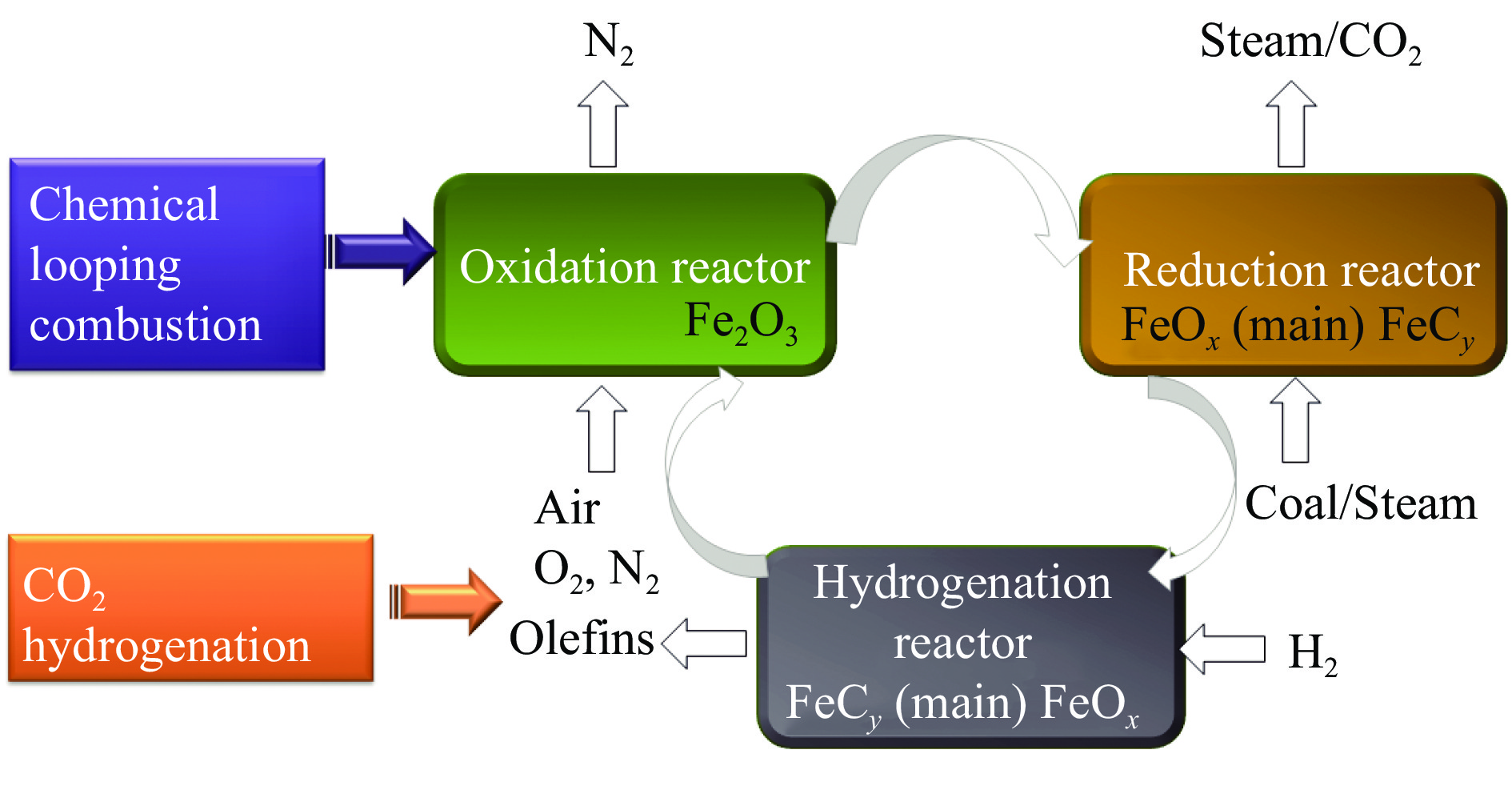
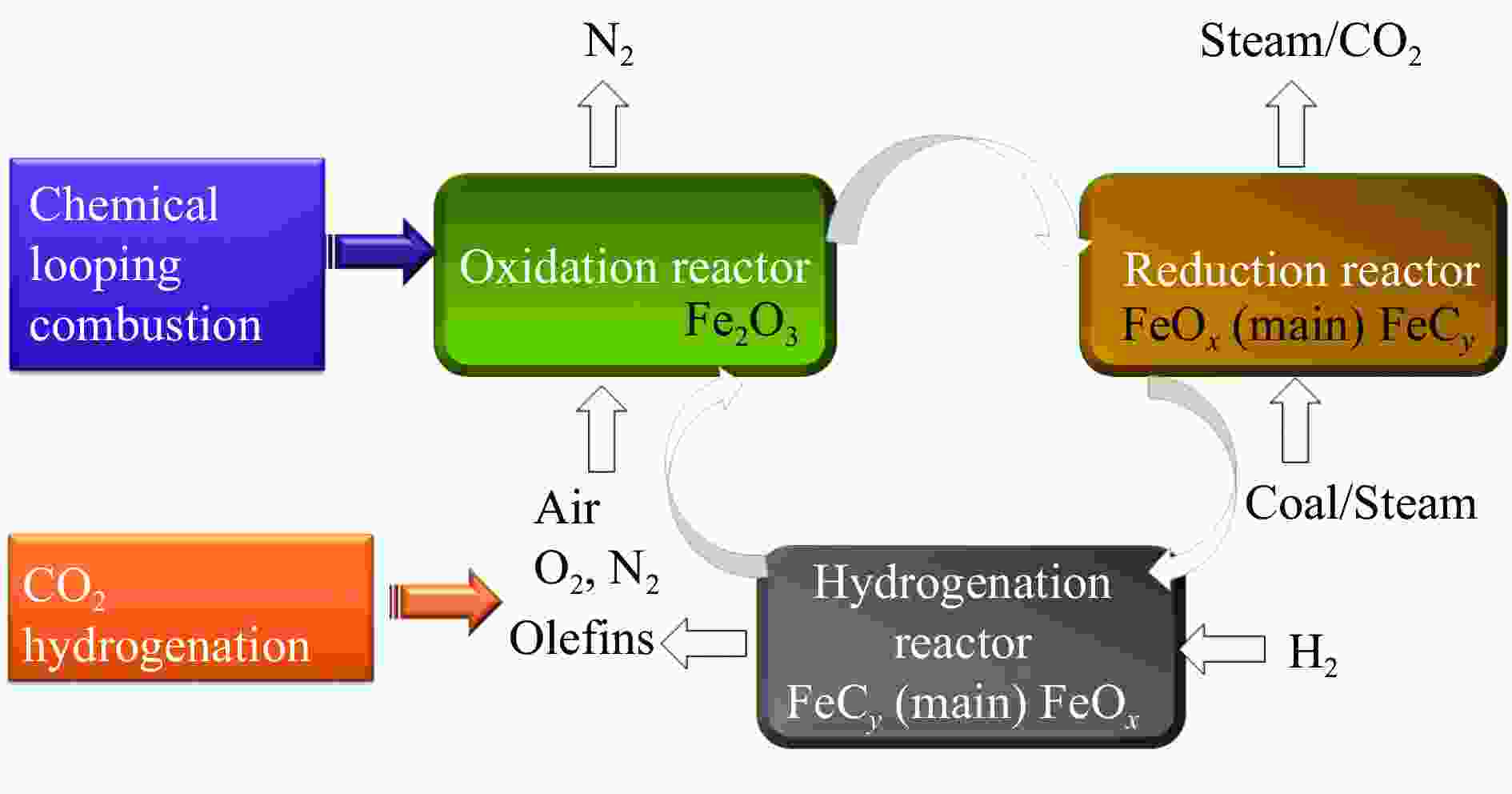
Effects of metal doping on the catalytic performance of LaFe-based perovskites for CO2 hydrogenation to light olefins
-
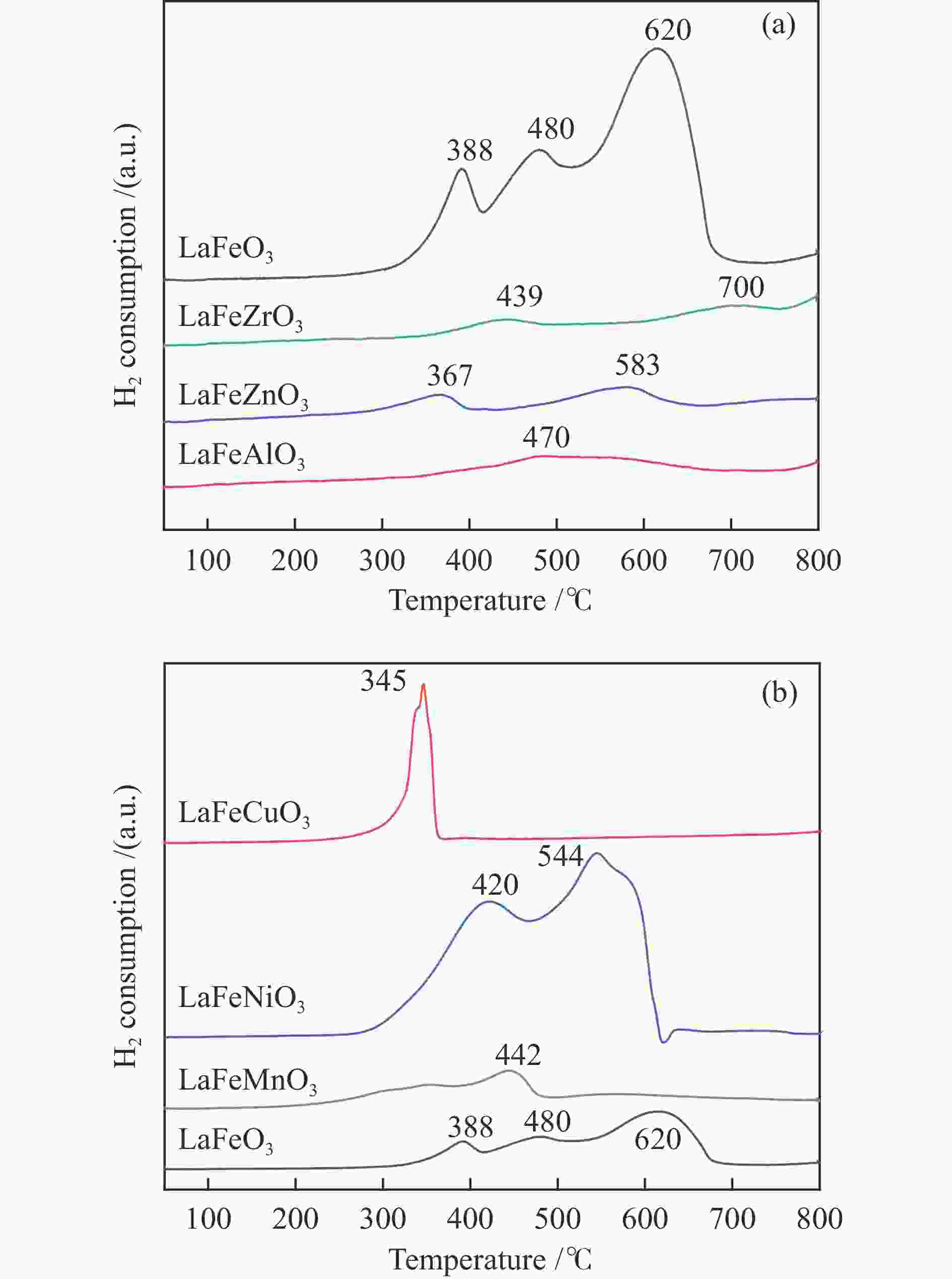
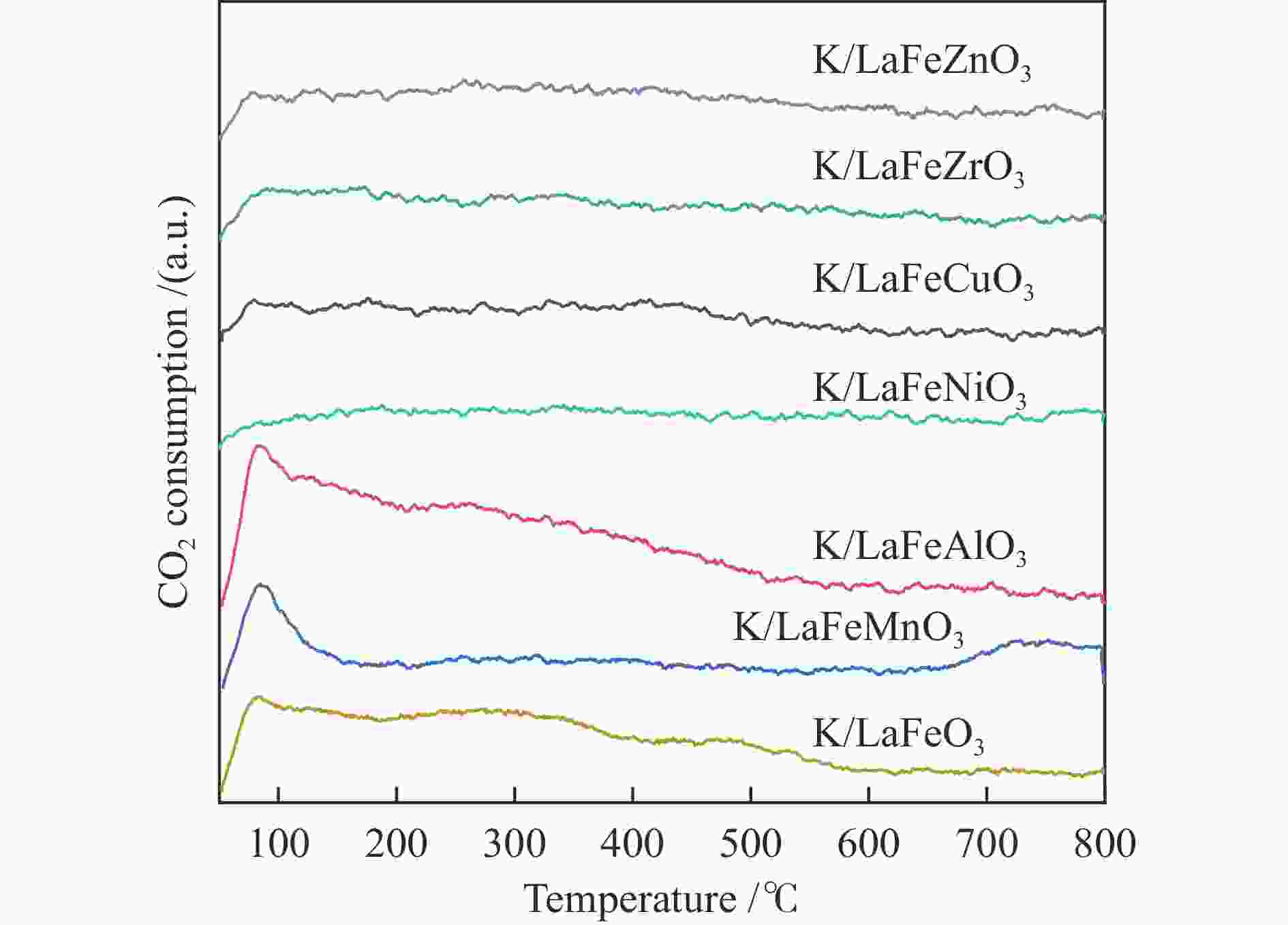
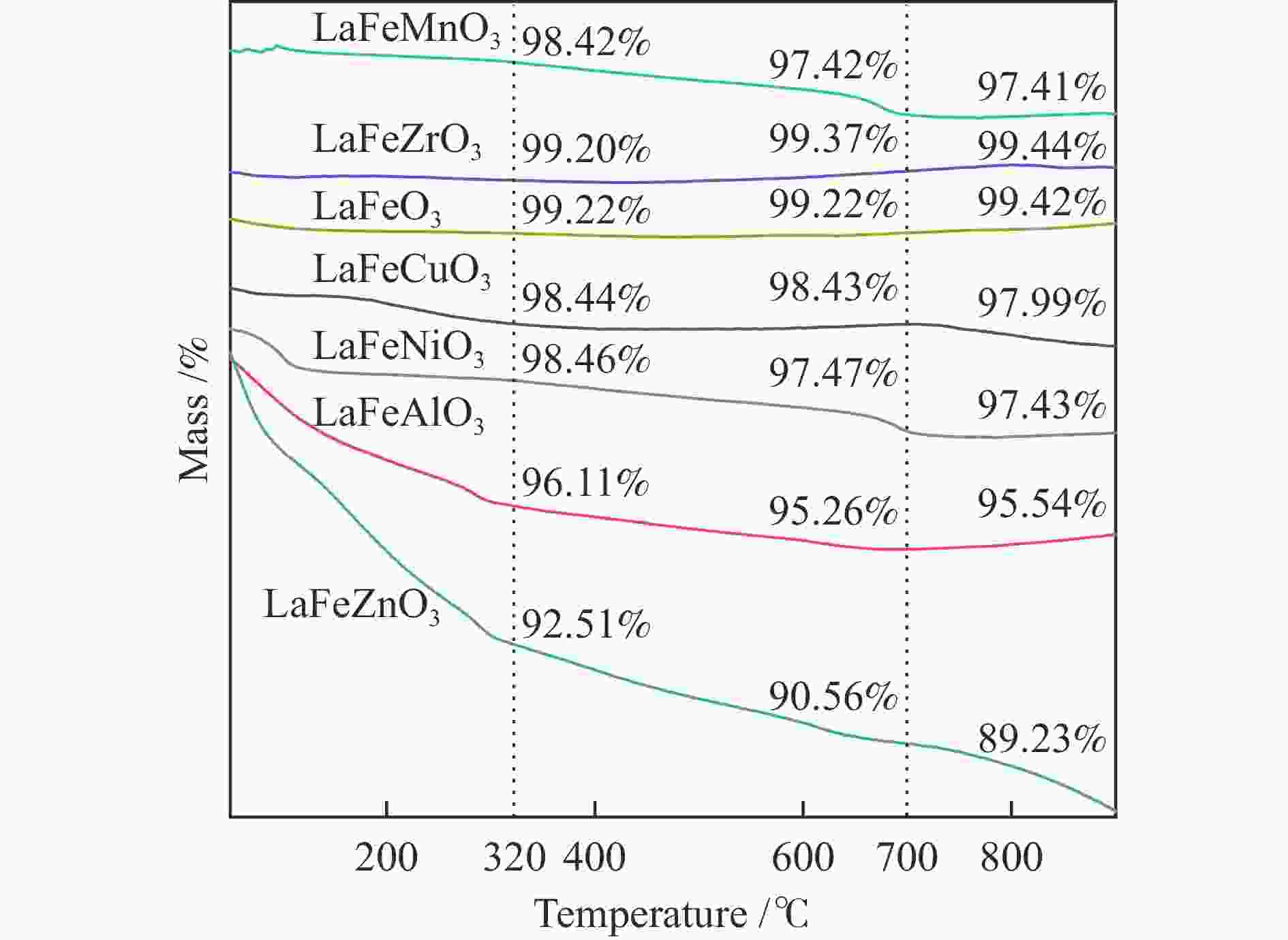
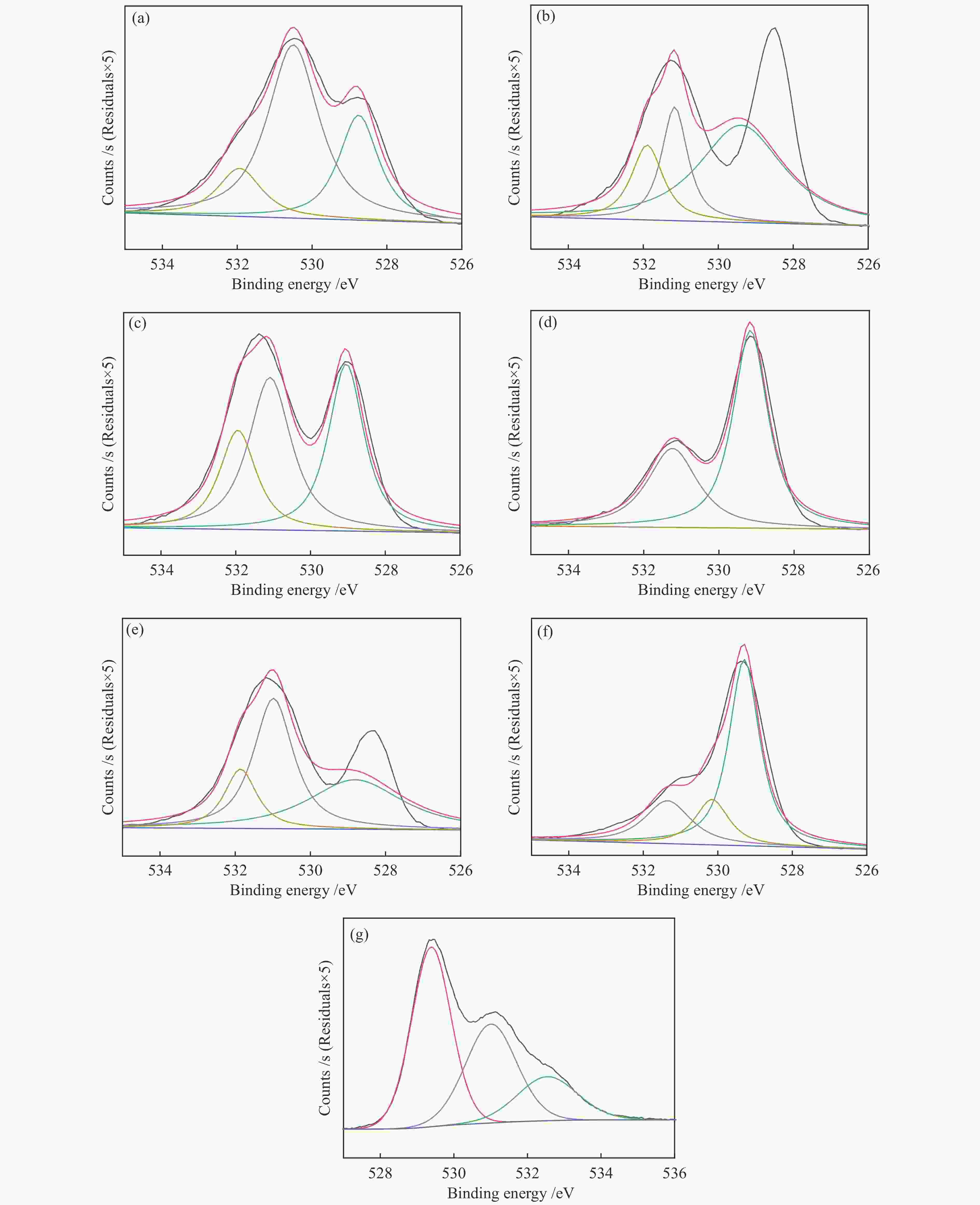
摘要: 通过溶胶-凝胶法和浸渍法制备K/LaFeBO3 (B =Cu、Zr、Al、Mn、Ni、Zn)钙钛矿催化剂,结合SEM、XRD、BET、H2-TPR、CO2-TPD、TG、XPS等表征,探究了金属掺杂对LaFe基钙钛矿催化CO2加氢制备低碳烯烃性能的影响。结果表明,Cu和Zn的加入有利于提高Fe分散度并降低还原温度,同时低温下氢的脱附增加且碱性位增多。氧空位迁移变化对催化活性和烯烃选择性有重要影响,当Cu和Zn在B位取代Fe时,氧迁移率增加明显,具有较低结合能的表面晶格氧富集,显著提高了催化活性,促进了低碳烯烃生成。
-
关键词:
- 二氧化碳加氢 /
- 钙钛矿 /
- LaFeBO3催化剂 /
- 氧迁移
Abstract: The catalytic behavior of K/LaFeBO3 (B =Cu, Zr, Al, Mn, Ni, and Zn) perovskite catalysts prepared by sol-gel and impregnation methods was investigated for CO2 hydrogenation to light olefins. The structure of various catalysts was characterized in detail by SEM, XRD, N2 adsorption-desorption, H2-TPR, CO2-TPD, TG, and XPS analysis. With the addition of Cu and Zn, the particles size decreased with high dispersion of Fe, while the exposed basic sites increased with lower hydrogen desorption temperature. The oxygen mobility in perovskites exhibited a considerable impact on catalytic activity and olefins selectivity, which considerably increased when Fe was substituted by Cu and Zn at the B site. Olefins were formed preferentially from oxygen species of the surface lattice with low binding energies (BEs). In addition, a faster diffusion rate of oxygen would lead to an enrichment of lattice oxygen species on the surface and increase the production of olefins.-
Key words:
- CO2 hydrogenation /
- perovskite /
- LaFeBO3 catalyst /
- oxygen mobility
-
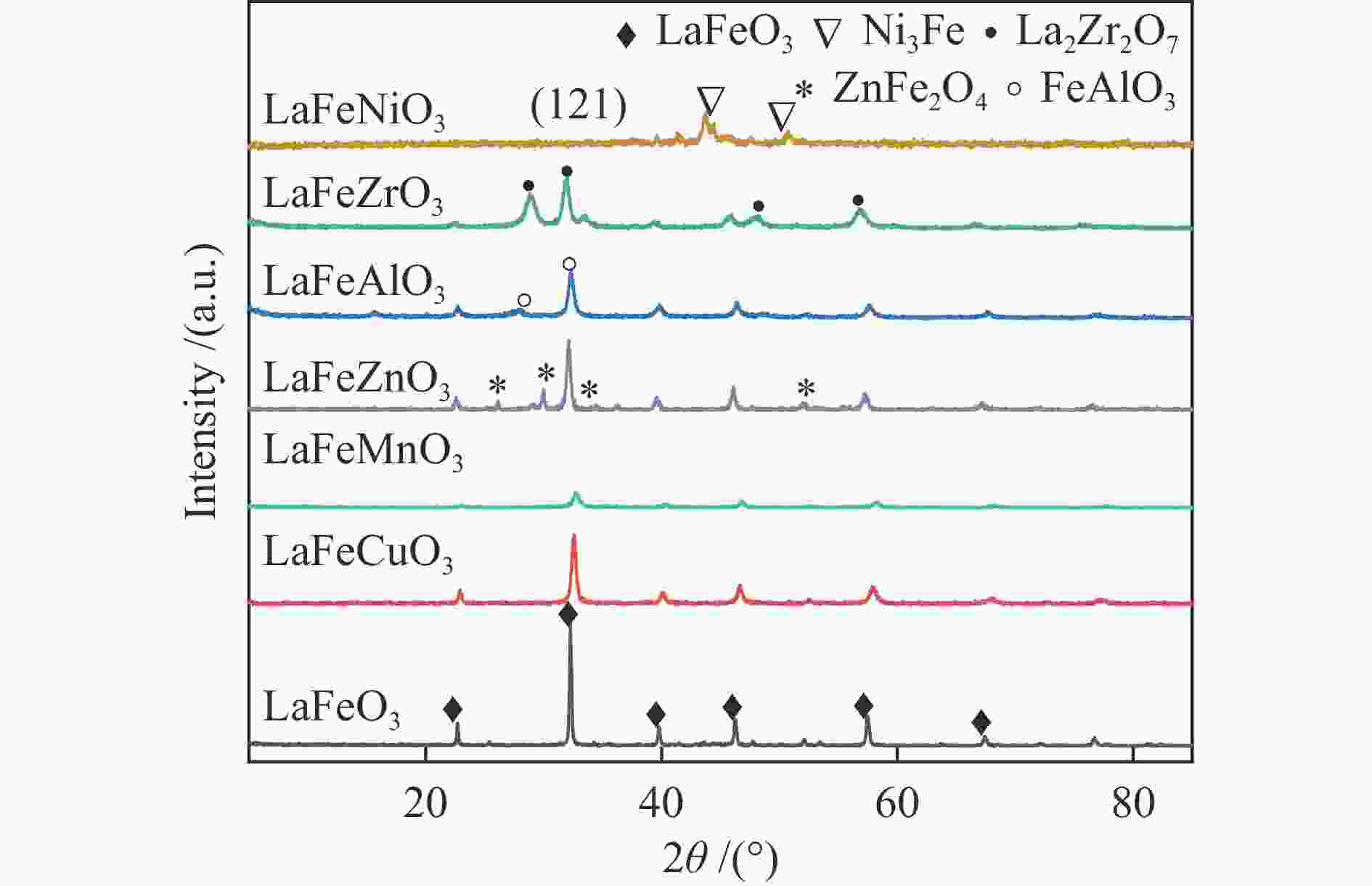
Table 1 Crystalline size of various catalyst samples
Catalyst Crystalline size/nma Lattice parameter/ Å LaFeO3 96 3.5628 LaFeMnO3 14.8 3.8754 LaFeCuO3 30.8 3.8903 LaFeAlO3- 16.7 4.4676 LaFeZrO3 22.8 3.6622 LaFeZnO3 28.9 4.6819 LaFeNiO3 29.8 6.4846 a: Caculated by Scherrer equation Table 2 Texture properties of LaFeBO3 catalyst samples
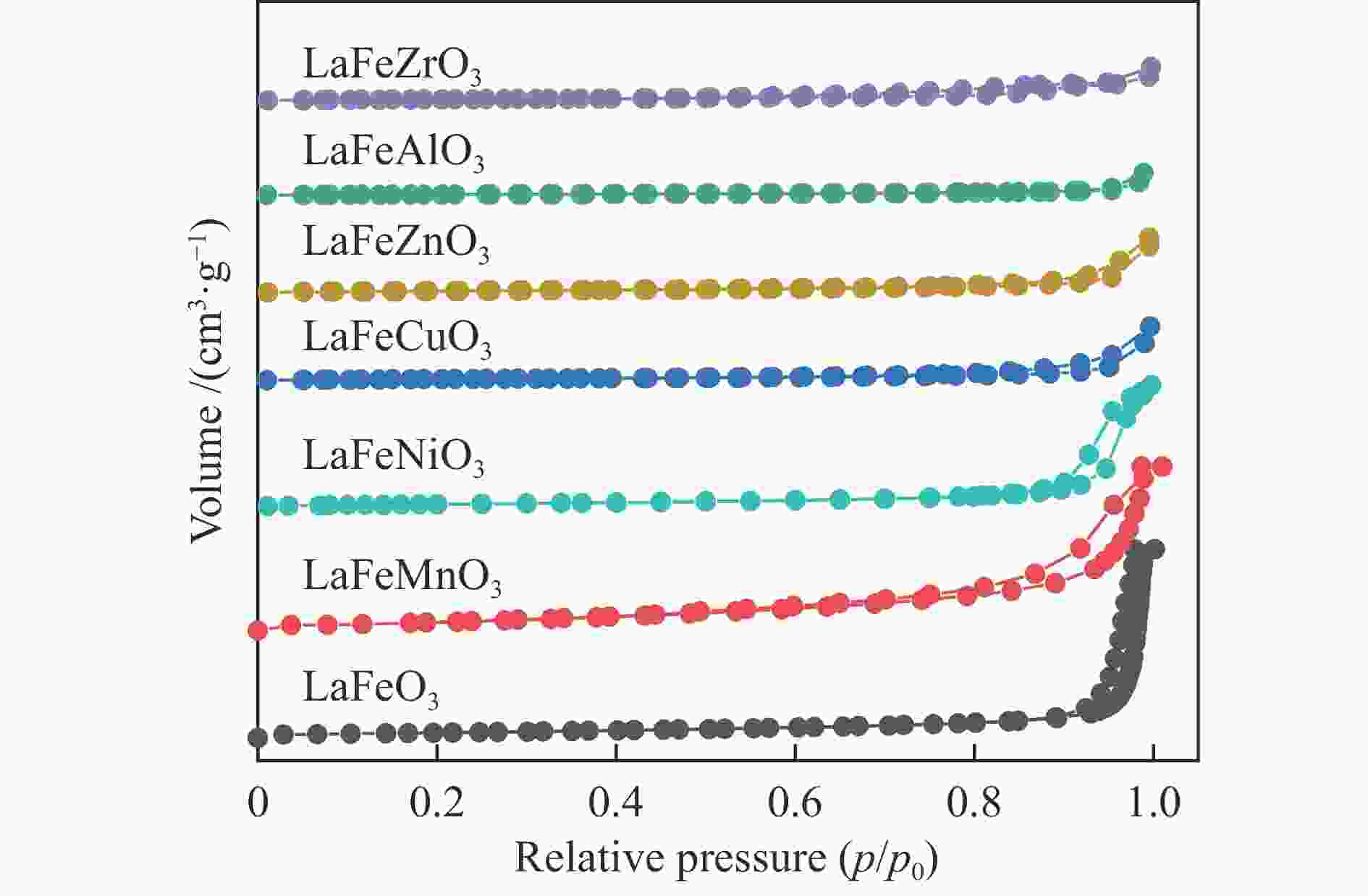
Catalysta BET/
(m2·g−1)bVtotal/
(cm3·g−1)Average pore
diameterc/nmLaFeO3 11.5 0.118 40.96 LaFeMnO3 27.5 0.108 15.68 LaFeCuO3 3.8 0.023 24.83 LaFeAlO3− 2.1 0.014 26.74 LaFeZrO3 3.4 0.011 12.55 LaFeZnO3 4.2 0.010 9.58 LaFeNiO3 9.5 0.071 29.80 a: Fresh samples, b: BET desorption cumulative volume,
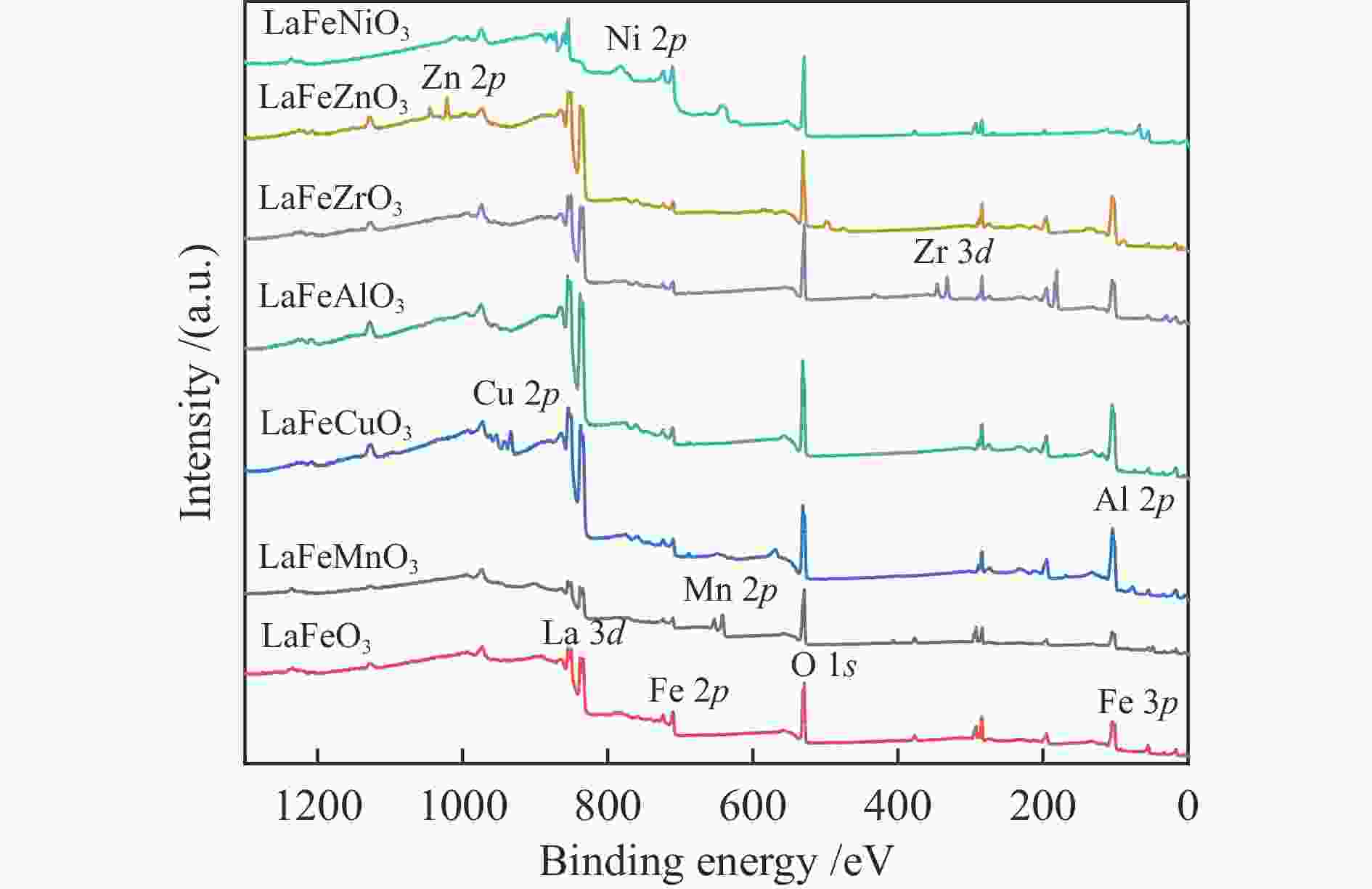
c: BET desorption average pore diameterTable 3 Surface composition of the catalysts determined by XPS
Sample Surface element content wmol/% Fe/B Fe/B* Fe O B LaFeMnO3 3.81 59.01 4.09 0.93 0.69 LaFeCuO3 5.87 59.29 6.09 0.96 0.98 LaFeAlO3 4.15 60.1 6.03 0.69 0.59 LaFeZrO3 4.77 54.95 8.02 0.59 0.53 LaFeZnO3 3.8 57.41 4.67 0.81 0.87 LaFeNiO3 7.48 59.74 8.05 0.93 0.89 * Determined by ICP Table 4 Catalytic activity of different catalysts
Catalyst sample CO2 conv. /% CO sel. /% Hydrocarbon distribution C/% O/P CH4 ${\rm{C} }_{2}^= - {\rm{C} }_{4}^=$ ${\rm{C} }_{2}^{0} - {\rm{C} }_{4}^{0}$ C5+ K/LaFeO3 5.6 24.2 90.4 0 9.6 0 0 K/LaFeMnO3 25.3 85.0 51.6 19.3 15.1 14.1 1.3 K/LaFeCuO3 35.6 62.3 41.3 27.2 22.7 8.8 1.2 K/LaFeZrO3 7.4 81.5 77.8 14.1 8.1 0 1.5 K/LaFeAlO3 8.2 87.6 78.9 10.0 11.1 0 0.9 K/LaFeNiO3 30.3 80.3 69.9 10.9 9.3 10 1.2 K/LaFeZnO3 48.5 25.7 15.62 41.0 16.9 26.5 2.4 Reaction conditions: H2/CO2 = 3, 320 °C, 2.0 MPa, 1000 h−1 and TOS=24 h -
[1] DO T N, KIM J. Green C2-C4 hydrocarbon production through direct CO2 hydrogenation with renewable hydrogen: Process development and techno-economic analysis[J]. Energy Convers Manage,2020,214:112866. doi: 10.1016/j.enconman.2020.112866 [2] GAO P, DANG S S, LI S G, BU X N, LIU Z Y, QIU M H, YANG C G, WANG H, ZHONG L S, HAN Y, LIU Q, WEI W, SUN Y H. Direct production of lower olefins from CO2 conversion via bifunctional catalysis[J]. ACS Catal,2018,8(1):571−578. doi: 10.1021/acscatal.7b02649 [3] WANG D, XIE Z H, POROSOFF M D, CHEN J G. Recent advances in carbon dioxide hydrogenation to produce olefins and aromatics[J]. Chem,2021,7(9):2277−2311. doi: 10.1016/j.chempr.2021.02.024 [4] ZHU H Y, ZHANG P F, DAI S. Recent advances of lanthanum-based perovskite oxides for catalysis[J]. ACS Catal,2015,5(11):6370−6385. doi: 10.1021/acscatal.5b01667 [5] LI D Y, XU R D, LI X Y, LI Z Q, ZHU X, LI K Z. Chemical looping conversion of gaseous and liquid fuels for chemical production: A review[J]. Energy Fuels,2020,34(5):5381−5413. doi: 10.1021/acs.energyfuels.0c01006 [6] QIU Y, MA L, ZENG D W, LI M, CUI D X, LV Y L, ZHANG S, XIAO R. Efficient CO2 to CO conversion at moderate temperatures enabled by the cobalt and copper co-doped ferrite oxygen carrier[J]. J Energy Chem,2020,46:123−132. doi: 10.1016/j.jechem.2019.10.025 [7] SHARMA P, ELDER T, GROOM L H, SPIVEY J J. Effect of structural promoters on Fe-Based Fischer-Tropsch synthesis of biomass derived syngas[J]. Top Catal,2013,57(6/9):526−537. [8] TIEN THAO N, SON L T. Production of cobalt-copper from partial reduction of La(Co, Cu)O3 perovskites for CO hydrogenation[J]. J Sci-Adv Mater and Dev,2016,1(3):337−342. [9] LIU Y, CHEN J F, BAO J, ZHANG Y. Manganese-modified Fe3O4microsphere catalyst with effective active phase of forming light olefins from syngas[J]. ACS Catal,2015,5(6):3905−3909. doi: 10.1021/acscatal.5b00492 [10] ZHAN H J, LI F, GAO P, ZHAO N, XIAO F K, WEI W, ZHONG L S, SUN Y. Methanol synthesis from CO2 hydrogenation over La-M-Cu-Zn-O (M=Y, Ce, Mg, Zr) catalysts derived from perovskite-type precursors[J]. J Power Sources,2014,251:113−121. doi: 10.1016/j.jpowsour.2013.11.037 [11] DING J, ZHAO W X, ZI L T, XU X, LIU Q, ZHONG Q, XU Y. Promotional effect of ZrO2 on supported FeCoK catalysts for ethylene synthesis from catalytic CO2 hydrogenation[J]. Int J Hydrogen Energy,2020,45(30):15254−15262. doi: 10.1016/j.ijhydene.2020.03.249 [12] ELISHAV O, SHENER Y, BEILIN V, LANDAU M V, HERSKOWITZ M, SHTER G E, GRADER G S. Electrospun Fe-Al-O nanobelts for selective CO2 hydrogenation to light olefins[J]. ACS Appl Mater Inter,2020,12(22):24855−24867. doi: 10.1021/acsami.0c05765 [13] GAO Q, MENG J, YANG Y, LIN Q Y, LU Y F, WEI X, LI J X, HAN G R, ZHANG Z. Zirconium doping in calcium titanate perovskite oxides with surface nanostep structure for promoting photocatalytic hydrogen evolution[J]. Appl Surf Sci,2021,542:148544. doi: 10.1016/j.apsusc.2020.148544 [14] WEI C Y, TU W F, JIA L Y, LIU Y Y, LIAN H L, WANG P, ZHANG Z Z. The evolutions of carbon and iron species modified by Na and their tuning effect on the hydrogenation of CO2 to olefins[J]. Appl Surf Sci,2020,525:146622. doi: 10.1016/j.apsusc.2020.146622 [15] HARE B J, MAITI D, RAMANI S, RAMOS A E, BHETHANABOTLA V R, KUHN J N. Thermochemical conversion of carbon dioxide by reverse water-gas shift chemical looping using supported perovskite oxides[J]. Catal Today,2019,323:225−232. doi: 10.1016/j.cattod.2018.06.002 [16] LINDENTHAL L, POPOVIC J, RAMESHAN R, HUBER J, SCHRENK F, RUH T, NENNING A, LÖFFLER S, OPITZ A K, RAMESHAN C. Novel perovskite catalysts for CO2 utilization-exsolution enhanced reverse water-gas shift activity[J]. Appl Catal B: Environ,2021,292:120183. doi: 10.1016/j.apcatb.2021.120183 [17] LINDENTHAL L, RAMESHAN R, SUMMERER H, RUH T, POPOVIC J, NENNING A, LÖFFLER S, OPITZ A K, BLAHA P, RAMESHAN C. Modifying the surface structure of perovskite-based catalysts by nanoparticle exsolution[J]. Catalysts,2020,10(3):268−282. doi: 10.3390/catal10030268 [18] CHANG H, BJØRGUM E, MIHAL O, YANG J, LEIN H L, GRANDE T, RAAEN S, ZHU Y A, HOLMEN A, CHEN D. Effects of oxygen mobility in La-Fe-based perovskites on the catalytic activity and selectivity of methane oxidation[J]. ACS Catal,2020,10(6):3707−3719. doi: 10.1021/acscatal.9b05154 [19] WU J X, ZHENG Y S, DACQUIN J P, DJELAL N, CORDIER C, DUJARDIN C, GRANGER P. Impact of dual calcium and manganese substitution of La-deficient perovskites on structural and related catalytic properties: Future opportunities in next three-way-catalyst generation[J]. Appl Catal A: Gen,2021,619:118137. doi: 10.1016/j.apcata.2021.118137 [20] ABBAS M, ZHANG J, MANSOUR T S, CHEN J G. Hierarchical porous spinel MFe2O4 (M=Fe, Zn, Ni and Co) nanoparticles: Facile synthesis approach and their superb stability and catalytic performance in Fischer-Tropsch synthesis[J]. Int J Hydrogen Energy,2020,45(18):10754−10763. doi: 10.1016/j.ijhydene.2020.02.044 [21] YANG Q L, LIU G L, LIU Y. Perovskite-type oxides as the catalyst precursors for preparing supported metallic nanocatalysts: A review[J]. Ind Eng Chem Res,2018,57(1):1−17. doi: 10.1021/acs.iecr.7b03251 [22] WU M D, CHEN S Y, XIANG W G. Oxygen vacancy induced performance enhancement of toluene catalytic oxidation using LaFeO3 perovskite oxides[J]. Chem Eng J,2020,387:124101. doi: 10.1016/j.cej.2020.124101 [23] FANG Y Z, LIU Y, ZHANG L H. LaFeO3-supported nano Co-Cu catalysts for higher alcohol synthesis from syngas[J]. Appl Catal A: Gen,2011,397(1/2):183−191. [24] YIN K J, SHEN Y L. Theoretical insights into CO2 hydrogenation to HCOOH over FexZr1-xO2 solid solution catalyst[J]. Appl Surf Sci,2020,528:146926. doi: 10.1016/j.apsusc.2020.146926 [25] SIM Y, KWON D, ANA S, HAB J M, OHC T S, JUNG J H. Catalytic behavior of ABO3 perovskites in the oxidative coupling of methane[J]. Mol Catal,2020,489:110925. doi: 10.1016/j.mcat.2020.110925 [26] SHESHKO T F, MARKOVA E B, SHARAEVA A A, KRYUCHKOVA T A, ZVEREVA I A, CHISLOVA I V, YAFAROVA L V. Carbon monoxide hydrogenation over Gd (Fe/Mn) O3 perovskite-type catalysts[J]. Petrol Chem,2019,59(12):1307−1313. doi: 10.1134/S0965544119120107 [27] MATTSSON A, LEJON C, BAKARDJIEVA S, ŠTENGL V, ÖSTERLUND L. Characterisation, phase stability and surface chemical properties of photocatalytic active Zr and Y co-doped anatase TiO2 nanoparticles[J]. J Solid State Chem,2013,199:212−223. doi: 10.1016/j.jssc.2012.12.018 [28] EZBIRI M, BECATTINI V, HOES M, MICHALSKY R, STEINFELD A. High redox capacity of Al-Doped La1−xSrxMnO3−δ perovskites for splitting CO2 and H2O at Mn-enriched surfaces[J]. ChemSusChem,2017,10(7):1517−1525. doi: 10.1002/cssc.201601869 [29] GROSVENOR A P, KOBE B A, BIESINGER M C, MCINTYRE N S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds[J]. Surf Interface Anal,2004,36(12):1564−1574. doi: 10.1002/sia.1984 [30] WU J X, ZHENG Y S, DACQUIN J P, DJELAL N, CORDIER C, DUJARDIN C, GRANGER P. Impact of dual calcium and manganese substitution of La-deficient perovskites on structural and related catalytic properties: Future opportunities in next three-way-catalyst generation[J]. Appl Catal A: Gen,2021,619(11):118−137. [31] XI X Y, ZENG F, ZHANG H, WU X F, REN J, BISSWANGER T, STAMPFER C, HOFMANN J P, PALKOVITS R, HEERES H J. CO2 hydrogenation to higher alcohols over K-promoted bimetallic Fe–In catalysts on a Ce-ZrO2 support[J]. ACS Sustainable Chem Eng,2021,9(18):6235−6249. doi: 10.1021/acssuschemeng.0c08760 [32] SHI J M, CHANG Y, TANG Y S, WANG X B, WANG X F, ZHANG X C, CAO J L. Hydrogenated LaFeO3 with oxygen vacancies for enhanced visible light photocatalytic performance[J]. Ceram Inter,2020,46(4):5315−5322. doi: 10.1016/j.ceramint.2019.10.282 [33] ZHANG X H, PEI C L, CHANG X, CHEN S, LIU R, ZHAO Z J, MU R T, GONG J L. FeO6 octahedral distortion activates lattice oxygen in perovskite ferrite for methane partial oxidation coupled with CO2 splitting[J]. J Am Chem Soc,2020,142(26):11540−11549. doi: 10.1021/jacs.0c04643 -




 下载:
下载: