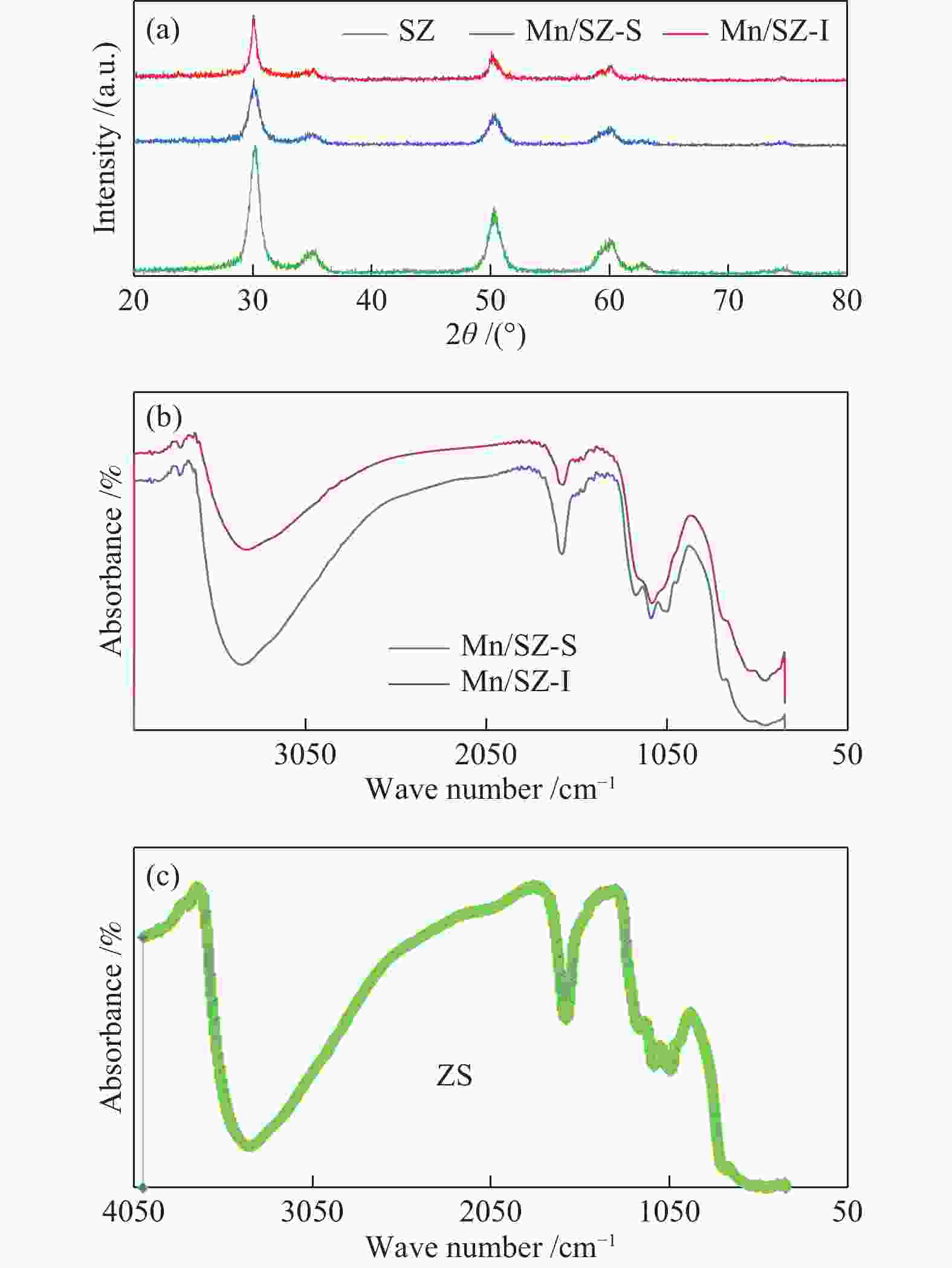
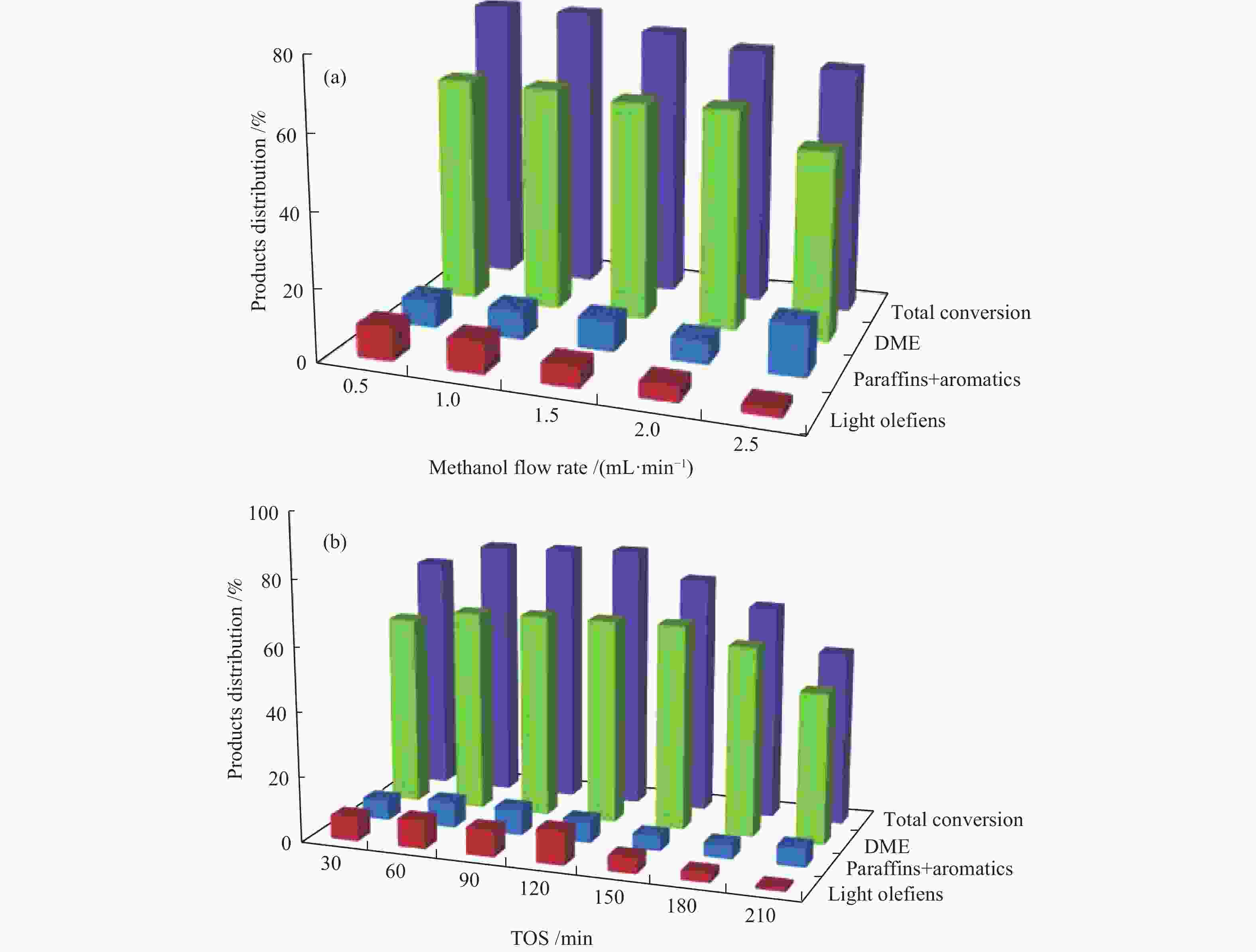
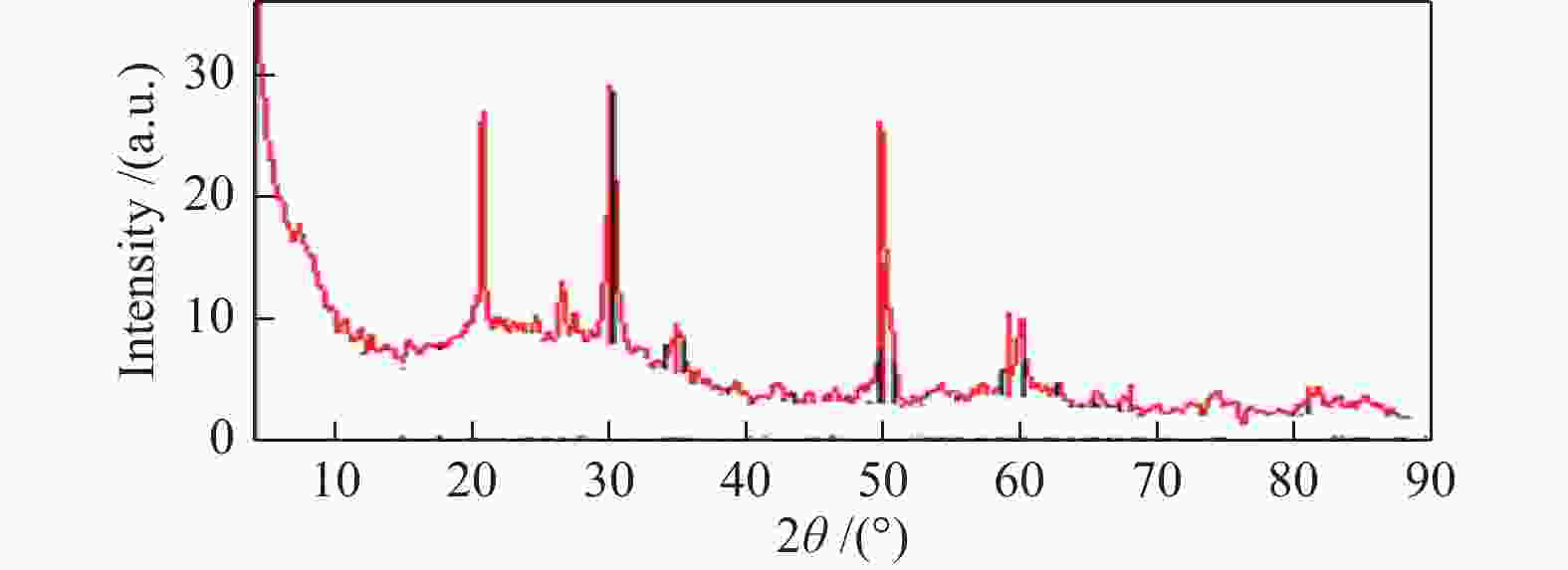
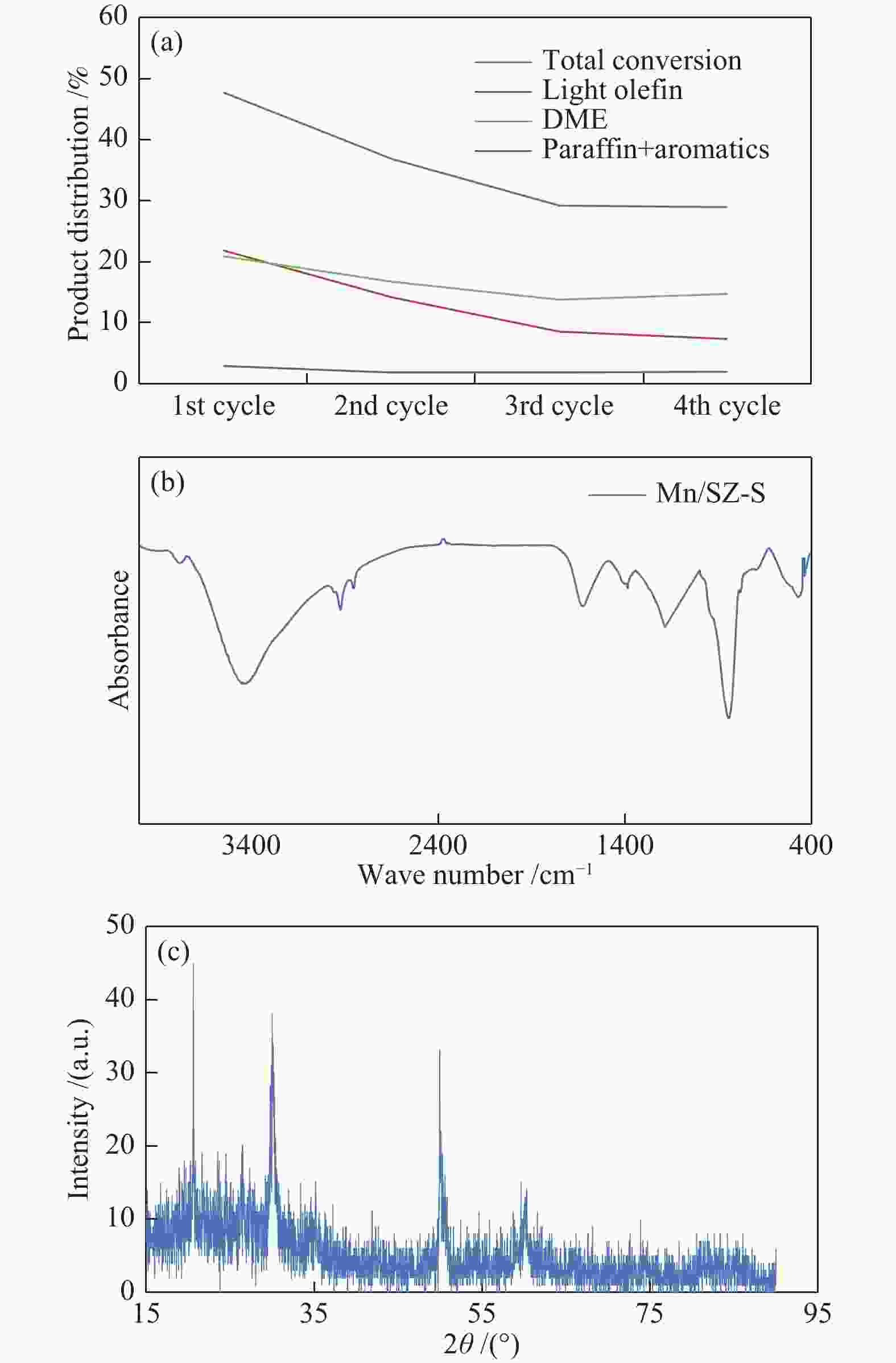
| [1] |
SOLYMAN S M, ABOUL-GHEIT N A K, TAWFIK F M, SADEK M, AHMED H A. Performance of ultrasonic - treated nano-zeolites employed in the preparation of dimethyl ether[J]. Egypt J Pet,2013,22:91−99. doi: 10.1016/j.ejpe.2012.09.003
|
| [2] |
SOLYMAN S M, ABOUL-GHEIT N A K, SADEK M, TAWFIK F M, AHMED H A. The effect of physical and chemical treatment on nano-zeolite characterization and their performance in dimethyl ether preparation[J]. Egypt J Pet,2015,24:289−297. doi: 10.1016/j.ejpe.2015.07.007
|
| [3] |
ABOUL-FOTOUH S M K, ALI L I, NAGHMASH M A, ABOUL-GHEIT N A K. Effect of the Si/Al ratio of HZSM-5 zeolite on the production of dimethyl ether before and after ultrasonication[J]. J Fuel Chem Technol,2017,45(5):581−588. doi: 10.1016/S1872-5813(17)30030-0
|
| [4] |
ABOUL-GHEIT A K, ABOUL-FOTOUH S M, ALI L I, NAGHMASH M A. Ultrasonication of H-MOR zeolite catalysts for dimethylether (DME) production as a clean fuel[J]. J Pet Technol Altern Fuels,2014,5(2):13−25. doi: 10.5897/JPTAF2014.0101
|
| [5] |
ABOUL-FOTOUH S M, ABOUL-GHEIT N A K, NAGHMASH M A. Dimethylether production on zeolite catalysts activated by Cl−, F− and/or ultrasonication[J]. J Fuel Chem Technol,2016,44(4):428−436. doi: 10.1016/S1872-5813(16)30022-6
|
| [6] |
SOLYMAN S M, BETIHA M A. The performance of chemically and physically modified local kaolinite in methanol dehydration to dimethyl ether[J]. Egypt J Pet,2014,23:247−254. doi: 10.1016/j.ejpe.2014.08.001
|
| [7] |
RADWAN D, SAAD L, MIKHAIL S, SELIM S A. Catalytic evaluation of sulfated zirconia pillared clay in N-hexane transformation[J]. J Appl Sci Res,2009,5(12):2332−2342.
|
| [8] |
IBRAHIM A H, ISMAIL H A S, EL-DESOUKI D S, ABDEL-AZIM S A, ABOUL-GHEIT N A K. Preparation of sulfated zirconia catalyst loaded by copper in “nano-scale: Green application to synthesis of biolubricant[J]. Egypt J Chem,2018,61:503−516.
|
| [9] |
LIU N, WANG X, SHI L, MENG X. Metallic oxide-modified sulfated zirconia: an environment-friendly solid acid catalyst[J]. New J Chem,2019,43(8):3625−3632. doi: 10.1039/C8NJ04636K
|
| [10] |
FÖTTINGER K, HALWAX E, VINEK H. Deactivation and regeneration of Pt containing sulfated zirconia and sulfated zirconia[J]. Appl Catal A: Gen,2006,301:115−122. doi: 10.1016/j.apcata.2005.11.024
|
| [11] |
KEDIA A O, ZAIDI H A. Conversion of methanol to hydrocarbons over Ni-ZSM-5 catalyst[J]. Int J Adv Res Sci Eng Technol,2014,3(1):350−356.
|
| [12] |
ABOUL-GHEIT A K, GAD F K, ABDEL-ALEEM G M, EL-DESOUKI D S, ABDEL-HAMID S M, GHONEIM S A, IBRAHIM A H. Pt, Re and Pt-Re incorporation in sulfated zirconia as catalysts for n-pentane isomerization[J]. Egypt J Pet,2014,23:303−314. doi: 10.1016/j.ejpe.2014.08.006
|
| [13] |
SOYLU G S P, ISIK A B, BOZ I. Dehydroisomerization of n-butane over metal promoted sulfated zirconia[J]. Turkish J Eng Env Sci,2009,33:273−279.
|
| [14] |
HSU C Y, HEIMBUCH C R, ARMES C T, GATES B C. A highly active solid superacid catalyst for n-butane isomerization: a sulfated oxide containing iron, manganese and zirconium[J]. J Chem Soc, Chem Comm,1992:1645−1646.
|
| [15] |
WITOON T, PERMSIRIVANICH T, KANJANASOONTORN N, AKKARAPHATAWORN C, SEUBSAI A, FAUNGNAWAKIJ K, WARAKULWIT C, CHAREONPANICH M, LIMTRAKUL J. Direct synthesis of dimethyl ether from CO2 hydrogenation over Cu-ZnO-ZrO2/SO4 2--ZrO2 hybrid catalysts: Effects of sulfur-to-zirconia ratios[J]. Catal Sci Technol,2015,5:2347−2357. doi: 10.1039/C4CY01568A
|
| [16] |
SAID A E A A, EL-AAL M A. Effect of different metal sulfate precursors on structural and catalytic performance of zirconia in dehydration of methanol to dimethyl ether[J]. J Fuel Chem Technol,2018,46(1):67−74. doi: 10.1016/S1872-5813(18)30004-5
|
| [17] |
POPOVA M, SZEGEDI Á, LAZAROVA H, DIMITROV M, KALVACHEV Y, ATANASOVA G, RISTIĆ A, WILDE N, GLÄSER R. Influence of the preparation method of sulfated zirconia nanoparticles for levulinic acid esterification[J]. React Kinet Mech Catal,2017,120:55−67. doi: 10.1007/s11144-016-1088-4
|
| [18] |
CHEN W H, KO H H, SAKTHIVEL A, HUANG S J, LIU S H, LO A Y, TSAI T C, LIU S B. A solid-state NMR, FT-IR and TPD study on acid properties of sulfated and metal-promoted zirconia: Influence of promoter and sulfation treatment[J]. Catal Today,2006,116:111−120. doi: 10.1016/j.cattod.2006.01.025
|
| [19] |
SRINIVASAN R, WATKINS T R, HUBBARD C R, DAVIS B H. Sulfated zirconia catalysts. the crystal phases and their transformations[J]. Chem Mater,1995,7:725−730. doi: 10.1021/cm00052a018
|
| [20] |
SHI G L, YU F, YAN X L, LI R F. Synthesis of tetragonal sulfated zirconia via a novel route for biodiesel production[J]. J Fuel Chem Technol,2017,45(3):311−316. doi: 10.1016/S1872-5813(17)30019-1
|
| [21] |
SOHN J R, LIM J S. Catalytic properties of NiSO4/ZrO2 promoted with Fe2O3 for acid catalysis[J]. Mater Res Bull,2006,41:1225−1241. doi: 10.1016/j.materresbull.2006.01.010
|
| [22] |
BENITO H E, ALAMILLA R G, ENRÍQUEZ J M H, DELGADO F P, GUTIÉRREZ D L, GARCÍA P. Porous silicates modified with zirconium oxide and sulfate ions for alcohol dehydration reactions[J]. Adv Mater Sci Eng,2015,2015:1−11.
|
| [23] |
REN K, KONG D, MENG X, WANG X, SHI L, LIU N. The effects of ammonium sulfate and sulfamic acid on the surface acidity of sulfated zirconia[J]. J Saudi Chem Soc,2019,23:198−204. doi: 10.1016/j.jscs.2018.06.006
|
| [24] |
VLASOV E A, MYAKIN S V, SYCHOV M M, AHO A, POSTNOV A Y, MAL’TSEVA N V, DOLGASHEV A O, OMAROV S O, MURZIN D Y. On synthesis and characterization of sulfated alumina-zirconia catalysts for isobutene alkylation[J]. Catal Lett,2015,145:1651−1659. doi: 10.1007/s10562-015-1575-7
|
| [25] |
VAHID B R, SAGHATOLESLAMI N, NAYEBZADEH H, MASKOOKI A. Preparation of nano-size Al-promoted sulfated zirconia and the impact of calcination temperature on its catalytic activity[J]. Chem Biochem Eng Q,2012,26(2):71−77.
|
| [26] |
YADAV G D, MURKUTE A D. Preparation of a novel catalyst UDCaT-5: Enhancement in activity of acid-treated zirconia - Effect of treatment with chlorosulfonic acid vis-à-vis sulfuric acid[J]. J Catal,2004,224:218−223. doi: 10.1016/j.jcat.2004.02.021
|
| [27] |
BENOMAR S, MASSÓ A, SOLSONA B, ISSAADI R, NIETO J L. Vanadium supported on alumina and/or zirconia catalysts for the selective transformation of ethane and methanol[J]. Catalysts,2018,8:126. doi: 10.3390/catal8040126
|
| [28] |
MA T, IMAI H, YAMAWAKI M, TERASAKA K, LI X. Selective synthesis of gasoline-ranged hydrocarbons from syngas over hybrid catalyst consisting of metal-loaded ZSM-5 coupled with copper-zinc oxide[J]. Catalysts,2014,4:116−128. doi: 10.3390/catal4020116
|
| [29] |
MORADI G R, YARIPOUR F, VALE-SHEYDA P. Catalytic dehydration of methanol to dimethyl ether over mordenite catalysts[J]. Fuel Process Technol,2010,91:461−468. doi: 10.1016/j.fuproc.2009.12.005
|
| [30] |
STÖCKER M. Methanol-to-hydrocarbons: Catalytic materials and their behavior[J]. Microporous Mesoporous Mater,1999,29:3−48. doi: 10.1016/S1387-1811(98)00319-9
|
| [31] |
SOHN J R, LEE D G. Characterization of zirconium sulfate supported on TiO2 and activity for acid catalysis[J]. Korean J Chem Eng,2003,20:1030−1036. doi: 10.1007/BF02706933
|
| [32] |
TESTA M, LA PAROLA V, MESRAR F, OUANJI F, KACIMI M, ZIYAD M, LIOTTA L. Use of zirconium phosphate-sulphate as acid catalyst for synthesis of glycerol-based fuel additives[J]. Catalysts,2019,9:148. doi: 10.3390/catal9020148
|
| [33] |
HUSSAIN S T, MAZAR M, GUL S, CHUANG K T, SANGER A R. Dehydration of methanol to dimethyl ether, ethylene and propylene over silica-doped sulfated zirconia[J]. Bull Korean Chem Soc,2006,27(11):1844−1850. doi: 10.5012/bkcs.2006.27.11.1844
|
| [34] |
PALOMO J, RODRIGUEZ-MIRASOL J, CORDERO T. Methanol dehydration to dimethyl ether on Zr-loaded P-containing mesoporous activated carbon catalysts[J]. Materials,2019,12:2204−2220. doi: 10.3390/ma12132204
|
| [35] |
GLUKHOVA I O, ASABINA E A, PET’KOV V I, MIRONOVA E Yu, ZHILYAEVA N A, KOVALSKII A M, YAROSLAVTESEV A B. Zirconium d-transition metal phosphates as catalysts for selective dehydration of methanol to dimethyl ether[J]. Inorg Mater,2020,56(4):395−401. doi: 10.1134/S0020168520040056
|





 下载:
下载: