Effect of nickel cobalt co-catalyst on catalytic activity of molybdenum naphthenate for the hydroprocessing of coal tar pitch in suspension bed
-
摘要: 合成了钼、镍、钴的五种分散型油溶性均相催化剂,选用高压釜反应器进行悬浮床加氢催化反应,控制反应条件在370 ℃、10 MPa氢压,反应时间4 h,考察环烷酸钼加入量、钼-镍、钼-钴双金属等比例对加氢反应的影响。通过反应结果的液体收率来衡量催化体系的煤沥青加氢催化效果。综合运用了元素分析、ICP-MS、透射电镜、X射线光电子能级、四组分分析等多种分析方法,探索煤沥青悬浮床加氢反应的最优催化体系。得出最优催化体系为:2.0×10−3下,环烷酸钼&环烷酸镍(1∶1)。此体系下,液体收率达85.3%,残渣量10.6%,气体4.1%。Abstract: Five dispersed molybdenum, nickel and cobalt oil soluble homogeneous catalysts were synthesized. The hydrogenation of coal tar pitch was under the conditions of 370 °C, 10 MPa hydrogen pressure, and 4 h reaction time in an autoclave reactor. The effects of molybdenum naphthenate addition, molybdenum nickel and molybdenum cobalt bimetallic ratios on hydrogenation were investigated. The catalytic hydrogenation effect was evaluated by the liquid yield. A variety of analytical methods, such as elemental analysis, ICP-MS, TEM, XPS, and four component separation, were used to explore the optimal catalytic system for the slurry bed hydrogenation of coal tar pitch. The results show that the optimal catalytic system is molybdenum naphthenate and nickel naphthenate ratio of 1∶1 at catalyst amount of 2×103. Under optimal conditions, the liquid yield is 85.3%, the residue yield is 10.6%, and the gas yield is 4.1%.
-
Key words:
- coal tar pitch /
- hydro-upgrading /
- homogeneous catalyst /
- slurry bed hydrocracking
-
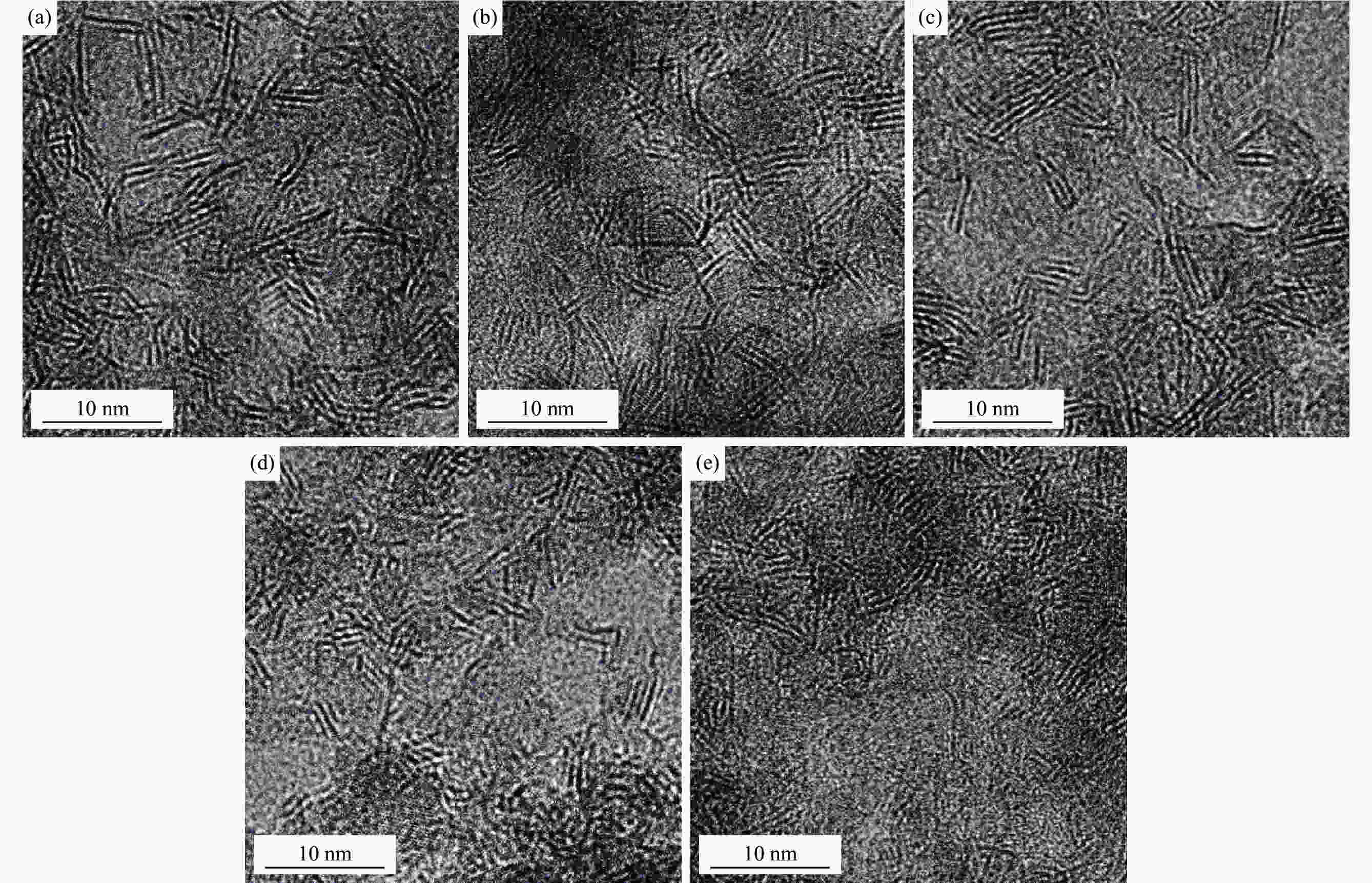
图 5 硫化催化剂透射电镜照片
Figure 5 Transmission electron microscope photographs of sulfide catalyst
(a): molybdenum naphthenate; (b): molybdenum naphthenate & nickel naphthenate; (c): molybdenum naphthenate & nickel iso-octanate; (d): molybdenum naphthenate & cobalt naphthenate; (e): molybdenum naphthenate & cobalt iso-octanate
表 1 (Co/Ni)MoS2晶片的形貌参数计算方法
Table 1 Formulae for the morphology parameters of (Co/Ni)MoS2 slabs
Formula Symbolic meaning $ \bar L $ $\bar L = \dfrac{ {\sum { {L _i} } } }{n}$ the average slab length $\bar N $ $\bar N = \dfrac{ {\sum { { {n_i}{N} }_i} } }{n}$ the average stacking numbers n'i $n'_i = \dfrac{{10 \times \dfrac{{\overline L }}{{3.2}} + 1}}{2}$ the number of Mo atoms along one side of a slab Me ${M_{\rm{e}}} = (6n'_i - 12) \times \overline L $ the number of Mo atoms at the edge sites Mc ${M_{\rm{c}}} = 6 \times \overline N $ the number of Mo atoms at the corner sites MT ${M_{\rm{T} } } = (3{ n '_i }^2 - 3n'_i + 1) \times \overline N$ the total number of Mo atoms fe,% ${f_{\rm{e} } },\% = \dfrac{ { {M_{\rm{e}}} } }{ { {M_{\rm{T} } } }} \times 100\%$ the fraction of Mo atoms at the edge sites fc,% ${f_{\rm{c}}},\% = \dfrac{{{M_{\rm{c}}}}}{{{M_{\rm{T}}}}} \times 100\% $ the fraction of Mo atoms at the corner sites 表 2 原料煤沥青元素分析数据
Table 2 Elemental analysis data of raw coal tar pitch
Element composition C N H S O C/H(atomic ratio) w/% 87.95 0.58 7.05 2.79 1.63 1.039 Four components saturated aromatic resin asphaltene toluene insoluble matter sum w/% 8.8 14.2 19.8 53.5 2.3 98.6 表 3 不同催化剂的活性金属含量
Table 3 The metal contents of various catalysts
Catalyst Theoretical metal
content w/%Metal
content w/%Relative metal
content w/%(a)Molybdenum naphthenate 11.68 9.5 81.3 Nickel naphthenate 14.6 12.9 88.4 Nickel iso-octoate 17.0 16.2 95.3 Cobalt iso-octoate 17.1 16.5 96.5 Cobalt naphthenate 14.6 12.26 83.4 a: the relative metal content is the percentage ratio of the measured metal content to the theoretical metal content 表 4 硫化催化剂表面活性物种的组成和浓度
Table 4 The composition and concentration of surface-active species of the sulfide catalysts
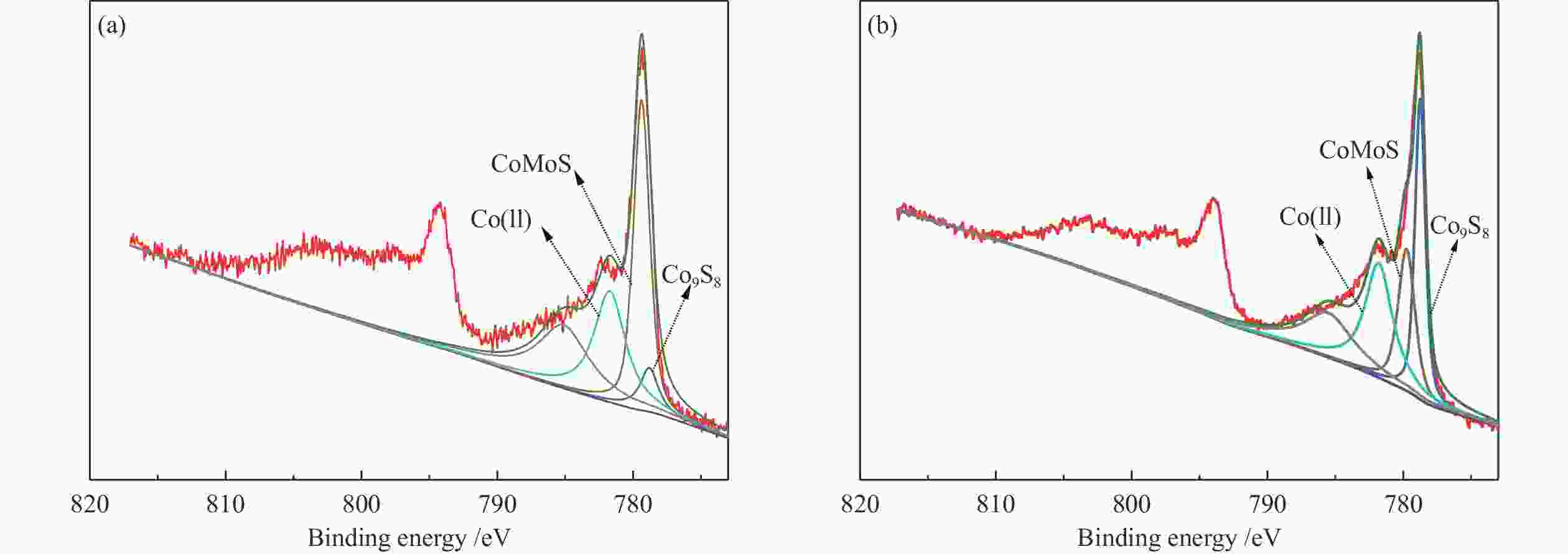
Catalyst Concentration w/% Mo(Ⅳ) Mo(Ⅴ) Mo(Ⅵ) Ni(Ⅱ) NiMoS NiSx Co(ll) CoMoS Co9S8 Molybdenum naphthenate 71.0 15.1 13.9 Molybdenum naphthenate & nickel naphthenate 85.1 10.3 4.6 36.3 47.8 15.9 Molybdenum naphthenate & nickel iso-octoate 80.0 12.2 7.8 48.3 38.7 13.0 Molybdenum naphthenate & cobalt naphthenate 75.6 11.2 13.2 31.5 55.5 13.0 Molybdenum naphthenate & cobalt iso-octoate 74.4 13.2 12.4 38.8 23.8 37.4 表 5 硫化催化剂透射电镜分析
Table 5 Transmission electron microscopy analysis of the sulfide catalyst
Catalyst $\bar L$/nm $\bar N$ fe /% fc /% Molybdenum naphthenate 4.35 3.04 22.89 4.32 Molybdenum naphthenate & nickel naphthenate 3.91 3.38 24.64 5.35 Molybdenum naphthenate & nickel iso-octoate 4.20 3.18 23.46 4.64 Molybdenum naphthenate & cobalt naphthenate 4.13 3.45 23.75 4.80 Molybdenum naphthenate & cobalt iso-octoate 4.28 3.10 23.17 4.47 表 6 环烷酸钼不同加入量下煤沥青加氢产物分布
Table 6 Hydrogenation product distribution of coal tar pitch with different addition amount of molybdenum naphthenate
The amount of catalyst added Liquid yield w/% Residue w/% Gas volume φ/% Molybdenum naphthenate 1.5×10−3 52.3 41.9 5.8 Molybdenum naphthenate 2.0×10−3 60.3 31.3 8.4 Molybdenum naphthenate 3.0×10−3 82.6 13.3 4.1 Molybdenum naphthenate 4.0×10−3 55.3 40.0 4.7 Molybdenum naphthenate 5.0×10−3 50.0 46.0 4.0 表 7 双金属等比例煤沥青加氢产物分布
Table 7 Distribution of hydrogenation products of catalysts with equal proportion of bimetal
Total amount of catalyst 2.0×10−3 Liquid yield w/% Residue w/% Gas volume φ/% Molybdenum naphthenate & nickel naphthenate 85.3 10.6 4.1 Molybdenum naphthenate & nickel iso-octoate 70.0 23.0 7.0 Molybdenum naphthenate & cobalt naphthenate 68.0 24.3 7.7 Molybdenum naphthenate & cobalt iso-octoate 66.7 26.3 7.0 表 8 四组分分析
Table 8 Results of four-component analyses
Content w/% saturated aromatic resin asphaltene toluene insoluble
mattersum Molybdenum naphthenate 9.3 31.1 13.9 36.1 7.2 97.6 Molybdenum naphthenate & nickel naphthenate 21.0 40.6 13.0 12.0 8.6 95.2 Molybdenum naphthenate & nickel iso-octoate 17.6 39.1 14.2 15.6 8.0 94.5 Molybdenum naphthenate & cobalt naphthenate 16.3 35.6 15.2 20.6 8.6 96.3 Molybdenum naphthenate & cobalt iso-octoate 10.6 33.5 11.3 29.8 9.0 94.2 表 9 不同环烷酸钼&环烷酸镍催化剂作用下煤沥青的加氢产物分布
Table 9 Distribution of hydrogenated products over catalysts with various ratios of molybdenum naphthenate to nickel naphthenate
Total amount of catalyst 2.0×10−3 Liquid yield w/% Residue w/% Gas volume φ/% Molybdenum naphthenate & nickel naphthenate(1∶2) 75.0 20.0 5.0 Molybdenum naphthenate & nickel naphthenate(2∶3) 76.0 17.3 6.7 Molybdenum naphthenate & nickel naphthenate(1∶1) 85.3 10.6 4.1 Molybdenum naphthenate & nickel naphthenate(3∶2) 68.0 22.6 9.4 Molybdenum naphthenate & nickel naphthenate(2∶1) 67.0 23.3 9.7 -
[1] HE D M, GUAN J, WU D, ZHAO S C, ZHANG Q M. Modification of coal tar pitch to reduce the carcinogenic polycyclic aromatic hydrocarbons[J]. Appl Mech and Mater,2013,295−298:3098−3103. doi: 10.4028/www.scientific.net/AMM.295-298.3098 [2] 肖劲, 王英, 刘永东, 赖廷清, 李劼. 煤沥青的改性研究进展[J]. 炭素技术,2010,29(2):31−37. doi: 10.3969/j.issn.1001-3741.2010.02.008XIAO Jin, WANG Ying, LIU Yong-dong, LAI Ting-qing, LI Jie. Progress in coal tar pitch modification[J]. Carbon Tech,2010,29(2):31−37. doi: 10.3969/j.issn.1001-3741.2010.02.008 [3] 党阿磊, 李铁虎, 张文娟, 赵廷凯, 方长青, 王珍. 煤沥青的最新研究进展[J]. 炭素技术,2011,30(6):19−23. doi: 10.3969/j.issn.1001-3741.2011.06.006DANG A-lei, LI Tie-hu, ZHANG Wen-juan, ZHAO Ting-kai, FANG Chang-qing, WANG Zhen. Newest research in progress of coal tar pitch[J]. Carbon Techn,2011,30(6):19−23. doi: 10.3969/j.issn.1001-3741.2011.06.006 [4] 常宏宏, 魏文珑, 王志忠, 杨怀旺, 姚润生. 煤沥青的性质及应用[J]. 山西焦煤科技,2007,(2):39−42+46. doi: 10.3969/j.issn.1672-0652.2007.02.014CHANG Hong-hong, WEI Wen-long, WANG Zhi-zhong, YANG Huai-wang, YAO Run-sheng. Properties and application of coal tar pitch[J]. Shanxi Coking Coal Sci & Technol,2007,(2):39−42+46. doi: 10.3969/j.issn.1672-0652.2007.02.014 [5] WANG L, WANG J, JIA F, WANG C, CHEN M. Nanoporous carbon synthesized with coal tar pitch and its capacitive performance[J]. J Mater Chem A,2013,01(33):9498−9507. doi: 10.1039/c3ta10426e [6] DIAZ C, BLANCO C G. NMR: A powerful tool in the characterization of coal tar pitch[J]. Energy Fuels,2003,17(4):907−913. [7] SUN Z, LI D, MA H, TIAN P, LI X, LI W, ZHU Y. Characterization of asphaltene isolated from low-temperature coal tar[J]. Fuel Process Technol,2015,138:413−418. doi: 10.1016/j.fuproc.2015.05.008 [8] 梁文杰. 重质油化学[M]. 东营: 石油大学出版社, 2000.LIANG Wen-jie. Heavy Oil Chemistry[M]. Dongying: Petroleum University Press, 2000. [9] LIM S H, GO K S, KWON E H, NHO N S, LEE J G. Investigation of asphaltene dispersion stability in slurry-phase hydrocracking reaction[J]. Fuel,2020,271:117509. doi: 10.1016/j.fuel.2020.117509 [10] 赵凌云. 临氢缓和条件下的煤油共炼技术研究及初步工艺设计[D]. 青岛: 中国石油大学(华东), 2016.ZHAO Lin-yun. Studies on coal/oil Co-Hydroprocessing with mild conditions and preliminary process design[D]. Qingdao: China University of Petroleum (East China), 2016. [11] 薛永兵, 凌开成, 邹纲明. 煤直接液化中溶剂的作用和种类[J]. 煤炭转化,1999,22(4):1−4. doi: 10.3969/j.issn.1004-4248.1999.04.001XUE Yong-bing, LIN Kai-cheng, ZOU Gang-ming. Functions and kinds of solvents in coal direct liquefaction[J]. Coal Convers,1999,22(4):1−4. doi: 10.3969/j.issn.1004-4248.1999.04.001 [12] 王学云, 赵渊, 颜丙峰. 不同重油作为煤油共炼溶剂的成浆性研究[J]. 煤质技术,2019,34(3):7−10+14. doi: 10.3969/j.issn.1007-7677.2019.03.002WANG Xue-yun, ZHAO Yuan, YAN Bing-feng. Study on the slurry ability of different heavy oils using as coal-oil co-processing solvent[J]. Coal Qual Technol,2019,34(3):7−10+14. doi: 10.3969/j.issn.1007-7677.2019.03.002 [13] 裴婷, 陈刚, 卢永斌, 李波. 煤油共炼技术的开发与催化剂研究[C]. 第十一届全国工业催化技术及应用年会论文集, 2014: 30−33.PEI Ting, CHEN Gang, LU Yong-bin, LI Bo. Development of Kerosene Co-Refining Technology and Catalyst Research[C]. Proceedings of the 11th Annual National Conference on Industrial Catalytic Technology and Applications, 2014: 30−33. [14] 黄传峰, 李大鹏, 杨涛. 煤油共炼技术现状及研究趋势讨论[J]. 现代化工,2016,36(8):8−13.HUANG Chuan-feng, LI Da-peng, YANG Tao. Status and research trends of co-processing of coal and oil[J]. Modern Chemical Industry,2016,36(8):8−13. [15] 郭强, 高雄成, 艾克利. 悬浮床加氢裂化技术在煤油共炼装置的应用[J]. 中国石油石化,2017,(4):45−46.GUO Qiang, GAO Xiong-cheng, AI Ke-li. Application of suspended bed hydrocracking technology in kerosene co-refining unit[J]. China Petrochem,2017,(4):45−46. [16] 石慕尔, 钟长文. 均相催化剂环烷酸钼的制备[J]. 大庆石油学院学报,1998,22(4):40−42+102.SHI Mu-er, ZHONG Chang-wen. Preparation of homogeneous catalyst molybdenum naphthenate[J]. J of Daqing Pet Inst,1998,22(4):40−42+102. [17] 胡志孟. 环烷酸钼的合成及其抗磨性[J]. 中国钼业,1999,23(6):33−34.HU Zhi-meng. Synthesis and antiwear performance of molybdenum naphthenate[J]. China Molybdenum Ind,1999,23(6):33−34. [18] 郭永辉, 杨占奎. 环烷酸镍的应用与制备[J]. 价值工程,2010,29(24):247. doi: 10.3969/j.issn.1006-4311.2010.24.228GUO Yong-hui, YANG Zhan-kui. Applications and preparation of nickel naphthenate[J]. Value Eng,2010,29(24):247. doi: 10.3969/j.issn.1006-4311.2010.24.228 [19] 贺晓慧. 异辛酸钴合成工艺的研究[J]. 石油化工,1999,28(3):36−38.HE Xiao-hui. Study on the synthesis of cobalt iso-octoate[J]. Petrochem Technol,1999,28(3):36−38. [20] 秦毅红, 何汉兵, 程玉贤, 黄草明. 异辛酸钴合成工艺研究[J]. 有色金属(冶炼部分),2005,(6):39−41+45.QIN Yi-hong, HE Han-bing, CHEN Yu-xian, HUANG Cao-ming. Study on the synthesis of cobalt iso-octoate[J]. Nonferrous Met (Smelting Part),2005,(6):39−41+45. [21] 王玉梅, 颜英. 环烷酸钴的制备[J]. 辽宁化工,2002,31(10):435−436+455. doi: 10.3969/j.issn.1004-0935.2002.10.007WANG Yu-mei, YAN Ying. Preparation of cobalt naphthenate[J]. Liaoning Chem Ind,2002,31(10):435−436+455. doi: 10.3969/j.issn.1004-0935.2002.10.007 [22] LI M, LI H, JIANG F, CHU Y, NIE H. The Relation between morphology of (Co)MoS2 phases and selective hydrodesulfurization for CoMo catalysts[J]. Catal Today,2010,149:35−39. doi: 10.1016/j.cattod.2009.03.017 [23] 窦民娜, 修远, 曹青, 徐华, 肖占敏. 石油沥青及渣油的组成分析方法研究进展[J]. 石化技术与应用,2019,37(5):356−360. doi: 10.3969/j.issn.1009-0045.2019.05.020DOU Min-na, XIU Yuan, CAO Qing, XU Hua, XIAO Zhan-min. Review of test methods for four components of residue and petroleum bitumen[J]. Petrocheml Technol & Appl,2019,37(5):356−360. doi: 10.3969/j.issn.1009-0045.2019.05.020 [24] 严方, 谢永杰. 大庆原油四组分分析及界面性质研究[J]. 化学分析计量,2009,18(4):20−24. doi: 10.3969/j.issn.1008-6145.2009.04.006YAN Fang, XIE Yong-jie. Investigation of separation of four fractions and interfacial properties of Da Qing crucial oil[J]. Chem Anal Meterage,2009,18(4):20−24. doi: 10.3969/j.issn.1008-6145.2009.04.006 [25] 李洪峰, 周子兵. 对沥青化学组分分析方法的研究[J]. 黑龙江交通科技,2004,(8):17−18. doi: 10.3969/j.issn.1008-3383.2004.08.013LI Hong-feng, ZHOU Zi-bing. Study on the method of chemical component analysis of asphaltene[J]. Heilongjiang Jiaotong Keji,2004,(8):17−18. doi: 10.3969/j.issn.1008-3383.2004.08.013 [26] 沐宝泉, 张淑霞. 重油四组分测定实验改进及优化教学[J]. 化学教育(中英文),2020,41(12):75−78.MU Bao-quan, ZHANG Shu-xia. Improvement and optimization teaching of heavy oil four components analysis test[J]. Chem Educ (Chinese and English),2020,41(12):75−78. [27] 郭淑香. 重质油组成四组分的分析实验[J]. 精细石油化工,2013,30(6):75−77. doi: 10.3969/j.issn.1003-9384.2013.06.021GUO Shu-xiang. Analysis of four component of heavy oil[J]. Spec Petrochem,2013,30(6):75−77. doi: 10.3969/j.issn.1003-9384.2013.06.021 [28] LI Y, ZHANG T, LIU D, LIU B, LU Y, CHAI Y, LIU C. Study of the promotion effect of citric acid on the active NiMoS phase in NiMo/Al2O3 catalysts[J]. Ind Eng Chem Res,2019,58(37):17195−17206. [29] CORTÉS-JÁCOME M A, ESCOBAR J, ANGELES C C, LÓPEZ-SALINAS E, ROMERO E, FERRAT G, TOLEDO-ANTONIO J A. Highly dispersed CoMoS phase on titania nanotubes as efficient HDS catalysts[J]. Catal Today,2008,130(01):56−62. doi: 10.1016/j.cattod.2007.07.012 [30] KIM K D, LEE Y K. Active phase of dispersed mos2 catalysts for slurry phase hydrocracking of vacuum residue[J]. J Catalysis,2019,369:111−121. doi: 10.1016/j.jcat.2018.10.013 [31] KRIJN P D J, LEON C A V D O, EELCO T C V, SONJA E, ABRAHAM J, HEINER F, PETRA E, DE J. High-resolution electron tomography study of an industrial Ni-Mo/-Al2O3 hydrotreating catalyst[J]. J Phys Chem B,2006,110(21):10209−10212. doi: 10.1021/jp061584f [32] DEVERS E, AFANASIEV P, JOUGUET B, VRINAT M. Hydrothermal syntheses and catalytic properties of dispersed molybdenum sulfides[J]. Cata Lett,2002,82:13−17. doi: 10.1023/A:1020512320773 [33] 黄澎, 李文博, 毛学锋, 赵鹏. 中温热解焦油重馏分悬浮床加氢裂化的研究[J]. 燃料化学学报,2020,48(2):154−162. doi: 10.3969/j.issn.0253-2409.2020.02.004HUANG Peng, LI Wen-bo, MAO Xue-feng, ZHAO Peng. Study on suspension bed hydrocracking of medium temperature pyrolytic heavy tar fraction[J]. J Fuel Chem Technol,2020,48(2):154−162. doi: 10.3969/j.issn.0253-2409.2020.02.004 [34] 戴鑫, 邓文安. 亲油型二硫化钼在渣油悬浮床加氢裂化中的应用[J]. 炼油技术与工程,2018,48(4):40−44. doi: 10.3969/j.issn.1002-106X.2018.04.011DAI Xin, DENG Wen-an. Application oil-soluble MoS2 in slurry-bed hydrocraking for heavy oil[J]. Pet Ref Eng,2018,48(4):40−44. doi: 10.3969/j.issn.1002-106X.2018.04.011 [35] HENRIK T. In situ mössbauer emission spectroscopy studies of unsupported and supported sulfided CoMo hydrodesulfurization catalysts: Evidence for and nature of a CoMoS phase[J]. J Catalysis,1981,68(02):433−452. doi: 10.1016/0021-9517(81)90114-7 [36] DAAGE M, CHIANELLI R R. Structure-function relations in molybdenum sulfide catalysts: The "Rim-Edge" model[J]. J Catal,1994,149(2):414−427. doi: 10.1006/jcat.1994.1308 [37] ZHENG P, LI T, CHI K, XIAO C, FAN J, WANG X, DUAN A. DFT Insights into the formation of sulfur vacancies over corner/edge site of Co/Ni-promoted MoS2 and WS2 under the hydrodesulfurization conditions[J]. Appl Cata B Environ,2019,257:117937. doi: 10.1016/j.apcatb.2019.117937 [38] LI C, HAN Y, YANG T, DENG W. Preliminary study on the influence of catalyst dosage on coke formation of heavy oil slurry-bed hydrocracking[J]. Fuel,2020,270:117489. doi: 10.1016/j.fuel.2020.117489 -





 下载:
下载: