CO2-assisted oxidative dehydrogenation of ethane to ethylene over the ZnO-ZrO2 catalyst
-
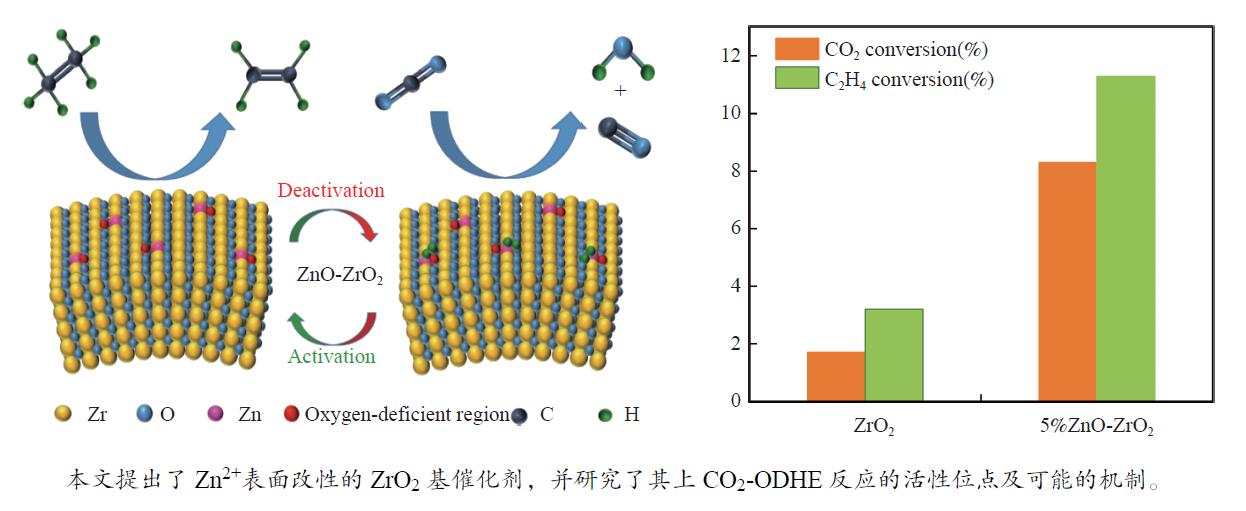
摘要: 以焙烧商用氢氧化锆(Zr(OH)4)得到的ZrO2为载体,通过沉积-沉淀法制备了ZnO-ZrO2催化剂,并在873 K下对该催化剂上CO2辅助的乙烷氧化脱氢反应(CO2-ODHE)的催化性能进行了评价。利用X射线衍射(XRD)、扫描电镜(SEM)、拉曼光谱(Raman)、高分辨透射电镜(HRTEM)、X射线光电子能谱(XPS)、CO2程序升温脱附(CO2-TPD)等技术对ZnO-ZrO2催化剂的表面物理化学性质和形貌进行了表征。结果表明,在5%ZnO-ZrO2催化剂上,ZnO掺入到了ZrO2的表面晶格之中,在催化剂表面产生了高度分散的ZnO物种和氧缺陷区域。5%ZnO-ZrO2催化剂可以选择性地剪裁乙烷C−H键,抑制C−C键的断裂,具备良好的催化性能。210 µmol/(gcat·min)的C2H4形成率可以与贵金属和过渡金属碳化物催化剂的报道数据接近。此外,还对ZnO-ZrO2催化剂上CO2-ODHE反应可能的反应机制进行了讨论。
-
关键词:
- 乙烯 /
- 乙烷氧化脱氢 /
- CO2辅助 /
- ZnO-ZrO2催化剂
Abstract: The ZnO-ZrO2 catalyst was prepared by the deposition-precipitation method using ZrO2 as the carrier obtained from calcining commercial zirconium hydroxide (Zr(OH)4). And the catalytic performance was evaluated at 873 K in CO2-assisted ethane oxidative dehydrogenation reaction (CO2-ODHE). The physical-chemical properties and morphology were characterized by X-ray diffraction (XRD), scanning electron microscope (SEM), Raman spectra, High-resolution transmission electron microscopy (HRTEM), X-ray photoelectron spectra (XPS), CO2 temperature-programmed desorption (CO2-TPD). The results show that ZnO were doped into the surface lattice of ZrO2 on the 5%ZnO-ZrO2 catalyst, generating highly dispersed ZnO species and oxygen-deficient regions on catalyst surface. 5%ZnO-ZrO2 catalyst could selectively breaking C−H bond instead of C−C bond, delivering excellent catalytic performance. 210 μmol/(gcat·min) of C2H4 formation rate could compare favorably with the data reported on noble metal and transition metal carbides. Additionally, the possible mechanism is discussed.-
Key words:
- ethylene /
- ethane oxidative dehydrogenation /
- CO2-assisted /
- ZnO-ZrO2 catalyst
-
Figure 7 (a) Ethane conversion of ZrO2 and 5%ZnO-ZrO2 catalysts; (b) Products yield, selectivity and CO2 conversion of ZrO2 and 5%ZnO-ZrO2 catalysts Reaction conditions: for all catalyst, test in a flow of C2H6/CO2/N2 = 1∶1∶2, the total flow of 60 mL/min, reaction temperature of 600 °C and atmospheric pressure, GHSV = 12000 mL/(gcat·h)
Table 1 Binding energy of Zr 3d, Zn 2p and O 1s spectra and surface oxygen-deficient region (OII) concentration on ZrO2, ZnO and 5%ZnO-ZrO2 catalysts
Catalyst Zr 3d Zn 2p OII/
(OI + OII)a /%Zr 3d5/2 Zr 3d3/2 Zn 2p3/2 Zn 2p1/2 ZrO2 182.40 184.80 − − 20 5%ZnO-ZrO2 182.06 184.46 1021.51 1044.62 30 ZnO − − 1021.26 1044.37 − a: relative content of surface oxygen-deficient regions calculated from XPS spectra Table 2 Details of catalytic performance over the ZrO2 and 5%ZnO-ZrO2 catalysts in CO2-ODHE and EDH reaction
Catalyst
CO2-ODHEa
EDHbC2H6
conversion
/%C2H4
selectivity
/%C2H4
yield
/%C2H4 STY /
(μmol·gcat−1·min−1)COtotal STYc /
(μmol·gcat−1·min−1)Carbon
balance
/%C2H6
conversion
/%C2H4
selectivity
/%C2H4
yield
/%ZrO2 3.2 93.5 3.0 − − 98.0 − − − 5%ZnO-ZrO2 11.2 84.0 9.4 210 195 96.6 8.6 91.3 7.8 a: reaction of ethane oxidative dehydrogenation with CO2 (CO2-ODHE), reaction conditions: for all catalyst, test in a flow of C2H6/CO2/N2 = 1∶1∶2, the total flow of 60 mL/min, reaction temperature of 873 K and atmospheric pressure, GHSV = 12000 mL/(gcat·h), b: reaction of ethane dehydrogenation without CO2 (EDH), reaction conditions: for 5%ZnO-ZrO2 catalyst, test in a flow of C2H6/N2 = 1∶3, the total flow of 60 mL/min, reaction temperature of 873 K and atmospheric pressure, GHSV = 12000 mL/(gcat·h), c: total CO formation rate from RWGS reaction, dry reforming reaction, and etc Table 3 Catalytic performance of various catalysts evaluated in CO2-ODHE reaction
Catalyst Mass /g T /K C2H6 flow rate /
(mL·${\rm{h}}^{- 1}\cdot{\rm{g}}_{\rm{cat}}^{-1} $)C2H6 conversion
/%C2H4 selectivity
/%STY /
(μmol·${\rm{g}}_{\rm{cat}}^{-1} \cdot {\rm{min}}^{-1} $)This work 0.3 873 3000 11 84 210 NiFe/CeO2[45] 0.1 873 6000 9.1 31 100 PtCe@MZ[17] 0.1 873 1500 39.4 84 326 CsRu/CeO2[46] 1.0 973 1080 33 60 163 1% Fe/Mo2C[47] 0.3 873 6000 7.3 75 235 Ni1.5Fe0.5/ZrO2-M[22] 0.2 873 3000 11 68 167 MoxCy[48] 0.1 873 3750 6.5 60 101 5% Cr-D-ERB-1[49] 0.5 973 600 35.6 94.5 150 Zn9.18/K0.74/NaS50[50] 2.0 923 180 57 79 61 Ga/TiSi-15[51] 0.2 923 270 46 84.9 78 3Cr/NaZSM-5-160[52] 0.2 923 270 61.3 78.9 89 -
[1] SCHARFE M, LIRA-PARADA P A, AMRUTE A P, MITCHELL S, PÉREZ-RAMÍREZ J. Lanthanide compounds as catalysts for the one-step synthesis of vinyl chloride from ethylene[J]. J Catal,2016,344:524−534. doi: 10.1016/j.jcat.2016.10.026 [2] WANG Y-C, CHENG P-Y, ZHANG Z-Q, FAN K-X, LU R-Q, ZHANG S, WU Y-X. Highly efficient terpolymerizations of ethylene/propylene/ENB with a half-titanocene catalytic system[J]. Polym Chem,2021,12(44):6417−6425. doi: 10.1039/D1PY01140E [3] BIKBAEVA V, NESTERENKO N, KONNOV S, NGUYEN T-S, GILSON J-P, VALTCHEV V. A low carbon route to ethylene: Ethane oxidative dehydrogenation with CO2 on embryonic zeolite supported Mo-carbide catalyst[J]. Appl Catal B: Environ,2023,320:122011. doi: 10.1016/j.apcatb.2022.122011 [4] SANFILIPPO D, MIRACCA I. Dehydrogenation of paraffins: Synergies between catalyst design and reactor engineering[J]. Catal Today,2006,111(1):133−139. [5] TIAN H, XU B. Oxidative co-dehydrogenation of ethane and propane over h-BN as an effective means for C–H bond activation and mechanistic investigations[J]. Chin J Catal,2022,43(8):2173−2182. doi: 10.1016/S1872-2067(21)64042-1 [6] ZHOU Y, LIN J, LI L, PAN X, SUN X, WANG X. Enhanced performance of boron nitride catalysts with induction period for the oxidative dehydrogenation of ethane to ethylene[J]. J Catal,2018,365:14−23. doi: 10.1016/j.jcat.2018.05.023 [7] ZHOU Y, LIN J, LI L, TIAN M, LI X, PAN X, CHEN Y, WANG X. Improving the selectivity of Ni-Al mixed oxides with isolated oxygen species for oxidative dehydrogenation of ethane with nitrous oxide[J]. J Catal,2019,377:438−448. doi: 10.1016/j.jcat.2019.07.050 [8] XIE Z, TIAN D, XIE M, YANG S-Z, XU Y, RUI N, LEE J H, SENANAYAKE S D, LI K, WANG H, KATTEL S, CHEN J G. Interfacial active sites for CO2 assisted selective cleavage of C–C/C–H bonds in ethane[J]. Chem,2020,6(10):2703−2716. doi: 10.1016/j.chempr.2020.07.011 [9] PAN Z-H, WENG Z-Z, KONG X-J, LONG L-S, ZHENG L-S. Lanthanide-containing clusters for catalytic water splitting and CO2 conversion[J]. Coord Chem Rev,2022,457:214419. doi: 10.1016/j.ccr.2022.214419 [10] GALADIMA A, MURAZA O. Catalytic thermal conversion of CO2 into fuels: Perspective and challenges[J]. Renewable Sustainable Energy Rev,2019,115:109333. doi: 10.1016/j.rser.2019.109333 [11] CHEN J G. Electrochemical CO2 reduction via low-valent nickel single-atom catalyst[J]. Joule,2018,2(4):587−589. doi: 10.1016/j.joule.2018.03.018 [12] JALID F, KHAN T S, HAIDER M A. CO2 reduction and ethane dehydrogenation on transition metal catalysts: Mechanistic insights, reactivity trends and rational design of bimetallic alloys[J]. Catal Sci Technol,2021,11(1):97−115. doi: 10.1039/D0CY01290D [13] GAMBO Y, ADAMU S, TANIMU G, ABDULLAHI I M, LUCKY R A, BA-SHAMMAKH M S, HOSSAIN M M. CO2-mediated oxidative dehydrogenation of light alkanes to olefins: Advances and perspectives in catalyst design and process improvement[J]. Appl Catal A: Gen,2021,623:118273. doi: 10.1016/j.apcata.2021.118273 [14] XIE Z, WANG X, CHEN X, LIU P, CHEN J G. General descriptors for CO2-assisted Selective C–H/C–C bond scission in ethane[J]. J Am Chem Soc,2022,144(9):4186−4195. doi: 10.1021/jacs.1c13415 [15] LI G, LIU C, CUI X, YANG Y, SHI F. Oxidative dehydrogenation of light alkanes with carbon dioxide[J]. Green Chem,2021,23(2):689−707. doi: 10.1039/D0GC03705B [16] DENG S, LI S, LI H, ZHANG Y. Oxidative dehydrogenation of ethane to ethylene with CO2 over Fe-Cr/ZrO2 catalysts[J]. Ind Eng Chem Res,2009,48(16):7561−7566. doi: 10.1021/ie9007387 [17] NUMAN M, EOM E, LI A, MAZUR M, CHA H W, HAM H C, JO C, PARK S-E. Oxidative dehydrogenation of ethane with CO2 as a soft oxidant over a PtCe bimetallic catalyst[J]. ACS Catal,2021,11(15):9221−9232. doi: 10.1021/acscatal.1c01156 [18] GAO Y, NEAL L, DING D, WU W, BAROI C, GAFFNEY A M, LI F. Recent advances in intensified ethylene production—A review[J]. ACS Catal,2019,9(9):8592−8621. doi: 10.1021/acscatal.9b02922 [19] HAN S, ZHAO D, OTROSHCHENKO T, LUND H, BENTRUP U, KONDRATENKO V A, ROCKSTROH N, BARTLING S, DORONKIN D E, GRUNWALDT J-D, RODEMERCK U, LINKE D, GAO M, JIANG G, KONDRATENKO E V. Elucidating the nature of active sites and fundamentals for their creation in Zn-containing ZrO2-based catalysts for nonoxidative propane dehydrogenation[J]. ACS Catal,2020,10(15):8933−8949. doi: 10.1021/acscatal.0c01580 [20] LIU J, HE N, ZHANG Z, YANG J, JIANG X, ZHANG Z, SU J, SHU M, SI R, XIONG G, XIE H-B, VILÉ G. Highly-dispersed zinc species on zeolites for the continuous and selective dehydrogenation of ethane with CO2 as a soft oxidant[J]. ACS Catal,2021,11(5):2819−2830. doi: 10.1021/acscatal.1c00126 [21] BUGROVA T A, DUTOV V V, SVETLICHNYI V A, CORTÉS CORBERÁN V, MAMONTOV G V. Oxidative dehydrogenation of ethane with CO2 over CrOx catalysts supported on Al2O3, ZrO2, CeO2 and CexZr1-xO2[J]. Catal Today,2019,333:71−80. doi: 10.1016/j.cattod.2018.04.047 [22] LI X, YANG Z, ZHANG L, HE Z, YAN Y, RAN J, KADIROVA Z C. Influence of ZrO2 crystal structure on the catalytic performance of Fe-Ni catalysts for CO2-assisted ethane dehydrogenation reaction[J]. Fuel,2022,322:124122. doi: 10.1016/j.fuel.2022.124122 [23] RORRER J E, TOSTE F D, BELL A T. Mechanism and kinetics of isobutene formation from ethanol and acetone over ZnxZryOz[J]. ACS Catal,2019,9(12):10588−10604. doi: 10.1021/acscatal.9b03045 [24] YANG G-Q, HE Y-J, SONG Y-H, WANG J, LIU Z-T, LIU Z-W. Oxidative dehydrogenation of propane with carbon dioxide catalyzed by ZnxZr1–xO2–x solid solutions[J]. Ind Eng Chem Res,2021,60(49):17850−17861. doi: 10.1021/acs.iecr.1c03476 [25] ZHU H, ROSENFELD D C, HARB M, ANJUM D H, HEDHILI M N, OULD-CHIKH S, BASSET J-M. Ni-M-O (M = Sn, Ti, W) catalysts prepared by a dry mixing method for oxidative dehydrogenation of ethane[J]. ACS Catal,2016,6(5):2852−2866. doi: 10.1021/acscatal.6b00044 [26] SALUSSO D, BORFECCHIA E, BORDIGA S. Combining X-ray diffraction and X-ray absorption spectroscopy to unveil Zn local environment in Zn-doped ZrO2 catalysts[J]. J Phys Chem C,2021,125(40):22249−22261. doi: 10.1021/acs.jpcc.1c06202 [27] TADA S, OCHIAI N, KINOSHITA H, YOSHIDA M, SHIMADA N, JOUTSUKA T, NISHIJIMA M, HONMA T, YAMAUCHI N, KOBAYASHI Y, IYOKI K. Active sites on ZnxZr1–xO2–x solid solution catalysts for CO2-to-methanol hydrogenation[J]. ACS Catal,2022,12(13):7748−7759. doi: 10.1021/acscatal.2c01996 [28] DEV G S, SHARMA V, SINGH A, BAGHEL V S, YANAGIDA M, NAGATAKI A, TRIPATHI N. Raman spectroscopic study of ZnO/NiO nanocomposites based on spatial correlation model[J]. RSC Adv,2019,9(46):26956−26960. doi: 10.1039/C9RA04555D [29] BAYLON R A L, SUN J, KOVARIK L, ENGELHARD M, LI H, WINKELMAN A D, WANG Y. Structural identification of ZnxZryOz catalysts for Cascade aldolization and self-deoxygenation reactions[J]. Appl Catal B: Environ,2018,234:337−346. doi: 10.1016/j.apcatb.2018.04.051 [30] CHEN H, CUI H, LV Y, LIU P, HAO F, XIONG W, LUO H A. CO2 hydrogenation to methanol over Cu/ZnO/ZrO2 catalysts: Effects of ZnO morphology and oxygen vacancy[J]. Fuel,2022,314:123035. doi: 10.1016/j.fuel.2021.123035 [31] SILVA-CALPA L D R, ZONETTI P C, RODRIGUES C P, ALVES O C, APPEL L G, DE AVILLEZ R R. The ZnxZr1−xO2−y solid solution on m-ZrO2: Creating O vacancies and improving the m-ZrO2 redox properties[J]. J Mol Catal A: Chem,2016,425:166−173. doi: 10.1016/j.molcata.2016.10.008 [32] SRIVASTAVA M, BERA P, BALARAJU J N, RAVISANKAR B. FESEM and XPS studies of ZrO2 modified electrodeposited NiCoCrAlY nanocomposite coating subjected to hot corrosion environment[J]. RSC Adv,2016,6(110):109083−109090. doi: 10.1039/C6RA20634D [33] LIU Y, XIA C, WANG Q, ZHANG L, HUANG A, KE M, SONG Z. Direct dehydrogenation of isobutane to isobutene over Zn-doped ZrO2 metal oxide heterogeneous catalysts[J]. Catal Sci Technol,2018,8(19):4916−4924. doi: 10.1039/C8CY01420E [34] BIESINGER M C, LAU L W M, GERSON A R, SMART R S C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn[J]. Appl Surf Sci,2010,257(3):887−898. doi: 10.1016/j.apsusc.2010.07.086 [35] WANG C, GARBARINO G, ALLARD L, WILSON F, BUSCA G, FLYTZANI-STEPHANOPOULOS M. Low-temperature dehydrogenation of ethanol on atomically dispersed gold supported on ZnZrOx[J]. ACS Catal,2016,6(1):210−218. doi: 10.1021/acscatal.5b01593 [36] HUANG C, WEN J, SUN Y, ZHANG M, BAO Y, ZHANG Y, LIANG L, FU M, WU J, YE D, CHEN L. CO2 hydrogenation to methanol over Cu/ZnO plate model catalyst: Effects of reducing gas induced Cu nanoparticle morphology[J]. Chem Eng J,2019,374:221−230. doi: 10.1016/j.cej.2019.05.123 [37] SUN J, ZHU K, GAO F, WANG C, LIU J, PEDEN C, WANG Y. Direct conversion of bio-ethanol to isobutene on nanosized ZnxZryOz mixed oxides with balanced acid-base sites[J]. J Am Chem Soc,2011,133(29):11096−11099. doi: 10.1021/ja204235v [38] ZHOU C, SHI J, ZHOU W, CHENG K, ZHANG Q, KANG J, WANG Y. Highly active ZnO-ZrO2 aerogels integrated with H-ZSM-5 for aromatics synthesis from carbon dioxide[J]. ACS Catal,2020,10(1):302−310. doi: 10.1021/acscatal.9b04309 [39] WITOON T, CHALORNGTHAM J, DUMRONGBUNDITKUL P, CHAREONPANICH M, LIMTRAKUL J. CO2 hydrogenation to methanol over Cu/ZrO2 catalysts: Effects of zirconia phases[J]. Chem Eng J,2016,293:327−336. doi: 10.1016/j.cej.2016.02.069 [40] TIAN H, JIAO J, ZHA F, GUO X, TANG X, CHANG Y, CHEN H. Hydrogenation of CO2 into aromatics over ZnZrO–Zn/HZSM-5 composite catalysts derived from ZIF-8[J]. Catal Sci Technol,2022,12(3):799−811. doi: 10.1039/D1CY01570B [41] HARE B J, MAITI D, DAZA Y A, BHETHANABOTLA V R, KUHN J N. Enhanced CO2 conversion to CO by silica-supported perovskite oxides at low temperatures[J]. ACS Catal,2018,8(4):3021−3029. doi: 10.1021/acscatal.7b03941 [42] XU Y, YU W, ZHANG H, XIN J, HE X, LIU B, JIANG F, LIU X. Suppressing C–C bond dissociation for efficient ethane dehydrogenation over the isolated Co(II) sites in SAPO-34[J]. ACS Catal,2021,11(21):13001−13019. doi: 10.1021/acscatal.1c03382 [43] PIDKO E A, VAN SANTEN R A. Activation of light alkanes over zinc species stabilized in ZSM-5 zeolite: A comprehensive DFT study[J]. J Phys Chem C,2007,111(6):2643−2655. doi: 10.1021/jp065911v [44] FAN H, NIE X, WANG H, JANIK M J, SONG C, GUO X. Mechanistic understanding of ethane dehydrogenation and aromatization over Zn/ZSM-5: Effects of Zn modification and CO2 co-reactant[J]. Catal Sci Technol,2020,10(24):8359−8373. doi: 10.1039/D0CY01566K [45] MYINT M, YAN B H, WAN J, ZHAO S, CHEN J G G. Reforming and oxidative dehydrogenation of ethane with CO2 as a soft oxidant over bimetallic catalysts[J]. J Catal,2016,343:168−177. doi: 10.1016/j.jcat.2016.02.004 [46] WANG X, WANG Y, ROBINSON B, WANG Q, HU J. Ethane oxidative dehydrogenation by CO2 over stable CsRu/CeO2 catalyst[J]. J Catal,2022,413:138−149. doi: 10.1016/j.jcat.2022.06.021 [47] YAO S Y, YAN B H, JIANG Z, LIU Z Y, WU QY, LEE J H, CHEN J G. Combining CO2 reduction with ethane oxidative dehydrogenation by oxygen-modification of molybdenum carbide[J]. ACS Catal,2018,8(6):5374−5381. doi: 10.1021/acscatal.8b00541 [48] MARQUART W, RASEALE S, CLAEYS M, FISCHER N. Promoted MoxCy-based catalysts for the CO2 oxidative dehydrogenation of ethane[J]. ChemCatChem,2022,14(13):e202200267. [49] WAN T Y, JIN F, CHENG X J, GONG J H, WANG C Y, WU G Y, LIU A L. Influence of hydrophilicity and titanium species on activity and stability of Cr/MWW zeolite catalysts for dehydrogenation of ethane with CO2[J]. Appl Catal A: Gen,2022,637:118542. doi: 10.1016/j.apcata.2022.118542 [50] LIU J X, ZHANG Z M, JIANG Y L, JIANG X, HE N, YAN S Y, GUO P, XIONG G, SU J, VILE G. Influence of the zeolite surface properties and potassium modification on the Zn-catalyzed CO2-assisted oxidative dehydrogenation of ethane[J]. Appl Catal B: Environ,2022,304:120947. doi: 10.1016/j.apcatb.2021.120947 [51] LEI T Q, CHENG Y H, MIAO C X, HUA W M, YUE Y H, GAO Z. Silica-doped TiO2 as support of gallium oxide for dehydrogenation of ethane with CO2[J]. Fuel Process Technol,2018,177:246−254. doi: 10.1016/j.fuproc.2018.04.037 [52] CHENG Y H, ZHANG F, ZHANG Y, MIAO C X, HUA W M, YUE Y H, GAO Z. Oxidative dehydrogenation of ethane with CO2 over Cr supported on submicron ZSM-5 zeolite[J]. Chin J Catal,2015,36(8):1242−1248. doi: 10.1016/S1872-2067(15)60893-2 -





 下载:
下载: