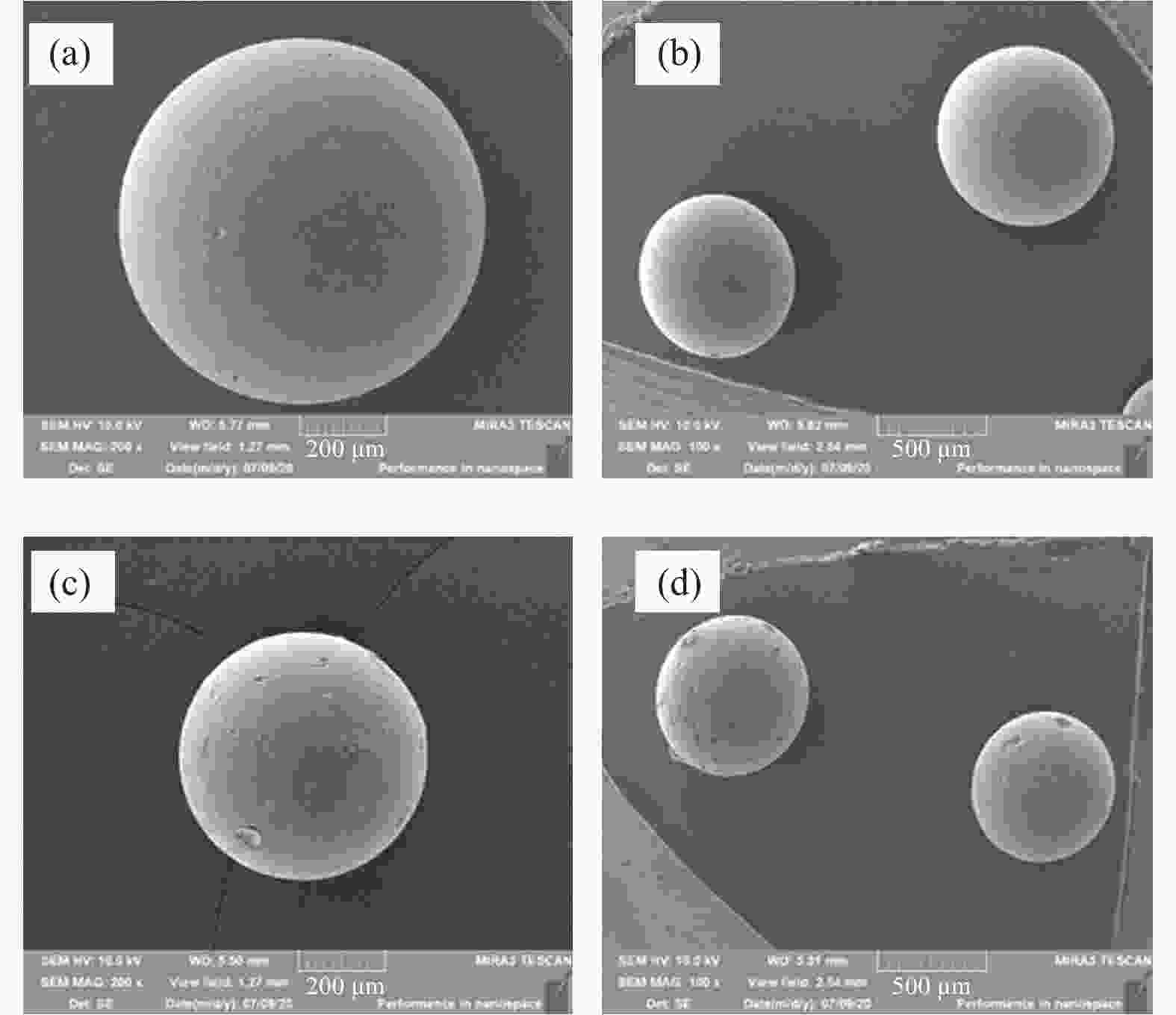
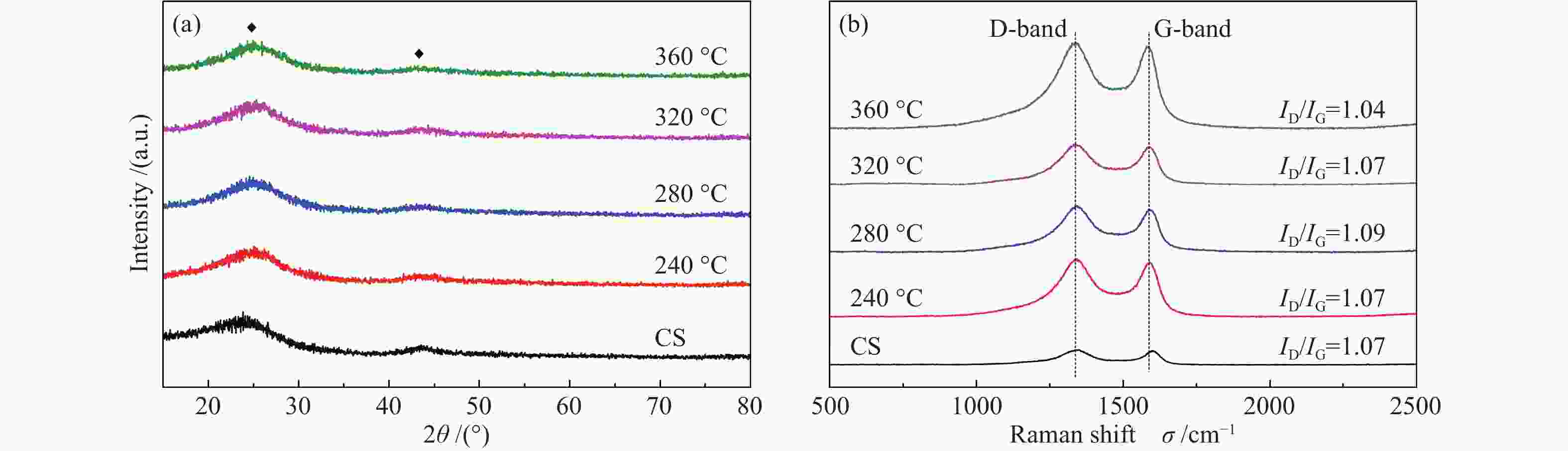
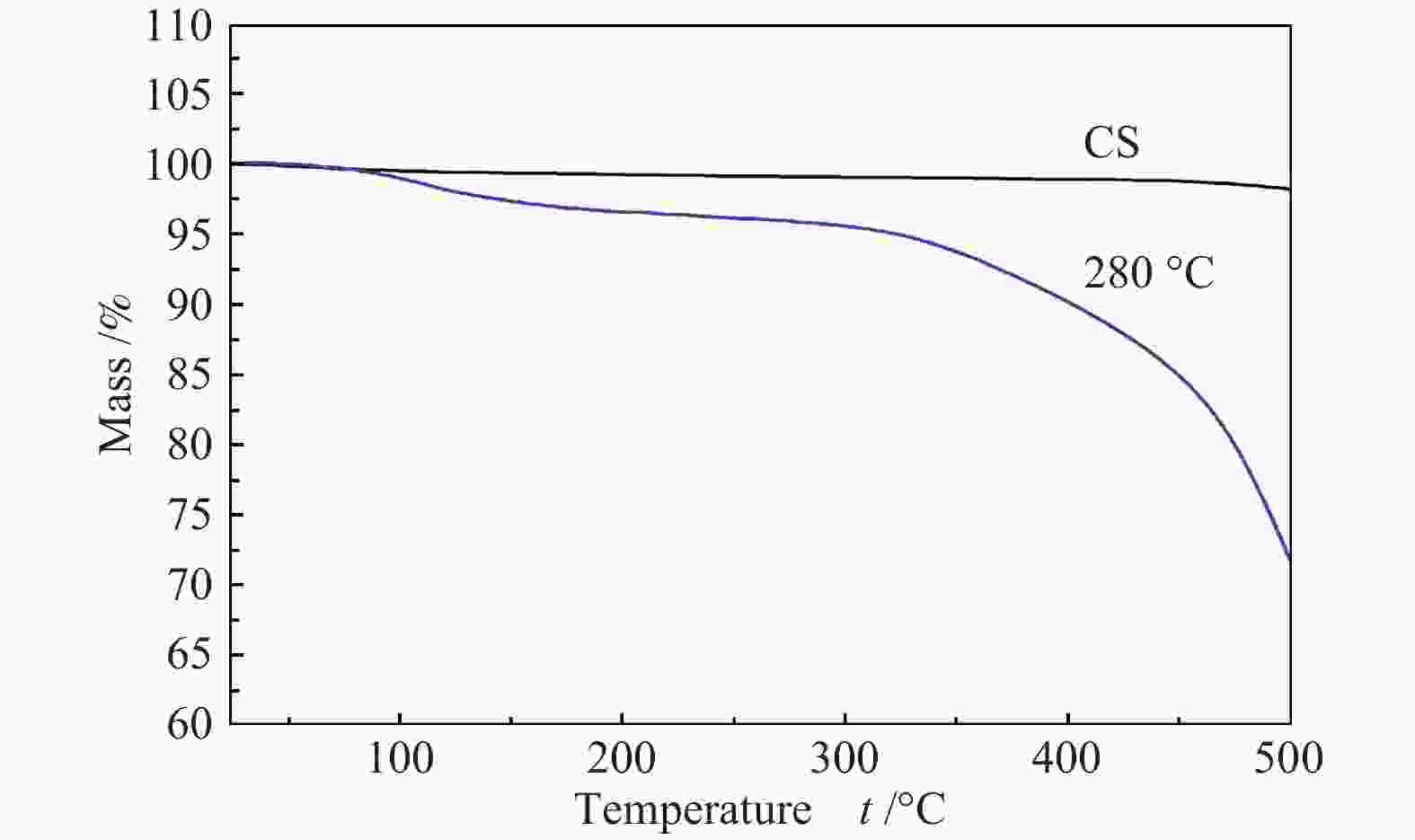
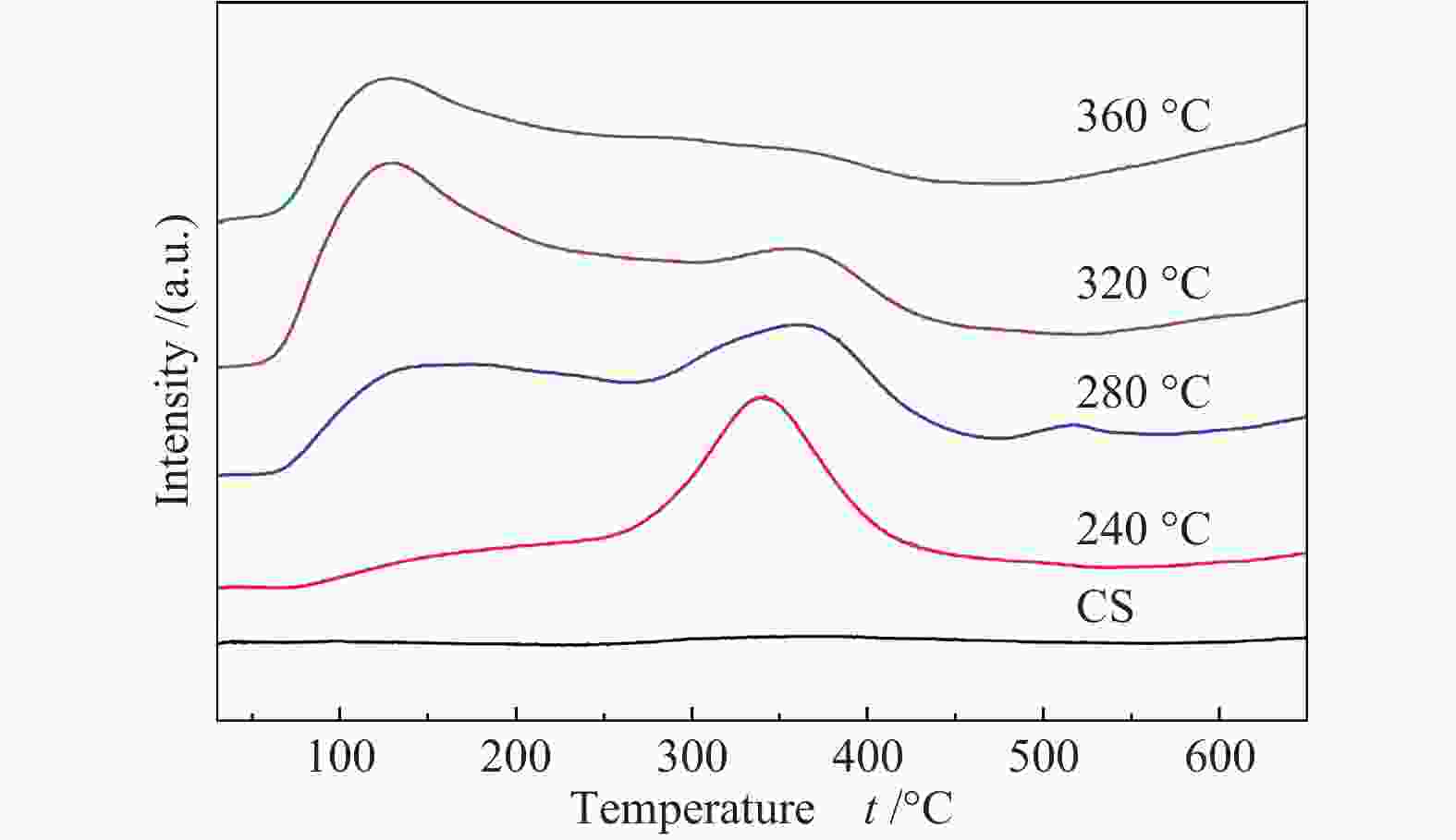
Effects of calcination temperature on the catalytic performance of Ti(SO4)2/CS for DME direct oxidation to polyoxymethylene dimethyl ethers
-
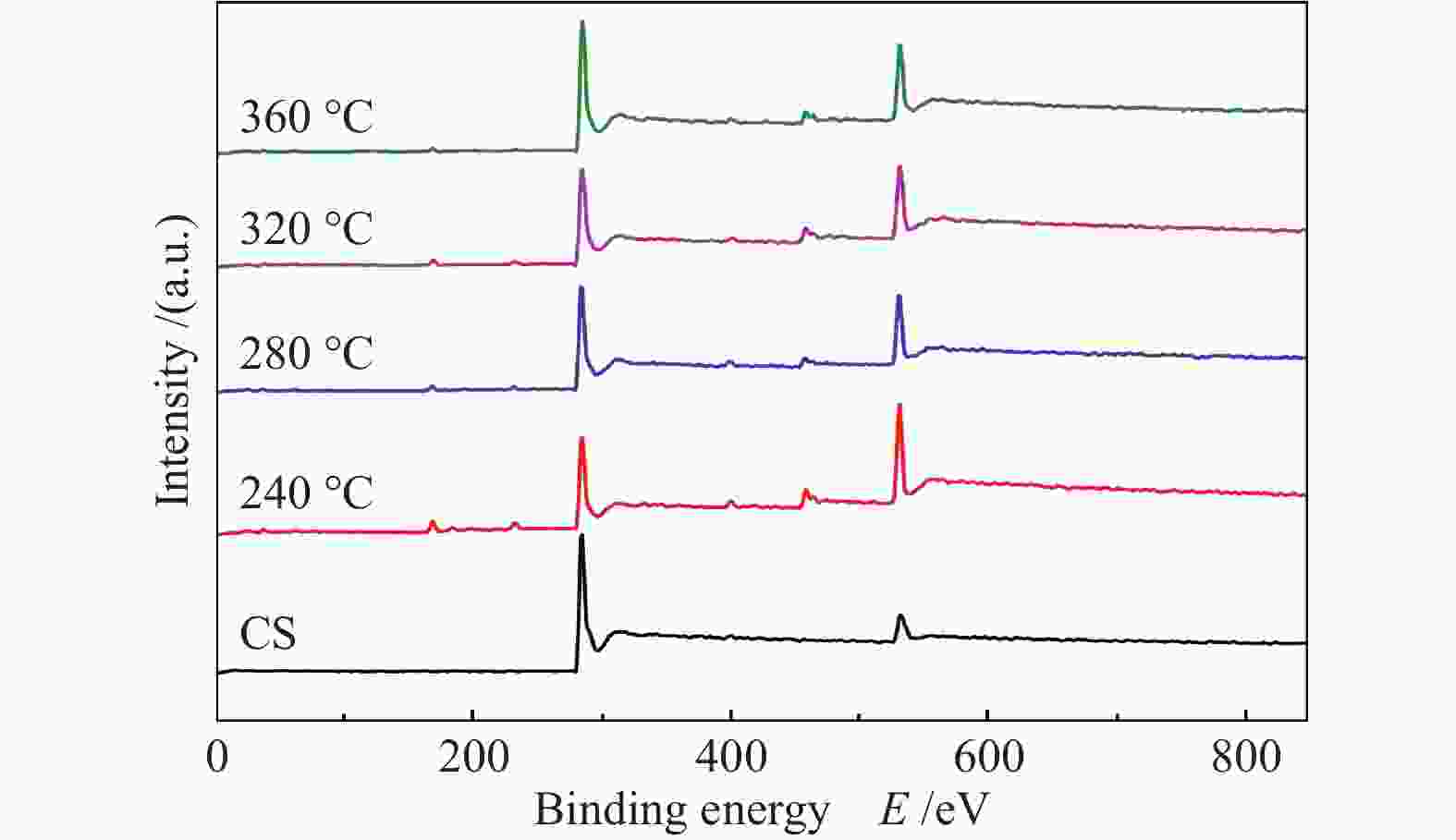
摘要: 采用等体积浸渍法制备了活性炭球(CS)负载Ti(SO4)2的双功能催化剂,考察了焙烧温度对Ti(SO4)2/CS催化剂选择氧化二甲醚(DME)直接合成聚甲氧基二甲醚(DMMx)催化性能的影响。结果表明,不同焙烧温度制备的Ti(SO4)2/CS催化剂表现出明显的催化活性差异,280 ℃焙烧的30% Ti(SO4)2/CS催化剂具有最佳性能,DME转化率为11.7%,DMM1−3的选择性达到75.8%,其中,DMM2, 3选择性在30%以上。采用SEM、XRD、Raman、TG、NH3-TPD及XPS等表征手段研究了催化剂的结构及表面性质。焙烧温度改变了活性炭球表面官能团的分布,进而影响了Ti(SO4)2的分散状态,调变了酸中心种类和数量,尤其是弱酸和中强酸的比例,使催化剂表面酸性强弱呈现不同梯度,催化剂的酸性和氧化还原性位达到比较合理的匹配,进一步促进了C−O链的增长。。
-
关键词:
- 二甲醚 /
- 直接氧化 /
- 聚甲氧基二甲醚 /
- 焙烧温度 /
- Ti(SO4)2/CS
Abstract: A series of Ti(SO4)2/activated carbon spheres (CS) bifunctional catalysts were designed and prepared by impregnation method, and the effect of calcination temperature of the catalysts on direct oxidation of dimethyl ether (DME) to polyoxymethylene dimethyl ethers (DMMx) was investigated. The results showed that the performance of Ti(SO4)2/CS catalysts was closely related to the calcination temperature. The 30% Ti(SO4)2/CS catalyst calcined under O2 atmosphere at 280 ℃ exhibited excellent activity over which the conversion of DME reached 11.7% with the selectivity of DMM1−3 up to 75.8%, wherein, the selectivity of DMM2−3 was over 30%. The texture and surface properties of the catalysts were characterized by SEM, XRD, Raman, TG, NH3-TPD and XPS. The suitable amount of weak acid sites and redox sites of the Ti(SO4)2/CS were beneficial to the direct oxidation of DME to DMMx. The calcination temperature changed the distribution of functional groups on the surface of CS which then affected the dispersion form of Ti(SO4)2. The type and amount of acid centers especially the ratio of weak acid and medium strong acid could also be adjusted, which can lead to different gradients of the surface acidity of the catalyst. The reasonable matching of the acidic and redox sites on the catalyst can evidently promote the growth of C−O chain. -
表 1 焙烧温度对Ti(SO4)2/CS催化性能的影响
Table 1 Effects of calcination temperature on the performance of Ti(SO4)2/CS for DME oxidation to DMMx
Catalyst DME Conv. x/% Selectivity sC-mol/% DMM DMM2 DMM3 DMM1−3 CH3OH HCHO MF COx CS 11.4 trace 0.0 0.0 trace 100 0.0 0.0 0.0 30% Ti(SO4)2/CS(O2, 240 ℃) 11.4 72.8 1.8 0.0 74.6 14.7 0.0 1.7 9.0 30% Ti(SO4)2/CS(O2, 280 ℃) 11.7 43.3 30.4 2.1 75.8 20.7 0.3 0.6 2.6 30% Ti(SO4)2/CS(O2, 320 ℃) 11.7 60.4 14.3 0.0 74.7 20.6 0.0 0.8 3.9 30% Ti(SO4)2/CS(O2, 360 ℃) 10.6 80.0 0.0 0.0 80.0 16.5 0.0 2.5 1.0 reaction conditions: 240 ℃, atmospheric pressure, cat: 1 mL, 3600 h−1, nDME:nO2 = 1:1, DMM: CH3OCH2OCH3;
DMM2: CH3OCH2OCH2OCH3; DMM3: CH3OCH2OCH2OCH2OCH3; MF: HCOOCH3表 2 NH3-TPD量化
Table 2 Quantitative analysis of NH3-TPD measurements
Catalyst AwA AMSA AMSA/AwA CS 108 1059 9.81 30% Ti(SO4)2/CS(O2, 240 ℃) 618 21555 34.88 30% Ti(SO4)2/CS(O2, 280 ℃) 14913 14586 0.98 30% Ti(SO4)2/CS(O2, 320 ℃) 28077 9736 0.35 30% Ti(SO4)2/CS(O2, 360 ℃) 20944 2494 0.12 AwA: area of weak acid; AMSA: area of medium strong acid 表 3 不同温度焙烧的Ti(SO4)2/CS催化剂的XPS C 1s谱图分析
Table 3 XPS C 1s spectra analysis of Ti(SO4)2/CS catalysts with different calcination temperature
Catalyst C−C/C−H w/% C−O−C/C−OH w/% C=O w/% COOH w/% π→π* w/% CS 63.84 20.51 3.82 5.00 6.85 30% Ti(SO4)2/CS(O2, 240 ℃) 57.45 26.86 4.56 6.13 5.00 30% Ti(SO4)2/CS(O2, 280 ℃) 63.22 17.66 9.09 4.23 5.81 30% Ti(SO4)2/CS(O2, 320 ℃) 56.25 19.70 8.44 4.25 11.36 30% Ti(SO4)2/CS(O2, 360 ℃) 58.70 21.01 5.00 5.92 9.36 -
[1] GA B V, THAI P Q. Soot emission reduction in a Biogas-DME hybrid dual-fuel engine[J]. Appl Sci-Basel,2020,10(10):3416−3434. [2] PALOMO J, RODRIGUEZ-CANO M A, RODRIGUEZ-MIRASOL J, CORDERO T. ZSM-5-decorated CuO/ZnO/ZrO2 fibers as efficient bifunctional catalysts for the direct synthesis of DME from syngas[J]. Appl Catal B: Environ,2020,270:118893. [3] SHENG Q T, YE R P, GONG W B, SHI X F, XU B, ARGYLE M, ADIDHARMA H, FAN M H. Mechanism and catalytic performance for direct dimethyl ether synthesis by CO2 hydrogenation over CuZnZr/ferrierite hybrid catalyst[J]. J Environ Sci,2020,92:106−117. [4] ZUO H M, MAO D S, GUO X M, YU J. Highly efficient synthesis of dimethyl ether directly from biomass-derived gas over Li-modified Cu-ZnO-Al2O3/HZSM-5 hybrid catalyst[J]. Renew Energy,2018,116:38−47. [5] ZENG L Y, WANG Y Z, MOU J, LIU F, YANG C L, ZHAO T X, WANG X D, CAO J X. Promoted catalytic behavior over gamma-Al2O3 composited with ZSM-5 for crude methanol conversion to dimethyl ether[J]. Int J Hydrogen Energy,2020,45(33):16500−16508. [6] PELAEZ R, MARIN P, ORDONEZ S. Synthesis of formaldehyde from dimethyl ether on alumina-supported molybdenum oxide catalyst[J]. Appl Catal A: Gen,2016,527:137−145. [7] 杨奇, 高秀娟, 冯茹, 李明杰, 张俊峰, 张清德, 韩怡卓, 谭猗生. 水热合成的MoO3-SnO2催化剂催化氧化二甲醚的性能研究[J]. 燃料化学学报,2019,47(8):934−941. doi: 10.3969/j.issn.0253-2409.2019.08.005YANG Qi, GAO Xiu-juan, FENG Ru, LI Ming-jie, ZHANG Jun-feng, ZHANG Qing-de, HAN Yi-zhuo, TAN Yi-sheng. MoO3-SnO2 catalyst prepared by hydrothermal synthesis method for dimethyl ether catalytic oxidation[J]. J Fuel Chem Technol,2019,47(8):934−941. doi: 10.3969/j.issn.0253-2409.2019.08.005 [8] 高秀娟, 王文峰, 张振洲, 张清德, 谭猗生, 韩怡卓. 二甲醚氧化制聚甲氧基二甲醚的研究进展[J]. 石油化工,2017,46(2):143−150. doi: 10.3969/j.issn.1000-8144.2017.02.001GAO Xiu-juan, WANG Wen-feng, ZHANG Zhen-zhou, ZHANG Qing-de, TAN Yi-sheng, HAN-Yi-zhuo. Progresses in synthesis of polymeyhylene dimethyl ethers from dimethyl ether[J]. Petrochem Technol,2017,46(2):143−150. doi: 10.3969/j.issn.1000-8144.2017.02.001 [9] PACHECO M A, MARSHALL C L. Review of dimethyl carbonate (DMC) manufacture and its characteristics as a fuel additive[J]. Energy Fuels,1997,11(1):2−29. [10] HMED M H M, MURAZA O, AL-AMER A M, MIYAKE K, NISHIYAMA N. Development of hierarchical EU-1 zeolite by sequential alkaline and acid treatments for selective dimethyl ether to propylene (DTP)[J]. Appl Catal A: Gen,2015,497:127−134. [11] ZHANG Q D, TAN Y S, YANG C H, XIE H J, HAN Y Z. Characterization and catalytic application of MnCl2 modified HZSM-5 zeolites in synthesis of aromatics from syngas via dimethyl ether[J]. J Ind Eng Chem,2013,19(3):975−980. [12] BARANOWSKI C J, BAHMANPOUR A M, KROCHER O. Catalytic synthesis of polyoxymethylene dimethyl ethers (OME): A review[J]. Appl Catal B: Environ,2017,217:407−420. [13] ZHENG Y Y, TANG Q, WANG T F, WANG J F. Kinetics of synthesis of polyoxymethylene dimethyl ethers from paraformaldehyde and dimethoxymethane catalyzed by ion-exchange resin[J]. Chem Eng Sci,2015,134:758−766. [14] ZHANG J Q, FANG D Y, LIU D H. Evaluation of Zr-alumina in production of polyoxymethylene dimethyl ethers from methanol and formaldehyde: Performance tests and kinetic investigations[J]. Ind Eng Chem Res,2014,53(35):13589−13597. [15] WU Y J, LI Z, XIA C G. Silica-gel-supported dual acidic ionic liquids as efficient catalysts for the synthesis of polyoxymethylene dimethyl Ethers[J]. Ind Eng Chem Res,2016,55(7):1859−1865. [16] LIU H C, IGLESIA E. Selective one-step synthesis of dimethoxymethane via methanol or dimethyl ether oxidation on H3+nVnMo12-nPO40 Keggin structures[J]. J Phys Chem B,2003,107(39):10840−10847. [17] ZHANG Q D, TAN Y S, YANG C H, LIU Y Q, HAN Y Z. Catalytic oxidation of dimethyl ether to dimethoxymethane over MnCl2-H4SiW12O40/SiO2 catalyst[J]. Chin J Catal,2006,27(10):916−920. [18] ZHANG Q D, TAN Y S, YANG C H, HAN Y Z, SHAMOTO J, TSUBAKI N. Catalytic oxidation of dimethyl ether to dimethoxymethane over Cs modified H3PW12O40/SiO2 catalysts[J]. J Nat Gas Chem,2007,16(3):322−325. [19] ZHANG Q D, TAN Y S, YANG C H, HAN Y Z. MnCl2 modified H2SiW12O40/SiO2 catalysts for catalytic oxidation of dimethyl ether to dimethoxymethane[J]. J Mol Catal A: Chem,2007,263(1/2):149−155. [20] ZHANG Q D, TAN Y S, LIU G B, ZHANG J F, HAN Y Z. Rhenium oxide-modified H3PW12O40/TiO2 catalysts for selective oxidation of dimethyl ether to dimethoxy dimethyl ether[J]. Green Chem,2014,16(11):4708−4715. [21] GERBER I C, SERP P. A theory/esperience description of support effects in carbon-supported catalysts[J]. Chem Rev,2020,120:1250−1349. [22] ZHANG Q D, WANG W F, ZHANG Z Z, ZHANG J F, BAI Y X, TSUBAKI N, HAN Y Z, TAN Y S. Application of modified CNTs with Ti(SO4)2 in selective oxidation of dimethyl ether[J]. Catal Sci Technol,2016,6(19):7193−7202. [23] GAO X J, WANG W F, GU Y Y, ZHANG Z Z, ZHANG J F, ZHANG Q D, TSUBAKI N, HAN Y Z, TAN Y S. Synthesis of polyoxymethylene dimethyl ethers from dimethyl ether direct oxidation over carbon-based catalysts[J]. ChemCatChem,2018,10(1):273−279. [24] ZHANG G L, GUAN T T, QIAO J L, WANG J L, LI K X. Free-radical-initiated strategy aiming for pitch-based dual-doped carbon nanosheets engaged into high-energy asymmetric supercapacitors[J]. Energy Storage Mater,2020,26:119−128. [25] ZHANG G L, GUAN T T, CHENG M, WANG Y X, XU N N, QIAO J L, XU F F, WANG Y Z, WANG J L, LI K X. Harvesting honeycomb-like carbon nanosheets with tunable mesopores from mild-modified coal tar pitch for high-performance flexible all-solid-state supercapacitors[J]. J Power Sources,2020,448:227446. [26] ZHANG D D, HE C, WANG Y Z, ZHAO J H, WANG J L, LI K X. Oxygen-rich hierarchically porous carbons derived from pitch-based oxidized spheres for boosting the supercapacitive performance[J]. J Colloid Interf Sci,2019,540:439−447. [27] ZHU Y P, JING Y, VASILEFF A, HEINE T, QIAO S Z. 3D synergistically active carbon nanofibers for improved oxygen evolution[J]. Adv Energy Mater,2017,7(14):1602928. [28] QIU S, XIAO L F, SUSHKO M L, HAN K S, SHAO Y Y, YAN M Y, LIANG X M, MAI L Q, FENG J W, CAO Y L, AI X P, YANG H X, LIU J. Manipulating adsorption-insertion mechanisms in nanostructured carbon materials for high-efficiency sodium ion storage[J]. Adv Energy Mater,2017,7(17):1700403. [29] MISHRA A K, RAMAPRABHU S. Functionalized graphene sheets for arsenic removal and desalination of sea water[J]. Desalination,2011,282:39−45. [30] WANG J L, LIU H, LIU Y, WANG W H, SUN Q, WANG X B, ZHAO X Y, HU H, WU M B. Sulfur bridges between Co9S8 nanoparticles and carbon nanotubes enabling robust oxygen electrocatalysis[J]. Carbon,2019,144:259−268. [31] QI W, LIU W, ZHANG B S, GU X M, GUO X L, SU D S. Oxidative dehydrogenation on nanocarbon: Identification and quantification of active Sites by chemical titration[J]. Angew Chem Int Ed,2013,52(52):14224−14228. [32] JUNG S M, GRANGE P. Characterization and reactivity of pure TiO2-SO42- SCR catalyst: Influence of SO42- content[J]. Cataly Today,2000,59(34):305−312. [33] YAMAGUCHI T, JIN T, TANABE K. Structure of acid sites on sulfur-promoted iron oxide[J]. J Phys Chem,1986,90(14):3148−3152. -





 下载:
下载: