Preparation of Ni/SiO2 by ammonia evaporation method for synthesis of 2-MTHF from 2-MF hydrogenation
-
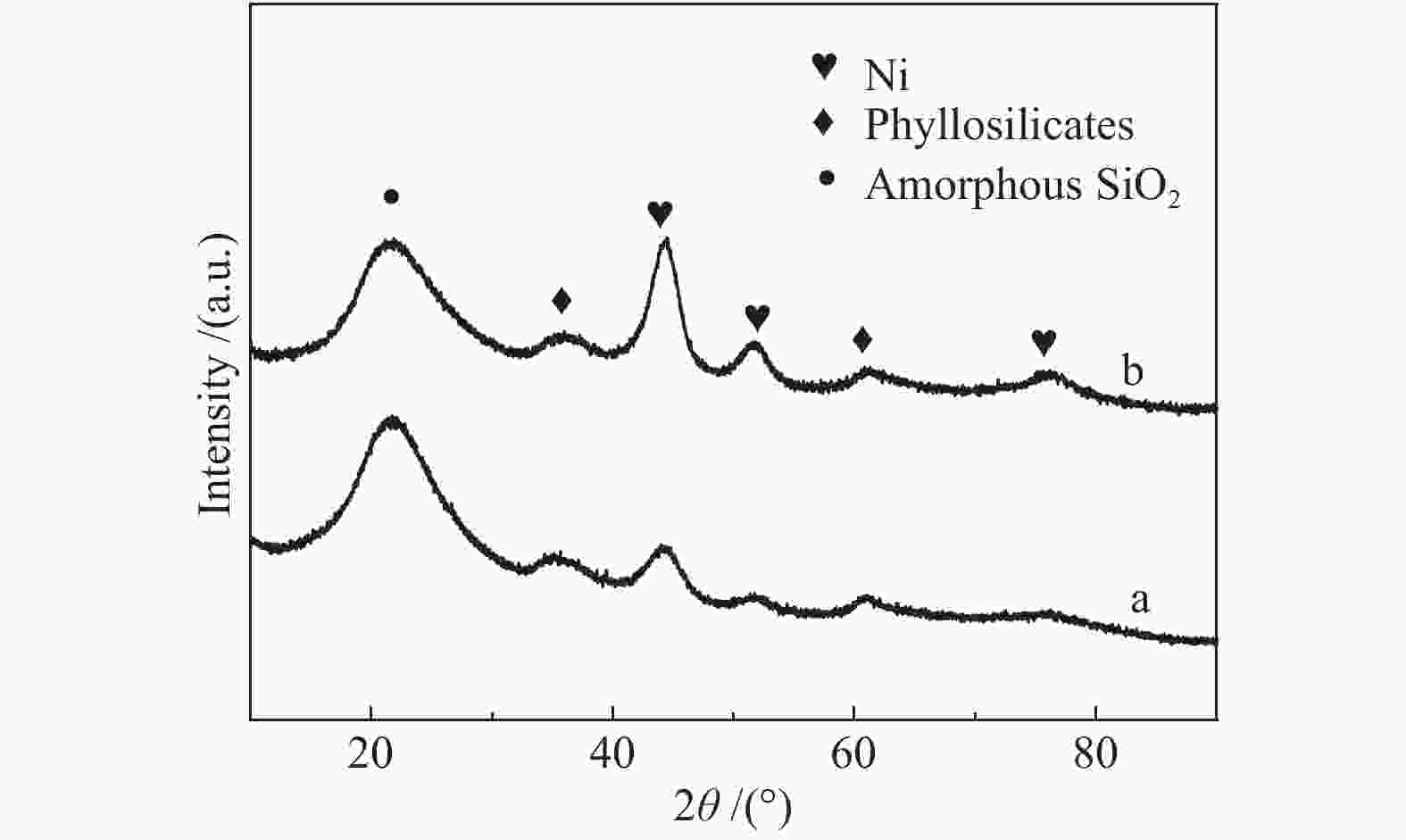
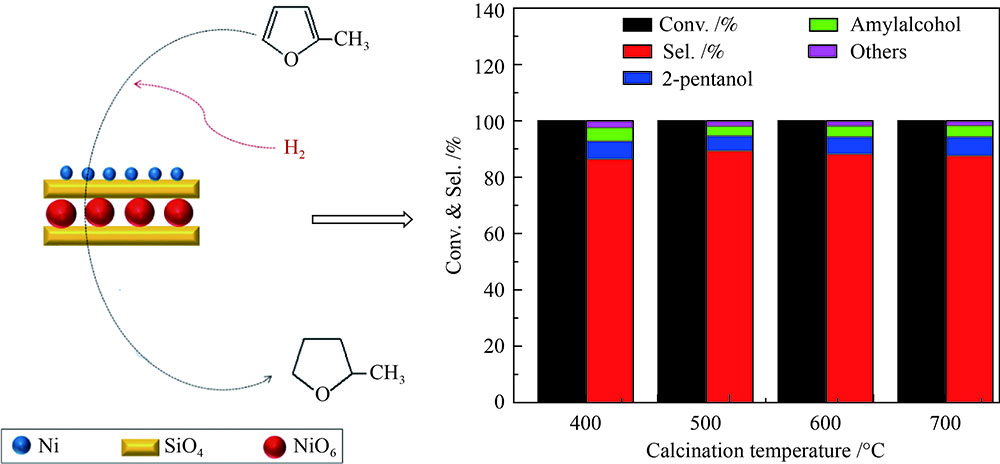
摘要: 以硝酸镍为镍源,碱性硅溶胶为硅源,采用蒸氨法制备了Ni/SiO2催化剂。通过XRD、N2等温吸附脱附、H2-TPR、NH3-TPD、XPS和TG对催化剂进行表征。采用固定床反应器,考察了催化剂焙烧温度及反应条件对催化剂应用于2-甲基呋喃(2-MF)气相加氢合成2-甲基四氢呋喃(2-MTHF)的反应性能。结果表明,蒸氨法制备的Ni/SiO2催化剂在焙烧后均呈现层状硅酸镍结构,还原后保持了该结构,活性组分Ni晶粒尺寸较小、金属载体相互作用较强,从而具有较高的活性。催化剂的焙烧温度影响催化剂Ni晶粒尺寸及催化剂的表面酸性。在500 ℃焙烧条件下制备的催化剂性能最佳,优化反应条件下,2-MF转化率100%,2-MTHF选择性为93.5%,反应15 h内催化性能稳定;含碳的有机物在催化剂表面沉积是催化剂失活的主要原因。Abstract: The Ni/SiO2 catalysts were prepared by ammonia evaporation method, with nickel nitrate as Ni source and silica sol as the SiO2 source, for synthesis of 2-MTHF from 2-MF hydrogenation. The catalytic performance of catalysts prepared at different calcination temperatures were tested on a fixed-bed reactor. XRD, N2 adsorption-desorption, H2-TPR, NH3-TPD, XPS and TG were employed to characterize the structure and surface properties of these catalysts. The effect of calcination temperature on the structure, surface property and catalytic performance of catalysts were investigated. The result indicated that all the catalysts had a phyllosilicate structure after calcination, and maintained the structure after reduction. Ni particles dispersed well with smaller size and strong metal support interaction showed high activity. The surface acidity of catalysts was influenced by the calcination temperature. The maximum catalytic activity and selectivity were obtained on the catalyst calcined by at 500 ℃, which exhibited a 2-MF conversion of 100% and the 2-MTHF selectivity of 93.5% at the optimized condition, due to smaller particle size and suitable surface acidity.
-
Key words:
- 2-methylfuran /
- 2-methyltetrahydrofuran /
- ammonia evaporation /
- hydrogenation /
- Ni/SiO2 catalysts
-
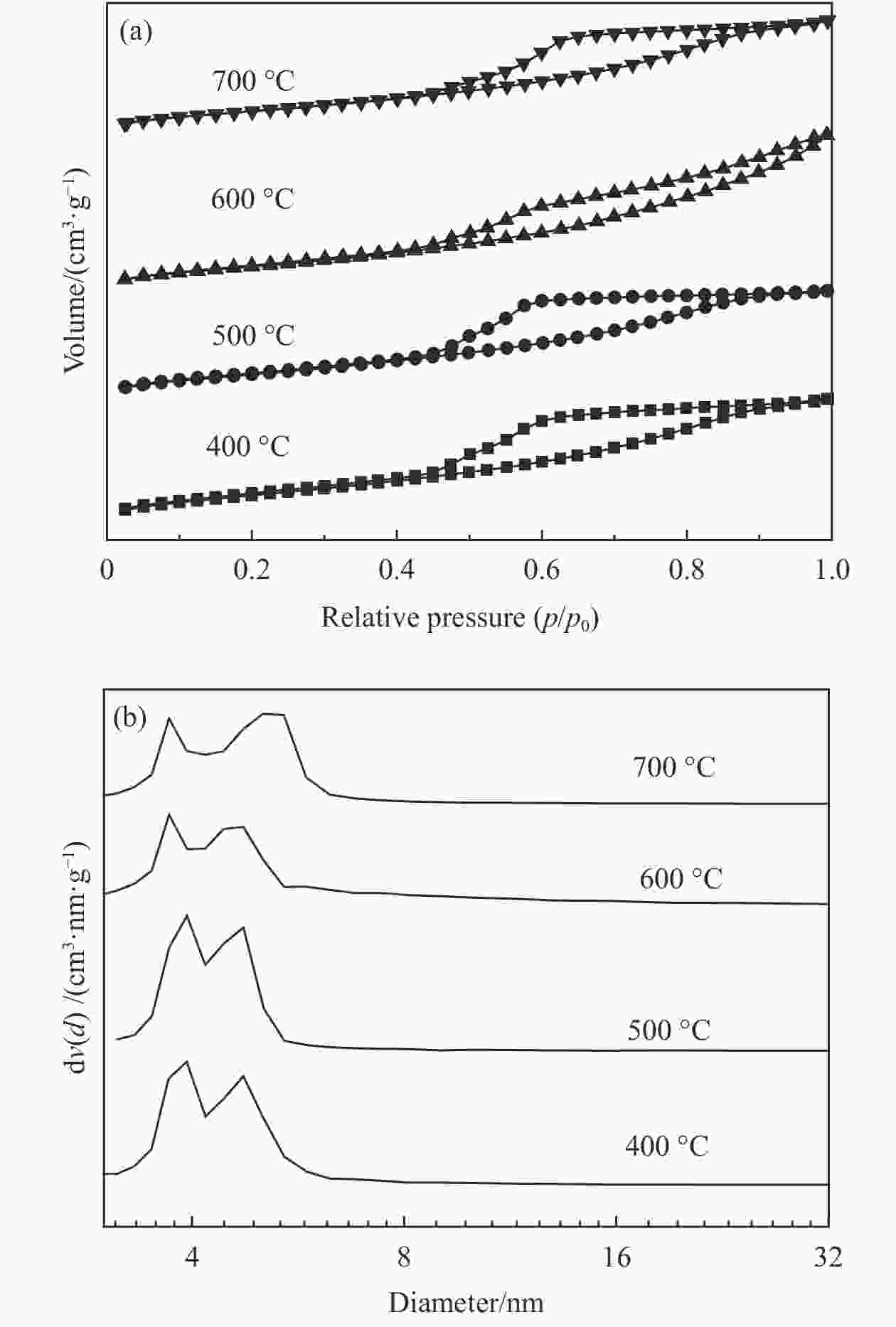
表 1 不同焙烧温度制备的Ni/SiO2催化剂的比表面积、孔容和孔径
Table 1 Specific surface area, pore volume and pore sizes of different catalysts
Catalysts calcination SBET /(m2·g−1) v /(cm3·g−1) d /nm 400 ℃ 391.1 0.510 3.9 500 ℃ 346.7 0.641 3.7 600 ℃ 345.6 0.462 3.9 700 ℃ 296.1 0.486 5.0 表 2 不同焙烧温度制备的Ni/SiO2催化剂的酸量
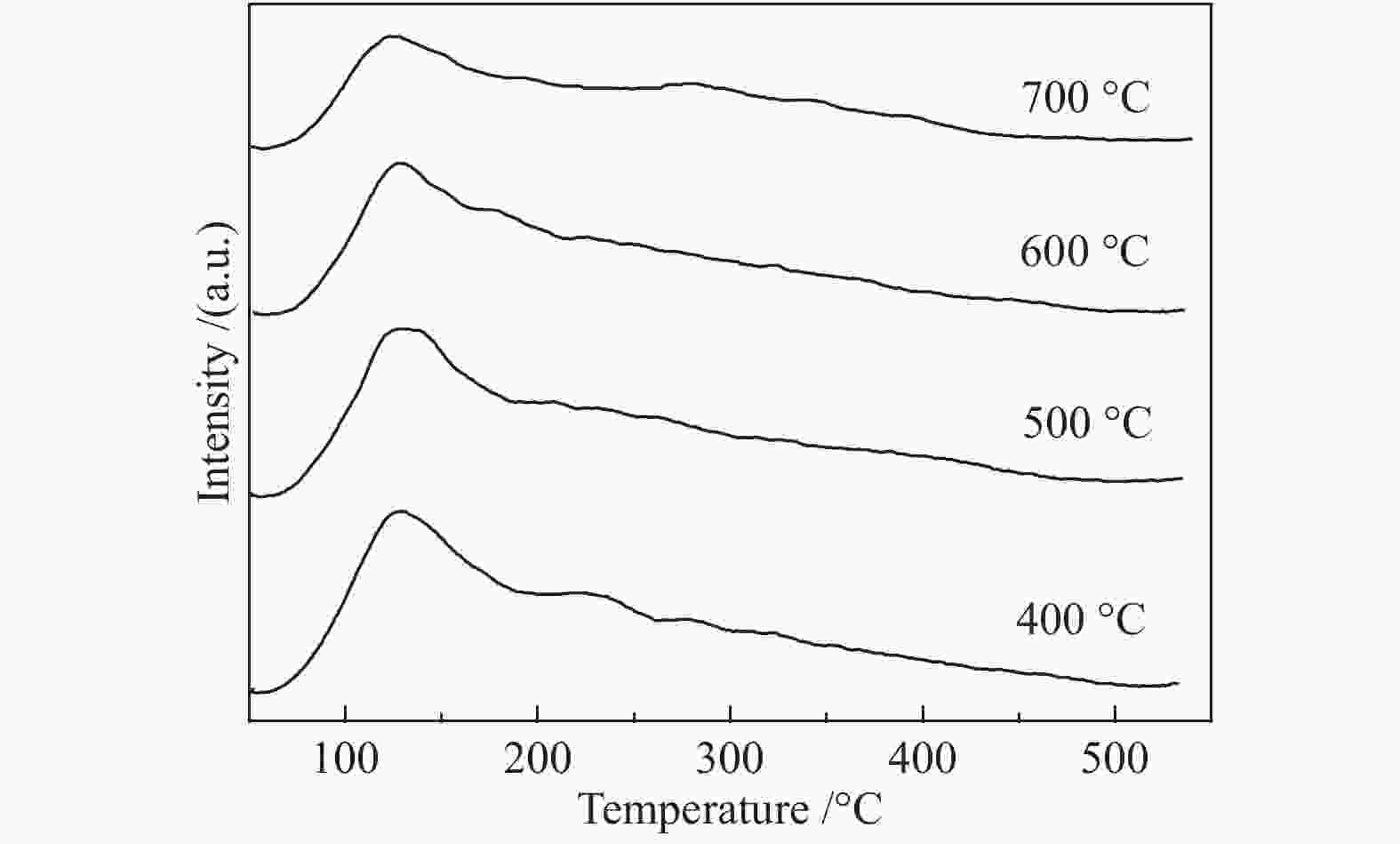
Table 2 Total acidity amount of different catalysts
Catalysts calcination 400 ℃ 500 ℃ 600 ℃ 700 ℃ Total acidity /(mmol $({\rm{NH} }_3)\cdot {\rm{g} }_{\rm{cat} }^{-1}$) 0.519 0.411 0.408 0.369 表 3 不同反应条件下催化剂的催化性能
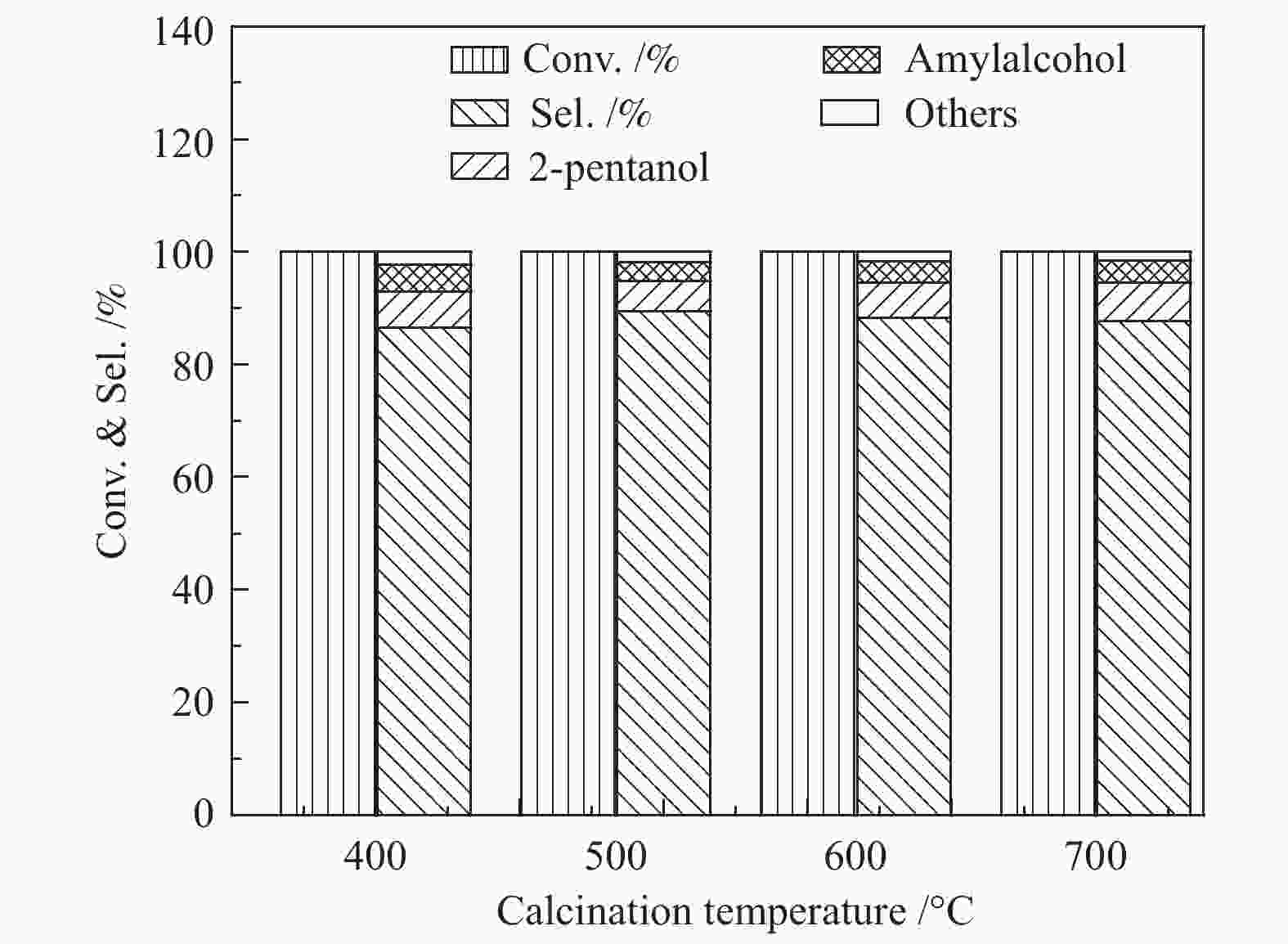
Table 3 Catalytic performance of Ni/SiO2 catalyst under different reaction conditions
p/MPa H2/2-MF(molar ratio) LHSV/h−1 t/℃ x(2-MF)/% Selectivity s/% 2-MTHF 2-pentanol amyl alcohol others 1.2 5.4 1.6 120 100 88.1 6.3 4.4 1.2 1.6 5.4 1.6 120 100 89.5 5.9 3.8 0.8 2.0 5.4 1.6 120 100 90.9 7.1 1.5 0.5 1.6 2.7 1.6 120 100 89.1 6.8 2.7 1.4 1.6 10.7 1.6 120 100 92.7 4.1 2.1 1.1 1.6 13.7 1.6 120 100 93.7 3.5 1.9 0.9 1.6 16.1 1.6 120 100 91.2 4.7 2.4 1.7 1.6 13.7 2.7 120 100 89.9 5.4 3.2 1.5 1.6 13.7 4.9 120 100 90.6 6.1 1.8 1.5 1.6 13.7 4.9 110 100 86.2 7.9 4.4 1.5 1.6 13.7 4.9 120 100 89.5 7.1 2.8 0.6 1.6 13.7 4.9 140 100 94.1 3.4 1.8 0.7 1.6 13.7 4.9 160 100 95.3 2.6 1.7 0.4 1.6 13.7 4.9 180 100 93.1 4.1 1.9 0.9 表 4 Ni/SiO2催化剂表面原子百分比
Table 4 Atom ratio on the surface of Ni/SiO2 catalysts
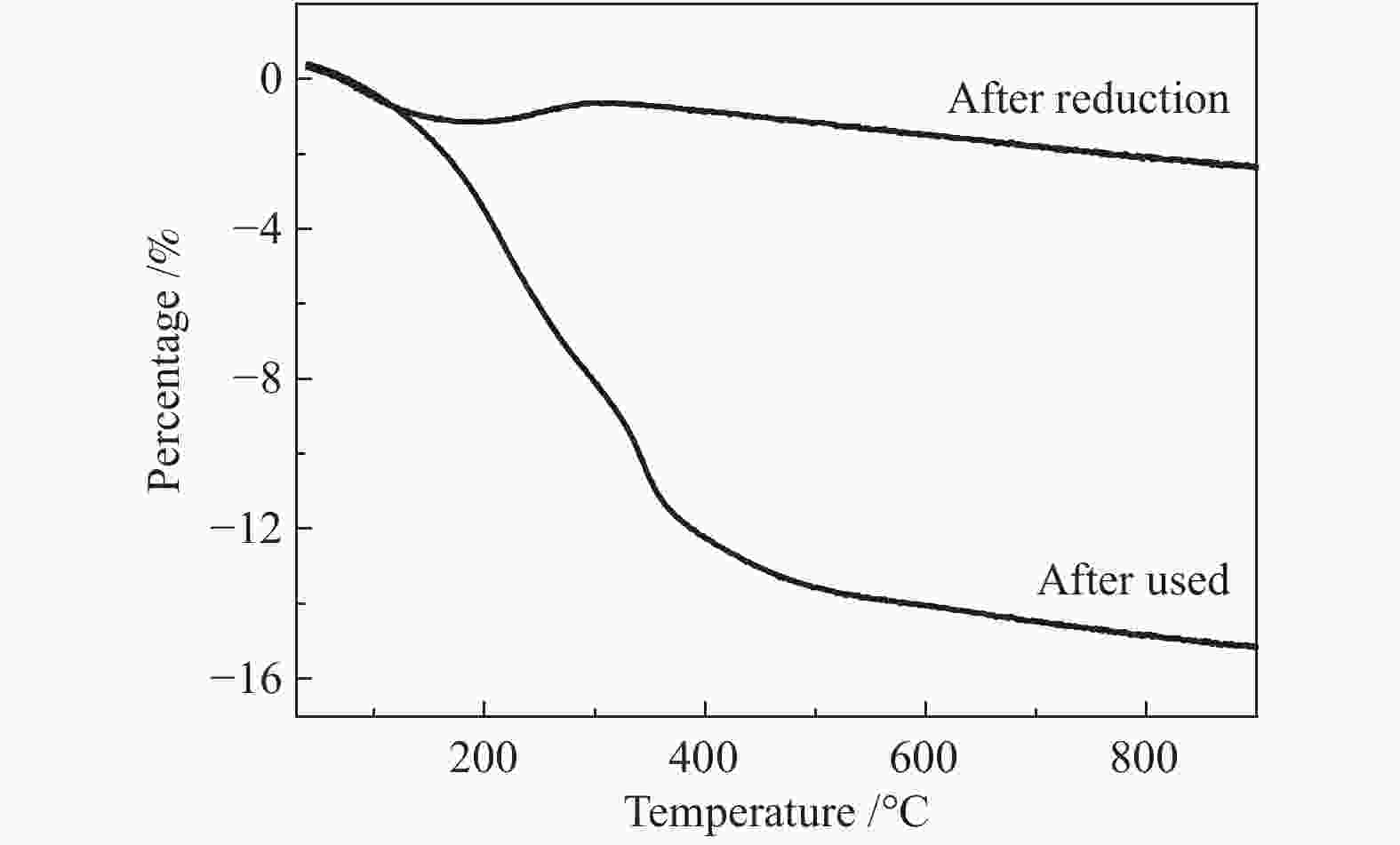
Atom After reduction /% After use /% C 9.10 26.76 O 57.35 48.62 Ni 7.33 4.30 SiO2 26.22 20.31 -
[1] 郭清泉, 陈焕钦. 乙酰丙酸及其衍生物的研究进展[J]. 精细石油化工,2003,20(3):45−48. doi: 10.3969/j.issn.1003-9384.2003.03.016GUO Qing-quan, CHEN Huan-qin. Development on prepartion of levulinic acid and its derivatives[J]. Spec Petrochem,2003,20(3):45−48. doi: 10.3969/j.issn.1003-9384.2003.03.016 [2] RAGAN J A, ENDE D J, BRENEK S J, EISENBEIS S A, SINGER R A, TICKNER D L, TEIXEIRA J J, VANDERPLAS B C, WESTONET N. Safe execution of a large-scale ozonolysis: Preparation of the bisulfite adduct of 2-hydroxyindan-2-carboxaldehyde and its utility in a reductive amination[J]. Org Process Res Dev,2003,7(2):155−160. doi: 10.1021/op0202235 [3] YANG J, ZHENG H, ZHU Y, ZHAO G, ZHANG C, TENG B, XIANG H, LI Y. Effects of calcination temperature on performance of Cu-Zn-Al catalyst for synthesizing c-butyrolactone and 2-methylfuran through the coupling of dehydrogenation and hydrogenation[J]. Catal Commun,2004,5(9):505−510. doi: 10.1016/j.catcom.2004.06.005 [4] 辛炳炜, 曲立强, 孙昌俊. 2-甲基四氢呋喃的制备研究进展[J]. 山东化工,2003,32:13−15.XIN Bing-wei, QU Li-qiang, SUN Chang-jun. Progress on synthesis of Effect of 2-methytetrahydrofuran[J]. Shangdong Chem Ind,2003,32:13−15. [5] DONALD B D, DOROTHY Z D, JOHN J G. Cyclodehydration of 1, 4-butanediols by pentaethoxy-phosphorane[J]. J Org Chem,1984,49:2831−2832. doi: 10.1021/jo00189a044 [6] SANEO J, FUKUMOTO T, NAKAO K. Cyclic ethers from lactones, Japan: 7333745 [P]. [7] DONG F, ZHU Y, DING G. One-step conversion of furfural into 2-methyltetrahydrofuran under mild conditions[J]. Chem Sus Chem,2015,8(9):1534−1537. doi: 10.1002/cssc.201500178 [8] MASAAKI Y, RYOZI H. Preparation of dihydropyran from furfural[J]. Kôgakuin Daigaku Kenkyû Hôkoku,1954,1:76. [9] PROSKURYAKOV V A, IVANOV P A, GENUSOV M L. Hydrogenation of furfural under pressure[J]. Trudy Leningrad Tekhnol Inst Im Lensoveta,1958,44:3−5. [10] 李增杰, 黄玉辉, 朱明. Ni/Al2O3催化2-甲基呋喃加氢制2-甲基四氢呋喃性能的研究[J]. 燃料化学学报,2018,46(1):54−58. doi: 10.3969/j.issn.0253-2409.2018.01.007LI Zeng-jie, HUANG Yu-hui, ZHU Ming. Catalytic performance of Ni/Al2O3 catalyst for hydrogenation of 2-methylfuran to 2-methyltetrahydrofuran[J]. J Fuel Chem Technol,2018,46(1):54−58. doi: 10.3969/j.issn.0253-2409.2018.01.007 [11] DING F, ZHANG Y. Synthesis and catalytic performance of Ni/SiO2 for hydrogenation of 2-methylfuran to 2-methyltetrahydrofuran[J]. J Nanomater,2015. [12] ASHOK J, KATHIRASER Y, ANG M L, KAWI S. Ni and/or Ni-Cu alloys supported over SiO2 catalysts synthesized via phyllosilicate structures for steam reforming of biomass tar reaction[J]. Catal Sci & Technol,2015,5:4398−4409. [13] ZHANG H, GAO X, MA Y, HAN X, NIU L, BAI G. A highly dispersed and stable Ni/mSiO2-AE nanocatalyst for benzoic acid hydrogenation[J]. Catal Sci Technol,2017,7:5993−5999. [14] THOMMES M, KANEKO K, NEIMARK A V. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report)[J]. Pure Appl Chem,2015,87(9/10):1051−1069. doi: 10.1515/pac-2014-1117 [15] CYCHOSZ K A, NICOLAS R G, MARTNEZ J G. Recent advances in the textural characterization of hierarchically structured nanoporous materials[J]. Chem Soc Rev,2017,46:389−414. doi: 10.1039/C6CS00391E [16] CHEN L, GUO P, QIAO M. Cu/SiO2 catalysts prepared by the ammonia-evaporation method: Texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol[J]. J Catal,2008,257(1):172−180. doi: 10.1016/j.jcat.2008.04.021 [17] KONG X, ZHU Y, ZHENG H. Ni Nanoparticles Inlaid Nickel Phyllosilicate as a metal-acid bifunctional catalyst for low-temperature hydrogenolysis reactions[J]. ACS Catal,2015,5:5914−5920. doi: 10.1021/acscatal.5b01080 [18] DONG F, DING G, ZHENG H. Highly dispersed Cu nanoparticles as an efficient catalyst for the synthesis of the biofuel 2-methylfuran[J]. Catal Sci Technol,2016,6:767−779. doi: 10.1039/C5CY00857C [19] LI Z, MO L, KATHIRASER Y. Yolk-Satellite-Shell structured Ni-Yolk@Ni@SiO2 nanocomposite: Superb catalyst toward methane CO2 reforming reaction[J]. ACS Catal,2014,4(5):1526−1536. doi: 10.1021/cs401027p [20] SUN K Q, MARCEAU E, CHE M. Evolution of nickel speciation during preparation of Ni-SiO2 catalysts: Effect of the number of chelating ligands in [Ni(en)x(H2O)6−2x]2+ precursor complexes[J]. Phys Chem Chem Phys,2006,8(14):1731−1738. doi: 10.1039/b513319j [21] ZHANG C, ZHU W, LI S. Sintering-resistant Ni-based reforming catalysts obtained via the nanoconfinement effect[J]. Chem Commun,2013,49:9383−9385. doi: 10.1039/c3cc43895c [22] BURATTIN P, CHE M, LOUIS C. Ni/SiO2 Materials prepared by deposition-precipitation: Influence of the reduction conditions and mechanism of formation of metal particles[J]. J Phys Chem B,2000,104(45):10482−10489. doi: 10.1021/jp0003151 [23] 刘吉, 王东旭, 肖显斌. 焙烧温度对Ni/γ-Al2O3还原条件及催化甲苯水蒸气重整反应的影响[J]. 燃料化学学报,2014,42(10):1225−1232. doi: 10.3969/j.issn.0253-2409.2014.10.011LIU Ji, WANG Dong-xu, XIAO Xian-bin. Effect of calcination temperature on Ni/γ-Al2O3 reduction and catalytic steam reforming of toluene[J]. J Fuel Chem Technol,2014,42(10):1225−1232. doi: 10.3969/j.issn.0253-2409.2014.10.011 [24] GILKEY M J, PANAGIOTOPOULOU P, MIRONENKO A V, JENNESS G R, VALCHOS DG, XU B[J]. ACS Catal, 2015, 5: 3988-3994. [25] SELVAM P, VISWANNATHAN B, SRINIVASAN V. XPS studies of the surface properties of CaNi5[J]. J Electron Spectrosc Relat Phemon,1989,49:203−211. doi: 10.1016/0368-2048(89)85009-1 -





 下载:
下载: