为主;当温度≥ 500℃,以纯相ZnO存在,ZnO晶粒粒径皆大于20 nm,ZnO耦合HZSM-5催化剂的产物以烃类为主。ZnO与HZSM-5的耦合方式对双功能催化剂的产物选择性有显著影响。Abstract: Zeolitic imidazolate frameworks (ZIF-8) were synthesized by solvothermal method. Used as precursor, ZIF-8 was decomposed into nanoparticles ZnO at different pyrolysis temperature in air atmosphere. The composition, structure and crystal size of ZnO were characterized by XRD, TEM, XPS, and Raman methods. The ZnO nanoparticles were coupled with HZSM-5 to form bifunctional catalysts. The catalytic performances of bifunctional catalysts in the syngas conversion were investigated in a fixed-bed tubular reactor. The results demonstrate that the pyrolysis temperature has an important influence on the particle size of ZnO. The temperature affects the rate of grain formation. High temperature promotes the aggregation of ZnO. The ZnO grain size by changing the temperature plays a role in changing the product distribution. When the pyrolysis temperature is near or below 450℃, carbon-coated ZnO nanoparticles are obtained, and the ZnO grain size is less than 20 nm. The carbon-coated ZnO coupled with HZSM-5 catalyzes syngas mainly into dimethyl ether (DME). When the temperature is higher than 450℃, pure phase ZnO nanoparticles are obtained, and the ZnO grain size is larger than 20 nm. The pure ZnO coupled with HZSM-5 catalyzes syngas mainly into hydrocarbons. Obviously, the coupling modes of ZnO and HZSM-5 have a significant effect on the product selectivity of bifunctional catalysts.
-
Key words:
- ZIF-8 /
- ZnO /
- bifunctional catalyst /
- syngas conversion /
- DME /
- hydrocarbon
-
表 1 不同温度ZnO催化剂的表面元素组成
Table 1 Data for catalysts with ZnO at different temperatures
Sample Ultimate analysis w/% OXPS NXPS ZnXPS ZnICP ZnO-600 22.01 - 77.98 82.85 ZnO-550 20.50 - 79.50 73.97 ZnO-500 19.97 - 80.03 74.04 ZnO-450 20.13 0.52 79.34 80.33 ZnO-400 24.48 11.36 64.16 50.86 ZnO-350 27.39 21.38 52.30 34.58 表 2 不同温度的ZnO耦合HZSM-5催化剂的催化性能
Table 2 Performance of the ZnO-t & HZSM-5 catalysts calcined at different temperatures
Sample CO CO2 C1 C2-4 C5+ CH3OCH3 wmol/% w/(mmol·gZn-1·h-1) ZnO-350 & HZSM-5 10.1 12.8 33.1 11.0 10.6 3.1 75.4 ZnO-400 & HZSM-5 8.6 5.7 32.4 11.4 9.9 3.0 75.6 ZnO-450 & HZSM-5 10.8 4.5 23.5 7.8 7.1 2.3 82.9 ZnO-500 & HZSM-5 7.5 3.5 37.9 20.1 47.9 32.0 - ZnO-550 & HZSM-5 4.7 2.2 39.8 22.3 56.6 21.2 - ZnO-600 & HZSM-5 6.3 2.6 29.4 34.6 49.5 15.8 - reaction conditions:t=350℃,p=3.0 MPa,GHSV=2000 h-1 -
[1] LIU X L, ZHOU W, YANG Y, CHENG K, KANG J, ZHAG L, ZHANG G Q, MIN X J, ZHANG Q H, WANG Y. Design of efficient bifunctional catalysts for direct conversion of syngas into lower olefins via methanol/dimethyl ether intermediates[J]. Chem Sci, 2018, 9(20):4708-4718. doi: 10.1039/C8SC01597J [2] SHIKADA T, OHNO Y, OGAWA T, ONO M, MIZUGUCHI M, TOMURA K, FUJIMOTA K. Direct synthesis of dimethyl ether form synthesis gas[J]. Stud Surf Sci Catal, 1998, 119:515-520. doi: 10.1016/S0167-2991(98)80483-7 [3] RAVEENDRA G, LI C, YANG C, MENG F H, LI Z. Direct transformation of syngas to lower olefins synthesis over hybrid Zn-Al2O3/SAPO-34 catalysts[J]. New J Chem, 2018, 42(10):4419-4431. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=e14ee91f96140d237fcefe4dfe58aabe [4] GENTZEN M, HABICHT W, DORONKIN D E, GRUNWALDT J D, SAUER J, BEHRENS S. Bifunctional hybrid catalysts derived from Cu/Zn-based nanoparticles for single-step dimethyl ether synthesis[J]. Catal Sci Technol, 2015, 6(4):10-21. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=08ace13e95921187c24846964d1116d2 [5] YANG J H, PAN X L, JIAO F, LI J, BAO X H. Direct conversion of syngas to aromatics[J]. Chem Commun, 2017, 53:11146-11149. doi: 10.1039/C7CC04768A [6] CHENG K, ZHOU W, KANG J C, HE S, SHI S L, ZHANG Q H, PAN Y, WEN W, WANG Y. Bifunctional catalysts for one-step conversion of syngas into aromatics with excellent selectivity and stability[J]. Chem, 2017, 3(2):1-14. https://www.sciencedirect.com/science/article/pii/S2451929417302206 [7] SARAVANAN K, HANM H, TSUBAKI N, JONG WOOK B. Recent progress for direct synthesis of dimethyl ether from syngas on the heterogeneous bifunctional hybrid catalysts[J]. Appl Catal B:Environ, 2017. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=c5ef8e43bdccfd2ccfa4cec961c21754 [8] CHENG K, GU B, LIU X L, KANG J C, ZHANG Q H, WANG Y. Direct and highly selective conversion of synthesis gas to lower olefins:design of a bifunctional catalyst combining methanol synthesis and carbon-carbon coupling[J]. Angew Chem Int Ed, 2016, 55(15):1-5. https://pubmed.ncbi.nlm.nih.gov/26961855/ [9] CHEN H Y, LAU S P, CHEN L, LIN J, HUAN C H A, TAN K L, PAN J S. Synergism between Cu and Zn sites in Cu/Zn catalysts for methanol synthesis[J]. Appl Surf Sci, 1999, 152(3/4):193-199. https://www.sciencedirect.com/science/article/abs/pii/S0169433299003177 [10] WILMER H, KURTZ M, KLEMENTIEV K V, TKACHENKO O P, GRVNERT W, HINRICHSEN O, BIRKNERA, RABE S, MERZ K, DRIESS M, WÖLL C, MUHLER M. Methanol synthesis over ZnO:A structure-sensitive reaction?[J]. PCCP, 2003, 5(20):4736-4742. doi: 10.1039/B304425D [11] NIU X J, GAO J, MIAO Q, DONG M, WANG G F, FAN W B, QIN Z F, WANG J G. Influence of preparation method on the performance of Zn-containing HZSM-5 catalysts in methanol-to-aromatics[J]. Microporous Mesoporous Mater, 2014, 197:25261. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=7dd90c7a9b1564bcd09ca55038d7ea55 [12] TAMADDON F, MORADI S. Controllable selectivity in Biginelli and Hantzsch reactions using nanoZnO as a structure base catalyst[J]. J Mol Catal A:Chem, 2013, 370:117-122. doi: 10.1016/j.molcata.2012.12.005 [13] YANG S J, IM J H, KIM T, LEE K, PARK C R. MOF-derived ZnO and ZnO@C composites with high photocatalytic activity and adsorption capacity[J]. J Hazard Mater, 2011, 186(1):376-382. doi: 10.1016/j.jhazmat.2010.11.019 [14] 姚显芳, 李映伟. MOFs作为牺牲模板制备纳米多孔碳材料的方法及其应用[J].中国科学, 2015, 60(20):1906-1914. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=kxtb201520006YAO Xian-fang, LI Ying-wei. Method for preparing nanoporous carbon material by using MOFs as sacrificial template and application[J]. Chin Sci Bull, 2015, 60(20):1906-1914. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=kxtb201520006 [15] VIETH J K, JANIAK C. MOFs, MILs and more:Concepts, properties and applications for porous coordination networks (PCNs)[J]. Chem Inform, 2010, 34(11):2366-2388. http://cn.bing.com/academic/profile?id=6c2c0580f1cc3c7e38960dcd23b585dd&encoded=0&v=paper_preview&mkt=zh-cn [16] PARK K, NI Z, COTE A, CHOI J Y, HUANG R D, URIBE-ROMO F J, CHAO H K, KEEFFE M, YAGHI O M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks[J].PNAS, 2006, 103(27):10186-10191. doi: 10.1073/pnas.0602439103 [17] BATS N, CHIZALLET C, LAZARE S, BAZER-BACHI D, BONNIER F, LECOCQ V, SOYER E, QUOINEAUD A, BATS N. Catalysis of transesterification by a nonfunctionalized metal-organic framework:Acido-basicity at the external surface of ZIF-8 probed by FTIR and ab initio calculations[J]. J Am Chem Soc, 2010, 132(35):12365-12377. doi: 10.1021/ja103365s [18] LEE J Y, FARHA O K, ROBERTS J, LEE Y, FARHA O K, ROBERTS J, SCHEIDT K A, NGUYEN S T, HUPP J T. Metal-organic framework materials as catalysts[J]. Chem Soc Rev, 2009, 38(5):1450-1459. doi: 10.1039/b807080f [19] YANG S J, KIM T, IM J H, KIM Y S, LEE K, JUNG H, PARK C R. MOF-derived hierarchically porous carbon with exceptional porosity and hydrogen storage capacity[J]. Chem Mater, 2012, 24(3):464-470. doi: 10.1021/cm202554j [20] ZHENG F, YANG Y, CHEN Q. High lithium anodic performance of highly nitrogen-doped porous carbon prepared from a metal-organic framework[J]. Nat Commun, 2014, 5(5):5261-5270. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=54abef17566b95fef792f68c65a10dd0 [21] SWIATOWSKA-MROWIECKA J, ZANNA S, OGLE K, MARCUS P. Adsorption of 1, 2-diaminoethane on ZnO thin films from p-xylene[J]. Appl Surf Sci, 2008, 254(17):5530-5539. doi: 10.1016/j.apsusc.2008.02.170 [22] JING L Q, XU Z L, SHANG J, SUN X J, CAI W M, GUO H C. The preparation and characterization of ZnO ultrafine particles[J]. Mater Sci Eng A, 2002, (1/2):356-361. doi: 10.1016-S0921-5093(01)01801-9/ [23] ANSARI S A, KHAN M M, KALATHIL S, NISAR A, LEE J, CHO M H. Oxygen vacancy induced band gap narrowing of ZnO nanostructures by an electrochemically active biofilm[J]. Nanoscale, 2013, 5(19):9238-9246. doi: 10.1039/c3nr02678g [24] HAN Y Z, QI P F, LI S W, FENG X, ZHOU J W, LI H W, SU S Y, LI X G, WANG B. A novel anode material derived from organic-coated ZIF-8 nanocomposites with high performance in lithium ion batteries[J]. Chem Commun, 2014, 50(59):8057-8060. doi: 10.1039/C4CC02691H [25] CHOI Y, FUTAGAMI K, FUJITANI T, J. NAKAMURA. The role of ZnO in Cu/ZnO methanol synthesis catalysts-morphology effect or active site model?[J]. Appl Catal A:Gen, 2001, 208(1/2):163-167. https://www.sciencedirect.com/science/article/abs/pii/S0926860X00007122 [26] KURTZ M, STRUNK J, HINRICHSEN O, MUHLER M, FINK K, MEYER B AND WÖLL C. Active sites on oxide surfaces:ZnO-catalyzed synthesis of methanol from CO and H2[J]. Angew Chem Int Ed, 2005, 44:2790-2794. doi: 10.1002/anie.200462374 -





 下载:
下载: