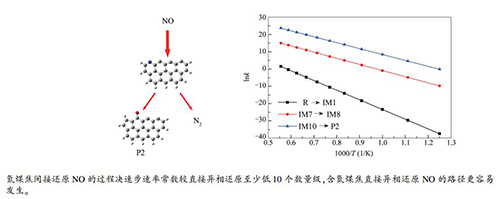
-
摘要: 采用量子化学密度泛函理论结合热力学和动力学分析研究了含氮煤焦还原NO的途径;从微观角度探究了含氮煤焦还原NO的间接还原和直接异相还原两种途径,分析了NO还原过程中的能量变化。结果表明,含氮煤焦先产生中间体NH2再还原NO(间接还原)的过程决速步能垒值较直接异相还原NO的决速步能垒值高183.76 kJ/mol;由能垒角度分析,含氮煤焦与NO直接发生异相还原的过程更为有利。从热力学角度分析,含氮煤焦直接异相还原NO为可自发进行的单向放热反应,较间接还原过程有利。动力学分析结果表明,含氮煤焦间接还原NO的过程决速步速率常数较直接异相还原至少低10个数量级,说明含氮煤焦直接异相还原NO的路径更容易发生。Abstract: Density functional theory was used to investigate the reaction pathways for the reduction of NO by nitrogen-containing char with char-bound nitrogen, viz., char(N); the reaction paths of heterogeneous reduction of NO by char(N) were analyzed from the thermodynamic and kinetic point of view. The results show that the energy barrier of the rate-determining step via the indirect NO reduction path by char(N), viz., first producing NH2 intermediate and then reducing NO, is 183.76 kJ/mol higher than that via the direct heterogeneous NO reduction path, suggesting that the later direct heterogeneous NO reduction path is more favorable. Thermodynamically, the direct heterogeneous NO reduction by char(N) is a spontaneous and exothermic process in the coal combustion system. Kinetically, the rate constant of the rate-determining step in indirect NO reduction path by char(N) is at least 10 orders of magnitude lower than that in direct heterogeneous reduction, also illustrating the superiority of the direct heterogeneous NO reduction path by char(N).
-
Key words:
- char(N) /
- indirect reduction /
- direct heterogeneous reduction /
- NO /
- thermodynamic /
- kinetic
-
表 1 由NBO计算的一些重要原子的自然居群
Table 1 Natural population of some important atoms calculated by NBO
Atom Species Charge Valence H1 TS6 0.37298 0.62531 IM8 0.38078 0.61231 N2 TS6 0.15304 4.80537 IM8 -0.03578 5.00058 -
[1] 苏胜, 宁星, 李亮国, 何金亮, 蔡兴飞, 向军.再燃条件下生物燃料及其焦还原NO特性研究[J].太阳能学报, 2013, 34(3):388-394. doi: 10.3969/j.issn.0254-0096.2013.03.006SU Ya-xin, NING Xing, LI Liang-guo, HE Jin-liang, CAI Xing-fei, XIANG Jun. Study on biofuel and its coke reduction NO characteristics under reburning conditions[J]. Acta Energ Sol Sin, 2013, 34(3):388-394. doi: 10.3969/j.issn.0254-0096.2013.03.006 [2] BURCH T E, CHEN W Y, LESTER T W, STERLING A M. Interaction of fuel nitrogen with nitric oxide during reburning with coal[J]. Combust Flame, 1994, 98(4):391-401. doi: 10.1016/0010-2180(94)90177-5 [3] THOMAS K M. The release of nitrogen oxides during char combustion[J]. Fuel, 1997, 76(6):457-473. doi: 10.1016/S0016-2361(97)00008-2 [4] MOLINA A, EDDINGS E G, PERSHIN D W, SAROFIM A F. Char nitrogen conversion:Implications to emissions from coal-fired utility boilers[J]. Prog Energy Combust Sci, 2000, 26(4/6):507-531. http://www.sciencedirect.com/science/article/pii/S0360128500000101 [5] PHONG-ANANT D, WIBBERLEY L J, WALL TF. Nitrogen oxide formation from australian coals[J]. Combust Flame, 1985, 62(1):21-30. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=J-STAGE_218526 [6] 张秀霞.焦炭燃烧过程中氮转化机理与低NOx燃烧技术的开发[D].杭州: 浙江大学, 2012.ZHANG Xiu-xia. Nitrogen conversion mechanism during char combustion and develepment of low nox technology[D]. Hangzhou: Zhejiang University, 2012. [7] ZHANG H, LIU J X, WANG X Y, LUO L, JIANG X M. DFT study on the C(N)-NO reaction with isolated and contiguous active sites[J]. Fuel, 2017, 203:715-724. doi: 10.1016/j.fuel.2017.05.023 [8] ZHANG H, LIU J X, SHEN J, JIANG X M. Thermodynamic and kinetic evaluation of the reaction between NO (nitric oxide) and char(N) (char bound nitrogen) in coal combustion[J]. Energy, 2015, 82:312-321. doi: 10.1016/j.energy.2015.01.040 [9] JIAO A Y, ZHANG H, LIU J X, JIANG X M. Quantum chemical and kinetics calculations for the NO reduction with char(N):Influence of the carbon monoxide[J]. Combust Flame, 2018, 196:377-385. doi: 10.1016/j.combustflame.2018.06.029 [10] RADOVIC L R. The mechanism of CO2 chemisorption on zigzag carbon active sites:A computational chemistry study[J]. Carbon, 2005, 43(5):907-915. doi: 10.1016/j.carbon.2004.11.011 [11] GIRIT C O, MEYER J C, ERNI R, ROSSELL M D, KISIELOWSKI C, YANG L, PARK C H, CROMMIE M F, COHEN M L, LOUIE S G, ZETTL A. Graphene at the edge:Stability and dynamics[J]. Science, 2009, 323(5922):1705-1708. doi: 10.1126/science.1166999 [12] ENOKI T, FUJⅡ S, TAKAI K. Zigzag and armchair edges in graphene[J]. Carbon, 2012, 50(9):3141-3145. doi: 10.1016/j.carbon.2011.10.004 [13] VALENTIM B, GUEDES A, BOAVIDA D. Nitrogen functionality in "oil window" rank range vitrinite rich coals and chars[J]. Org Geochem, 2012, 42(5):502-509. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ad11413968e9074617b26beae7a2fda6 [14] ZHANG Y C, ZHANG J, SHENG C D, CHEN J, LIU Y X, ZHAO L, XIE F. X-ray photoelectron spectroscopy (XPS) investigation of nitrogen functionalities during coal char combustion in O2/CO2 and O2/Ar atmospheres[J]. Energy Fuels, 2011, 25(1):240-245. doi: 10.1021/ef101134a [15] KYOTANI T, TOMITA A. Analysis of the reaction of carbon with NO/N2O using abinitio molecular orbital theory[J]. J Phys Chem B, 1999, 103(17):3434-3441. doi: 10.1021/jp9845928 [16] MONTOYA A, TRUONG T N, SAROFIM A F. Application of density functional theory to the study of the reaction of NO with char-bound nitrogen during combustion[J]. J Phys Chem A, 2000, 104(36):8409-8417. doi: 10.1021/jp001045p [17] ZHU Z H, FINNERTY J, LU G Q, YANG R T. A comparative study of carbon gasification with O2 and CO2 by density functional theory calculations[J]. Energy Fuels, 2002, 16(6):1359-1368. doi: 10.1021/ef0200020 [18] ZHU Z H, FINNERTY J, LU G Q, WILSON M A, YANG R T. Molecular orbital theory calculations of the H2O-carbon reaction[J]. Energy Fuels, 2002, 16(4):847-854. doi: 10.1021/ef010267z [19] SENDT K, HAYNES B S. Density functional study of the chemisorption of O2 on the zigzag surface of graphite[J]. Combus Flame, 2005, 143(4):629-643. doi: 10.1016/j.combustflame.2005.08.026 [20] SENDT K, HAYNES B S. Density functional study of the chemisorption of O2 across two rings of the armchair surface of graphite[J]. J Phys Chem C, 2007, 111(14):5465-5473. doi: 10.1021/jp067363r [21] LIU J, QU W Q, YUAN J Z, WANG S C, QIU J R, ZHENG C G. Theoretical studies of properties and reactions involving mercury species present in combustion flue gases[J]. Energy Fuels, 2010, 24(1):509-518. doi: 10.1021/ef9005143 [22] PHAM B Q, TRUONG T N. Electronic spin transitions in finite-size graphene[J]. Chem Phys Lett, 2012, 535(7):75-79. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=3293557cdb9c0888038bdc4a45cafa82 [23] CHEN P, GU M Y, CHEN X, CHEN J C. Study of the reaction mechanism of oxygen to heterogeneous reduction of NO by char[J]. Fuel, 2019, 236:1213-1225. doi: 10.1016/j.fuel.2018.09.094 [24] FRISCH M J, TRUCKS G W, SCHLEGEL H B, SCUSERIA G E, ROBB M A, et al. Gaussian09, revision E. 01[J]. Gaussian Inc., Wallingford, CT, 2009. [25] WIGNER E. Concerning the excess of potential barriers in chemical reactions[J]. Z Phys Chem B:Chem E, 1932, 19:203-216. [26] ALI M A, RAJAKUMAR B. Thermodynamic and kinetic studies of hydroxyl radical reaction with bromine oxide using density functional theory[J]. Comput Theor Chem, 2011, 964(1/3):283-290. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=744210536dd8e51a33da2b7a1c9fbe58 [27] LU T, CHEN F. Multiwfn:A multifunctional wavefunction analyzer[J]. J Comput Chem, 2012, 33(5):580-592. doi: 10.1002/jcc.v33.5 [28] ZHANG H, WANG X Y, LUO L, LIU J X, JIANG X M. First principles investigation on the two-state reactivity of N-exposure during coal combustion[J]. Fuel, 2017, 190:21-31. doi: 10.1016/j.fuel.2016.11.030 [29] RAJ A, ZAINUDDIN Z, SANDER M, KRAFT M. A mechanistic study on the simultaneous elimination of soot and nitric oxide from engine exhaust[J]. Carbon, 2011, 49(5):1516-1531. doi: 10.1016/j.carbon.2010.12.005 -





 下载:
下载: