A molecular simulation study on the adsorption of CH4 and CO2 on the mineral substances in oil shale
-
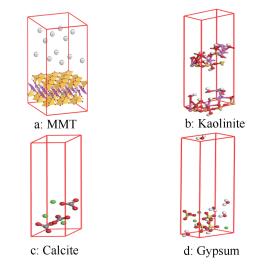
摘要: 利用Materials Studio2017模拟软件构建了蒙脱石、高岭石、方解石和生石膏四种矿物质分子模型。采用巨正则蒙特卡洛(GCMC)方法和分子动力学(MD)方法对四种模型的吸附量和吸附热进行了模拟计算。研究表明,相同温度和压力条件下四种矿物质对CH4和CO2分子吸附量大小为:蒙脱石>高岭石>生石膏>方解石;CH4和CO2分子的单组分吸附量随压力的增大而增大,两种气体吸附均符合Langmuir吸附规律;四种矿物质对CH4和CO2分子的等量吸附热均小于42 kJ/mol,即为物理吸附;随着温度的升高,CH4和CO2分子的吸附量和吸附热均减小,且CH4和CO2分子的等量吸附热和等温吸附量之间呈良好的正相关。Abstract: The models of montmorillonite, kaolinite, calcite and gypsum as the mineral substances in oil shale were built by using Materials Studio 2017 software; the adsorption of CH4 and CO2 on these mineral substances was then simulated by the GCMC and MD method. The results illustrated that the adsorption capacity of CH4 and CO2 on four mineral substances under the same temperature and pressure follows the order of montmorillonite > kaolinite > gypsum > calcite. The adsorption of single component CH4 and CO2 is in accordance with the Langmuir isotherm and the adsorption heats for both CH4 and CO2 on four mineral models all are less than 42 kJ/mol, suggesting that the adsorption belongs to physical category. With the increase of temperature, both the adsorption capacity and adsorption heat are reduced; there is a positive correlation between the adsorption heat and adsorption capacity for the CH4 and CO2 molecules.
-
Key words:
- oil shale /
- mineral /
- molecular simulation /
- adsorption /
- CH4 /
- CO2
-
表 1 CH4的等量吸附热
Table 1 Adsorption heat of CH4 on the surface of four mineral substances
Pressure p/MPa MMT Qst/(kJ·mol-1) Kaolinite Qst/(kJ·mol-1) Calcite Qst/(kJ·mol-1) Gypsum Qst/(kJ·mol-1) 303 K 333 K 363 K 303 K 333 K 363 K 303 K 333 K 363 K 303 K 333 K 363 K 1 8.330 8.146 8.021 13.665 13.075 12.920 9.894 9.966 9.807 6.673 6.389 6.372 2 8.594 8.335 8.314 14.184 13.631 13.472 10.109 10.016 10.068 6.971 6.682 6.816 3 8.983 8.560 8.289 14.677 14.259 13.619 10.410 10.238 10.159 7.109 7.075 6.912 4 9.142 8.816 8.399 15.100 14.385 13.849 10.523 10.372 10.121 7.519 7.226 7.205 5 9.129 8.824 8.590 15.360 14.941 14.351 10.694 10.489 10.406 7.678 7.448 7.406 6 9.322 8.891 8.728 15.468 15.067 14.636 10.887 10.690 10.623 8.033 7.607 7.464 7 9.548 9.042 8.673 15.690 15.284 14.975 10.941 10.745 10.774 8.184 7.699 7.527 8 9.669 9.075 8.870 15.686 15.514 14.878 11.104 10.853 10.746 8.431 8.017 7.556 9 9.644 9.217 8.799 15.949 15.443 14.970 11.242 10.974 10.807 8.464 7.966 7.799 10 9.858 9.468 9.033 15.999 15.518 15.113 11.276 11.121 10.824 8.924 8.280 8.042 Average 9.222 8.837 8.572 15.178 14.712 14.278 10.708 10.546 10.434 7.799 7.439 7.301 表 2 CO2的等量吸附热
Table 2 Adsorption heat of CO2 on the surface of four mineral substances
Pressure p/MPa MMT Qst/(kJ·mol-1) Kaolinite Qst/(kJ·mol-1) Calcite Qst/(kJ·mol-1) Gypsum Qst/(kJ·mol-1) 303 K 333 K 363 K 303 K 333 K 363 K 303 K 333 K 363 K 303 K 333 K 363 K 1 39.456 39.13 38.569 36.807 30.003 29.911 35.706 35.016 34.189 29.305 28.702 27.606 2 39.596 39.396 38.974 36.84 33.459 31.643 36.175 35.147 34.286 29.956 29.018 28.059 3 39.998 39.859 39.156 37.105 35.74 31.974 36.526 35.974 34.982 30.128 29.296 28.569 4 40.256 41.569 39.561 37.946 34.141 32.869 37.115 36.185 35.458 30.569 29.996 29.194 5 40.569 40.296 39.912 38.526 35.987 35.968 37.256 37.859 35.832 31.458 31.586 29.569 6 41.256 41.103 41.296 38.782 35.968 35.948 37.809 36.984 36.095 32.146 30.856 30.128 7 41.968 41.597 41.203 38.859 36.434 36.102 38.258 37.284 36.859 33.589 31.149 30.596 8 42.698 42.302 42.105 39.108 36.894 36.258 38.478 37.963 36.596 32.869 31.596 31.182 9 43.102 42.919 42.221 39.547 37.548 37.154 39.502 38.589 36.785 33.105 32.569 32.859 10 43.596 43.008 42.968 40.568 38.483 38.559 39.984 38.986 37.156 33.558 33.294 32.586 Average 41.249 41.117 40.596 38.408 35.465 34.638 37.680 36.998 35.823 31.668 30.806 30.034 -
[1] DENG S, WANG Z, GU Q. Extracting hydrocarbons from Huadian oil shale by sub-critical water[J]. Fuel Process Technol, 2011, 92(5):1062-1067. doi: 10.1016/j.fuproc.2011.01.001 [2] 姚宗惠, 张明山, 曾令邦.鄂尔多斯盆地北部断裂分析[J].石油勘探与开发, 2003, 30(2):20-23. http://d.wanfangdata.com.cn/Periodical/syktykf200302004YAO Zong-hui, ZHANG Ming-shan, ZENG Ling-bang. Analysis of the faults in the northern Ordos Basin[J]. Petrol Explor Dev, 2003, 30(2):20-23. http://d.wanfangdata.com.cn/Periodical/syktykf200302004 [3] 钱家麟, 王剑秋, 李术元.世界油页岩综述[J].中国能源, 2006, 28(8):16-19. http://d.wanfangdata.com.cn/Periodical/zgny200608007QIAN Jia-lin, WANG Jian-qiu, LI Shu-yuan. World oil shale[J]. Energy China, 2006, 28(8):16-19. http://d.wanfangdata.com.cn/Periodical/zgny200608007 [4] 邹永文. 桦甸油页岩及其半焦基础特性研究[D]. 吉林: 东北电力大学, 2010. doi: 10.3969/j.issn.1005-2992.2010.01.004ZOU Yong-wen. Study on the basic characteristics of the Huadian oil shales and their semi-cokes[D]. Jilin:Northeast Electric Power University, 2010. doi: 10.3969/j.issn.1005-2992.2010.01.004 [5] 吉利明, 邱军利, 张同伟.泥页岩主要黏土矿物组分甲烷吸附实验[J].地球科学-中国地质大学学报, 2012, 37(5):1043-1050. http://d.wanfangdata.com.cn/Periodical/dqkx201205016JI Li-ming, QIU Jun-li, ZHANG Tong-wei. Experiments on methane adsorption of common clay minerals in shale[J]. Earth Sci:J China Univ Geosci, 2012, 37(5):1043-1050. http://d.wanfangdata.com.cn/Periodical/dqkx201205016 [6] 吉利明, 邱军利, 夏燕青.常见黏土矿物电镜扫描微孔隙特征与甲烷吸附性[J].石油学报, 2012, 33(2):249-256. doi: 10.7623/syxb201202009JI Li-ming, QIU Jun-li, XIA Yan-qing. Micro-pore characteristics and methane adsorption properties of common clay minerals by electron microscope scanning[J]. Acta Pet Sin, 2012, 33(2):249-256. doi: 10.7623/syxb201202009 [7] 孙仁远, 张云飞, 范坤坤.页岩中黏土矿物吸附特性分子模拟[J].化工学报, 2015, 66(6):2118-2122. http://d.wanfangdata.com.cn/Periodical/hgxb201506018SUN Ren-yuan, ZHANG Yun-fei, FAN Kun-kun. Molecular simulations of adsorption characteristics of clay minerals in shale[J]. J Chem Ind Eng, 2015, 66(6):2118-2122. http://d.wanfangdata.com.cn/Periodical/hgxb201506018 [8] 侯新娟, 杨建丽, 李永旺.煤大分子结构的量子化学研究[J].燃料化学学报, 1999, 27(s1):143-149. http://d.wanfangdata.com.cn/Periodical/rlhxxb201107001HOU Xin-juan, YANG Jian-li, LI Yong-wang. Quantum chemistry study on coal molecular structure[J]. J Fuel Chem Technol, 1999, 27(s1):143-149. http://d.wanfangdata.com.cn/Periodical/rlhxxb201107001 [9] YANG X, ZHANG C. Structure and diffusion behavior of dense carbon dioxide fluid in clay-like slit pores by molecular dynamics simulation[J]. Chem Phys Lett, 2005, 407(4):427-432. [10] 茹鑫. 油页岩热解过程分子模拟及实验研究[D]. 吉林: 吉林大学, 2013.RU-Xin. Study on the experiment and molecular simulation of oil shale pyrolysis[D]. Jilin:Jilin University, 2013. [11] 王茂桢, 柳少波, 任拥军.页岩气储层粘土矿物孔隙特征及其甲烷吸附作用[J].地质论评, 2015, 61(1):207-216. http://d.wanfangdata.com.cn/Periodical/dzlp201501023WANG Mao-zhen, LIU Shao-bo, REN Yong-jun. Pore characteristics and methane adsorption of clay minerals in Shale gas reservoir[J]. Geol Rev, 2015, 61(1):207-216. http://d.wanfangdata.com.cn/Periodical/dzlp201501023 [12] JIN Z, FIROOZABADI A. Methane and carbon dioxide adsorption in clay-like slit pores by Monte Carlo simulations[J]. Fluid Phase Equilib, 2013, 360(1):456-465. [13] SKIPPER N T. Monte Carlo simulation of interlayer molecular structure in swelling clay minerals. 1. methodology[J]. Clays Clay Miner, 1995, 43(3):285-293. doi: 10.1346/CCMN [14] LEVY J H, DAY S J, KILLINGLEY J S. Methane capacities of Bowen Basin coals related to coal properties[J]. Fuel, 1997, 76(9):813-819. doi: 10.1016/S0016-2361(97)00078-1 [15] ASTASHOV A V, BELYI A A, BUNIN A V. Quasi-equilibrium swelling and structural parameters of coals[J]. Fuel, 2008, 87(15/16):3455-3461. doi: 10.1016-j.fuel.2008.04.027/ [16] LIU Y, WILCOX J. Effects of surface heterogeneity on the adsorption of CO2 in microporous carbons[J]. Environ Sci Technol, 2012, 46(3):1940. doi: 10.1021/es204071g [17] 王擎, 孙斌, 刘洪鹏.油页岩热解过程矿物质行为分析[J].燃料化学学报, 2013, 41(2):163-168. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18118.shtmlWANG-Qin, SUN-Bin, LIU Hong-peng. Analysis of mineral behavior during pyrolysis of oil shale[J]. J Fuel Chem Technol, 2013, 41(2):163-168. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18118.shtml [18] JI L, ZHANG T, MILLIKEN K L. Experimental investigation of main controls to methane adsorption in clay-rich rocks[J]. Appl Geochem, 2012, 27(12):2533-2545. doi: 10.1016/j.apgeochem.2012.08.027 [19] ROSS D J K, BUSTIN R M. The importance of shale composition and pore structure upon gas storage potential of shale gas reservoirs[J]. Mar Petrol Geol, 2009, 26(6):916-927. doi: 10.1016/j.marpetgeo.2008.06.004 [20] BUSTIN R M, CLARKSON C R. Geological controls on coalbed methane reservoir capacity and gas content[J]. Int J Coal Geol, 1998, 38(66):3-26. http://d.wanfangdata.com.cn/OAPaper/oai_doaj-articles_53b1231a8a59d0c8bb8c009350c4014e [21] KROOSS B M, BERGEN F V, GENSTERBLUM Y. High-pressure methane and carbon dioxide adsorption on dry and moisture-equilibrated Pennsylvanian coals[J]. Int J Coal Geol, 2002, 51(2):69-92. doi: 10.1016/S0166-5162(02)00078-2 [22] MASTALERZ M, GLUSKOTER H, RUPP J. Carbon dioxide and methane sorption in high volatile bituminous coals from Indiana, USA[J]. Int J Coal Geol, 2004, 60(1):43-55. doi: 10.1016/j.coal.2004.04.001 [23] NODZEŃSKI A. Sorption and desorption of gases (CH4, CO2) on hard coal and active carbon at elevated pressures[J]. Fuel, 1998, 77(11):1243-1246. doi: 10.1016/S0016-2361(98)00022-2 -





 下载:
下载: