Ti doped CeO2 nanosheets supported Pd catalyst for alcohol oxidation: Catalysis of interfacial sites
-
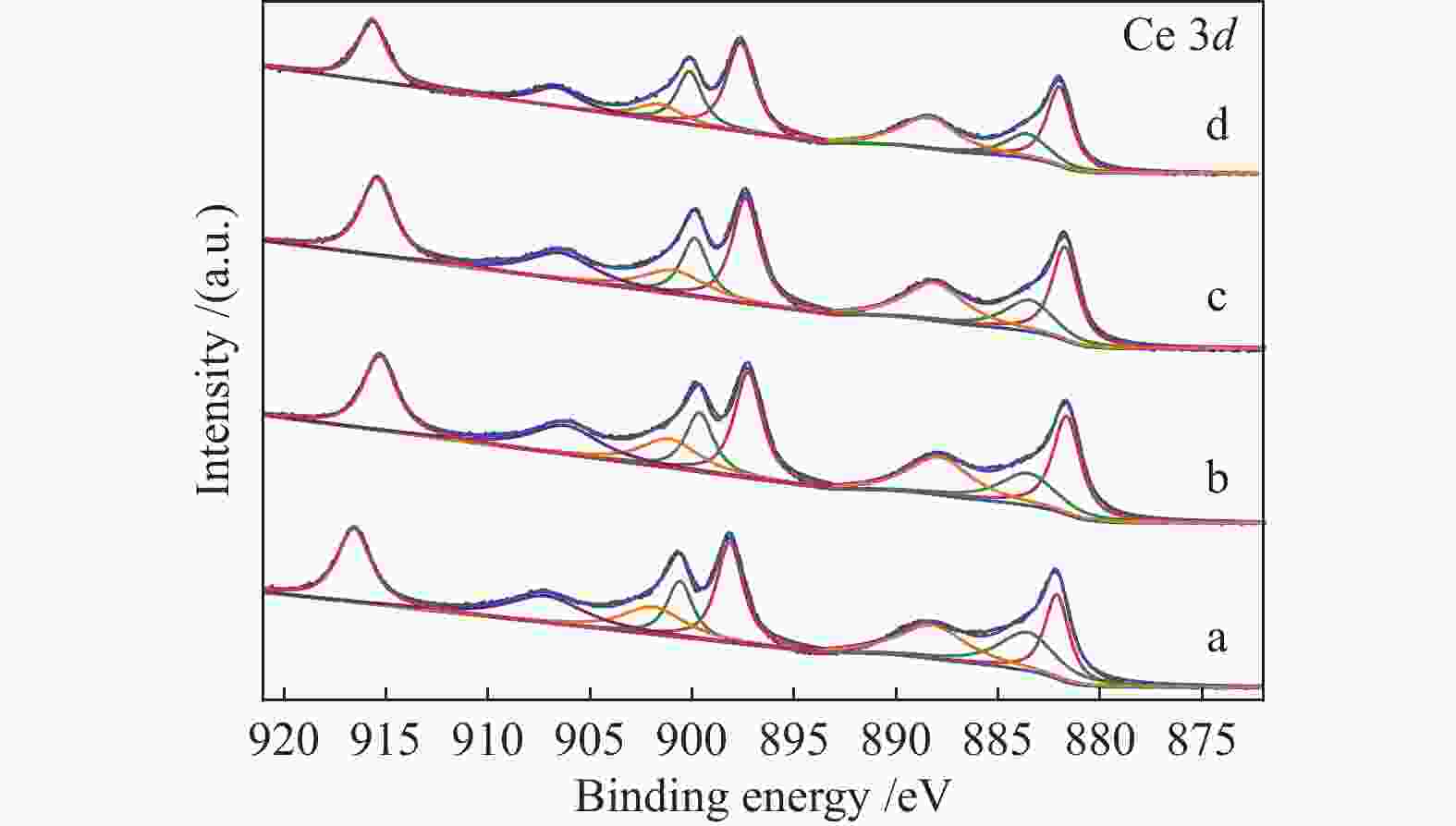
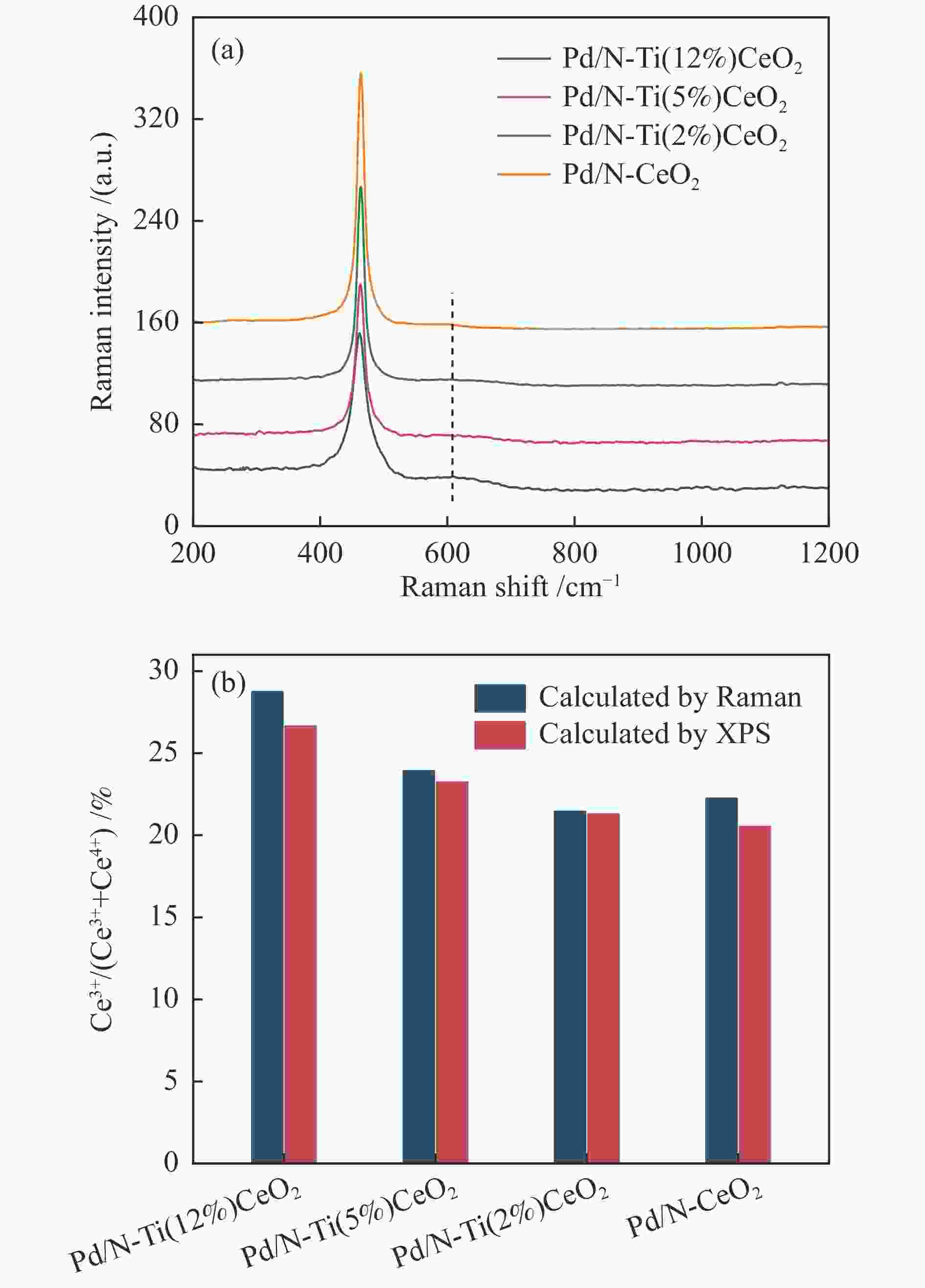
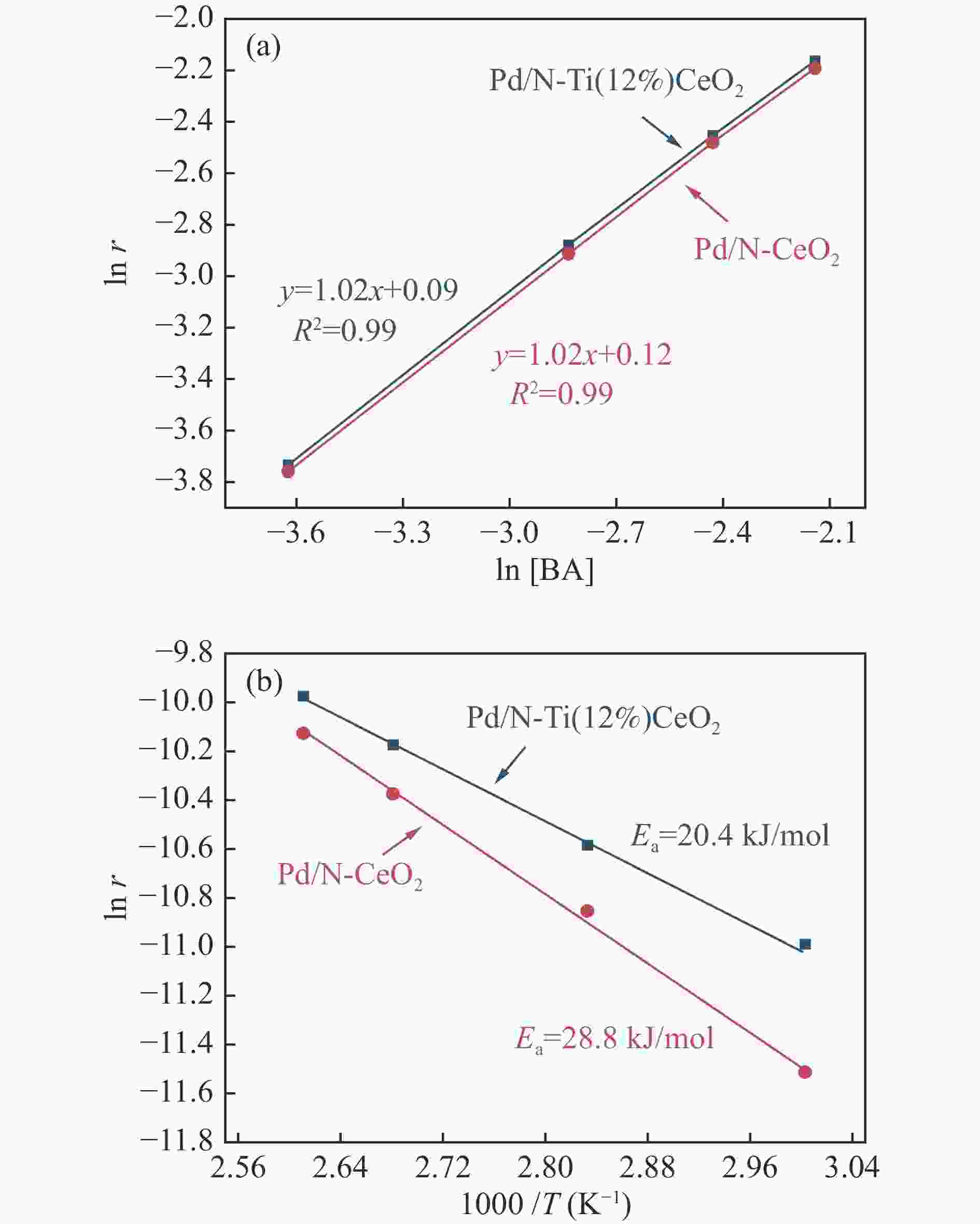
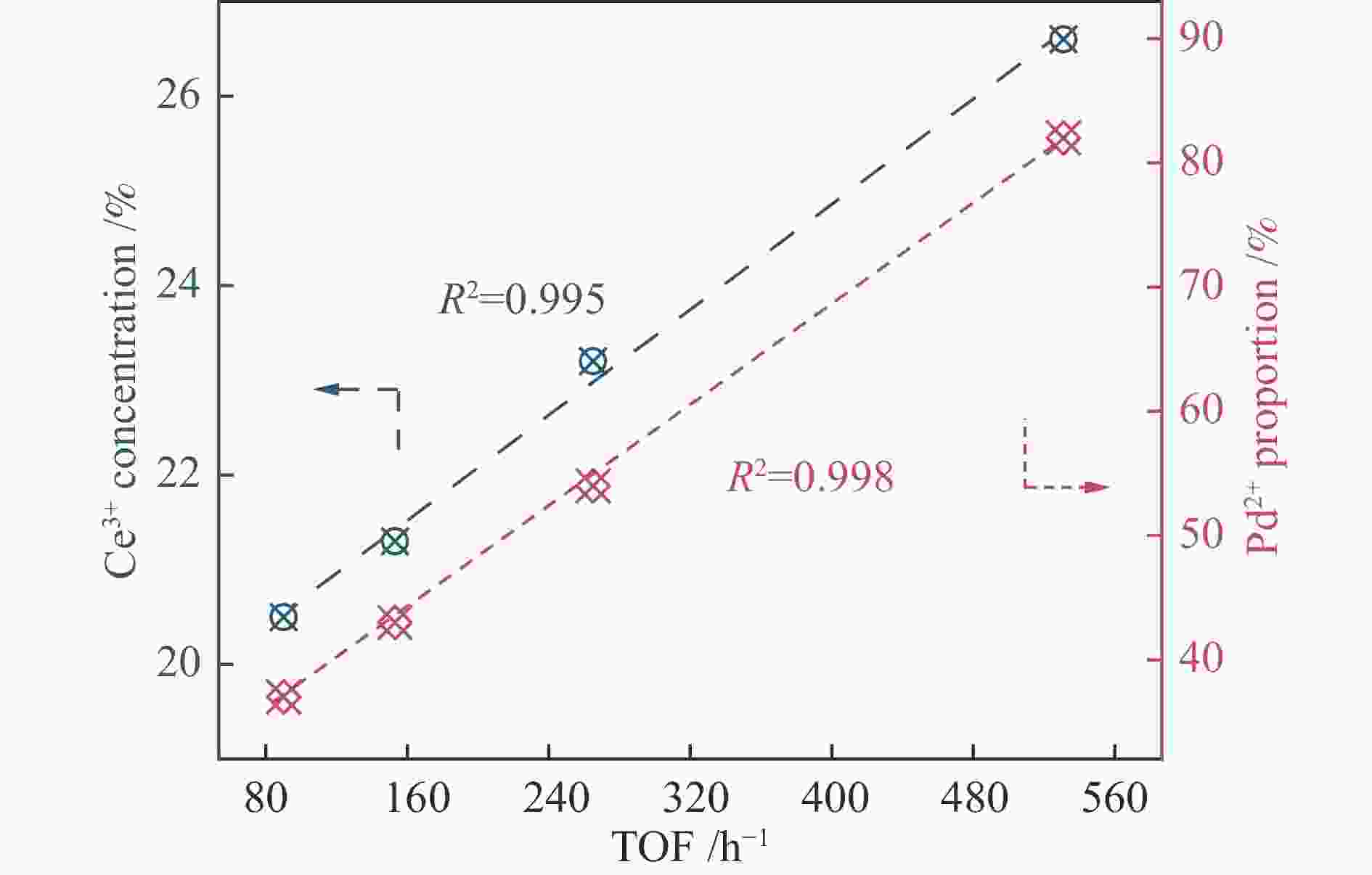
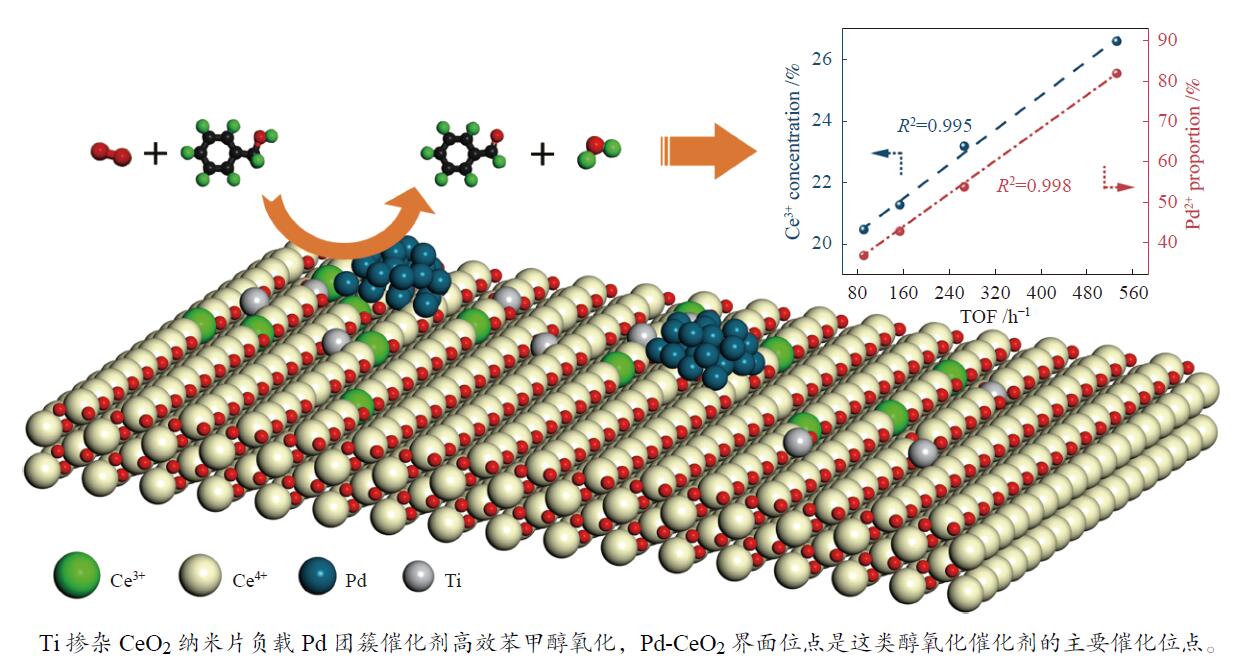
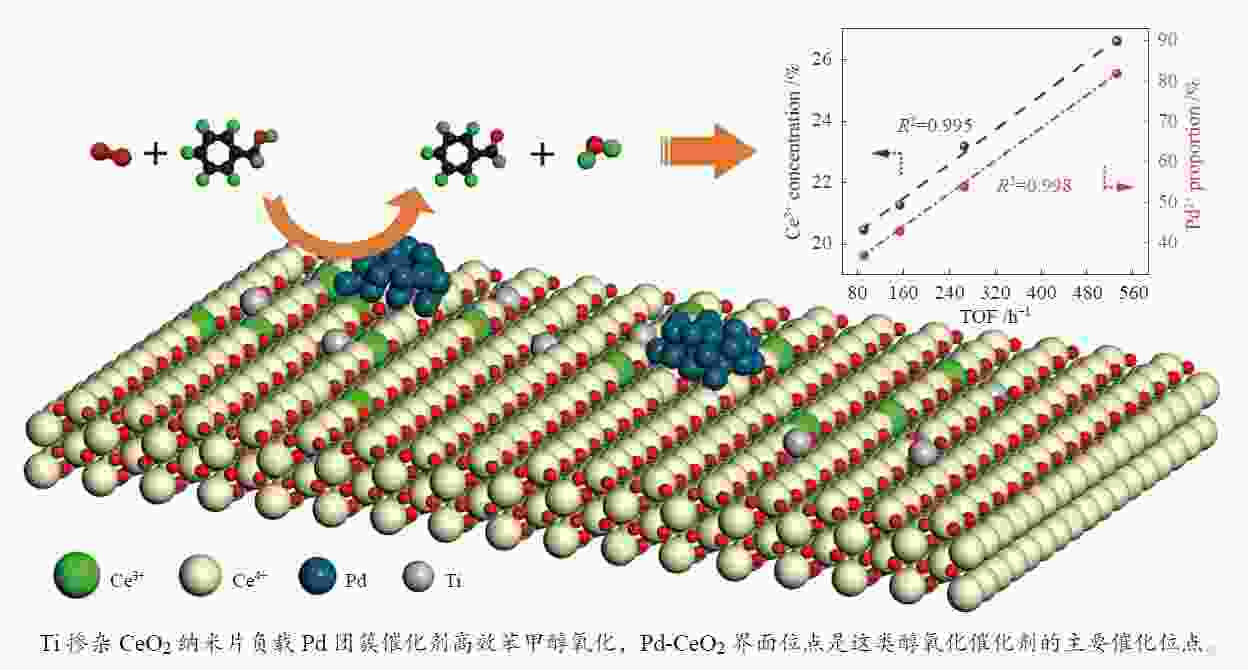
摘要: 本研究通过Ti掺杂以及改变Ti的掺杂量可控制备了表面氧空位浓度不同的CeO2纳米片,将其作为载体负载Pd物种后研究其醇类氧化性能。X射线光电子能谱、拉曼光谱以及X射线吸收谱的表征结果显示,CeO2表面氧空位浓度和Pd2 + 的比例正向相关。醇类氧化评价结果和构效关系研究显示,Pd2 + 比例和表面的Ce3 + 浓度分别与苯甲醇氧化反应的TOF值之间存在较好的线性关系,Pd与CeO2形成的界面位点(Pd–O–Ce)是这类醇氧化催化剂的主要催化位点。本研究有助于认识金属和氧化物载体界面位点的催化作用,从而开发更好的醇氧化催化剂。Abstract: The oxidation of alcohols is a significant chemical reaction, and the efficient oxidation of alcohols over heterogeneous catalysts using oxygen as oxidant has attracted much attention in recent years. Among them, Pd/CeO2 exhibits excellent alcohol oxidation performance. However, the structure-activity relationship between the catalyst’s structure and its catalytic performance for alcohol oxidation is still not clearly understood. This study involved the preparation of CeO2 nanosheets with different concentrations of surface oxygen vacancies (Ov) and their subsequent loading with Pd to explore their catalytic performance for alcohol oxidation. The findings obtained through XPS, Raman, and XAS indicated a positive correlation between the surface Ov concentration of CeO2 as well as the ratio of Pd2+ fraction. The alcohol oxidation results and structure-performance relationship studies showed that there was a good linear relationship between the Pd2+ ratio as well as the surface Ce3+ concentration and the TOF of benzyl alcohol oxidation reaction, respectively. And the interfacial site (Pd–O–Ce) formed by Pd and CeO2 was the main catalytic site for this type of alcohol oxidation catalysts. This study contributes to the understanding of the catalytic role of interfacial sites in metal and oxide support for the development of better alcohol oxidation catalysts.
-
Key words:
- Pd/CeO2 /
- interfacial catalytic site /
- CeO2 nanosheets /
- oxygen vacancies /
- alcohol oxidation
-
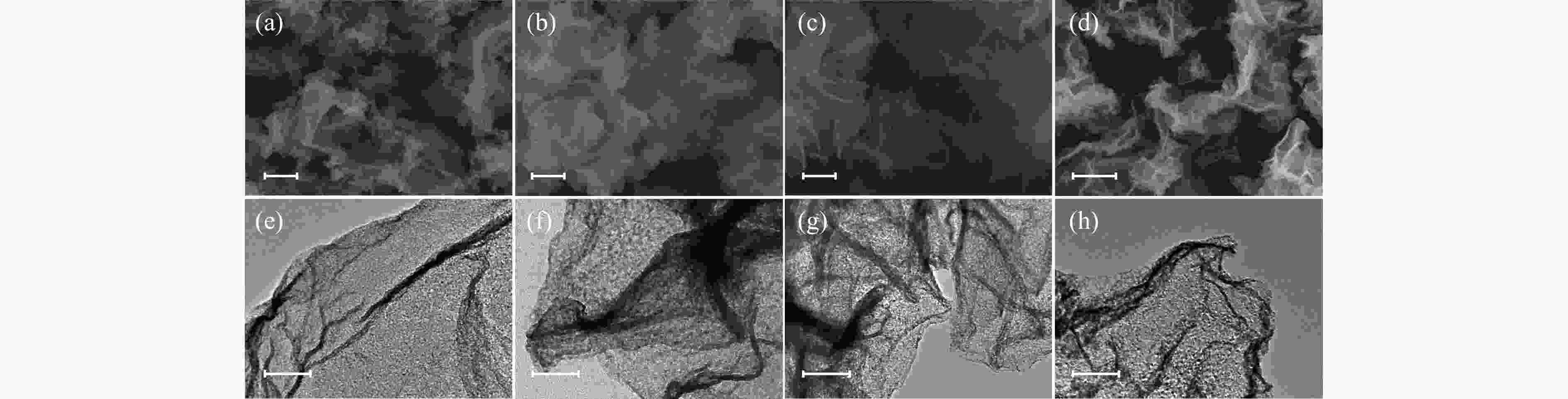
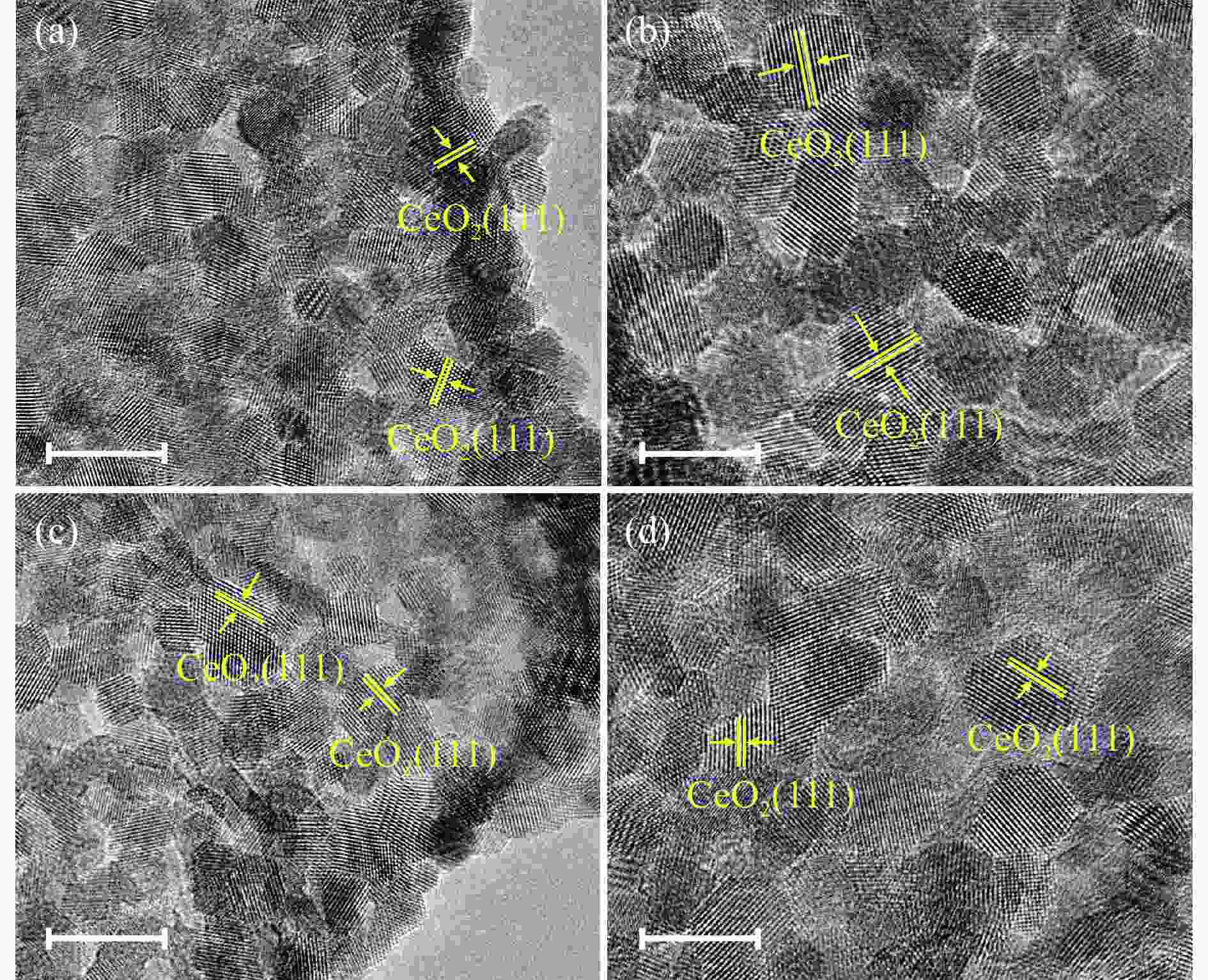
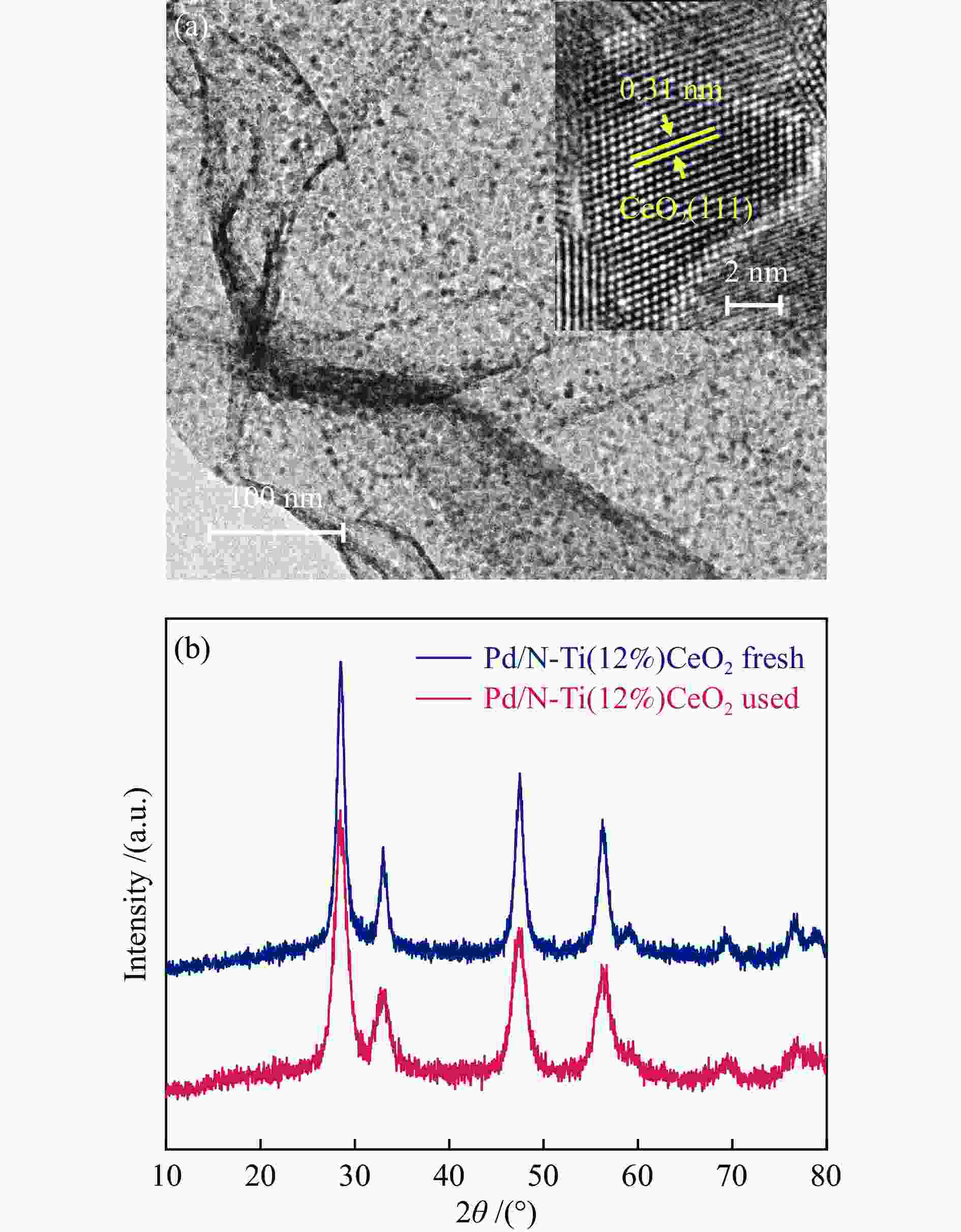
图 1 N-CeO2(a)、N-Ti(2%)CeO2(b)、N-Ti(5%)CeO2(c)和N-Ti(12%)CeO2(d)的扫描电镜照片;N-CeO2(e)、N-Ti(2%)CeO2(f)、N-Ti(5%)CeO2(g)和N-Ti(12%)CeO2(h)的透射电镜照片
Figure 1 SEM images of (a) N-CeO2, (b) N-Ti(2%)CeO2, (c) N-Ti(5%)CeO2, and (d) N-Ti(12%)CeO2; TEM images of (e) N-CeO2, (f) N-Ti(2%)CeO2, (g) N-Ti(5%)CeO2, and (h) N-Ti(12%)CeO2 Scale bars: ((a)–(d)): 500 nm, ((e)–(h)): 200 nm
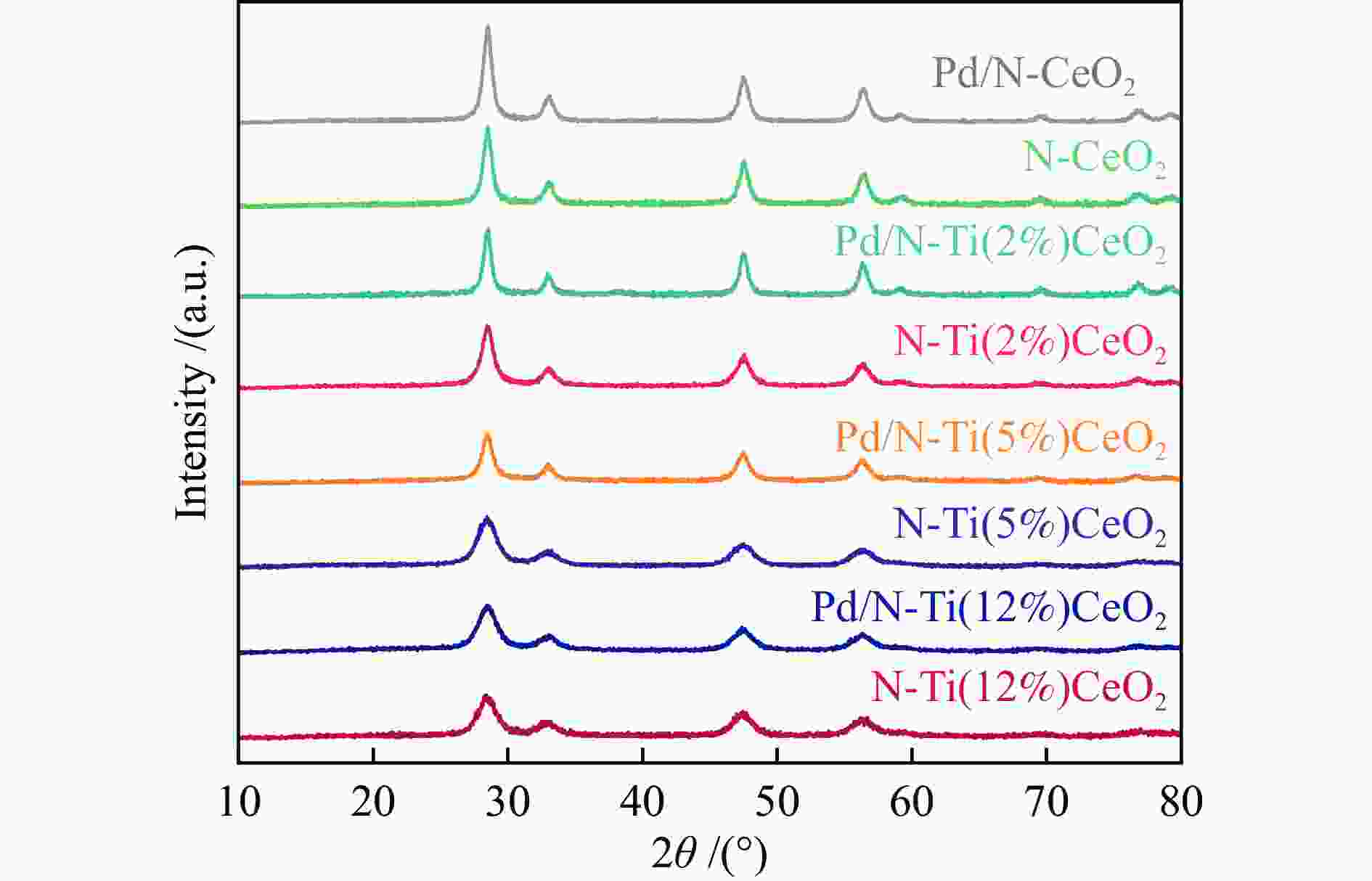
表 1 N-CeO2载体和Pd/N-CeO2催化剂的物理化学性质
Table 1 Physicochemical properties of prepared N-CeO2 and Pd/ N-CeO2 samples
Entry Sample Pd loading a w/% Surface areab /(m2·g–1) Crystal sizec /nm Cell parameterd /Å 1 N-CeO2 − 76.5 7.6 5.447 2 N-Ti(2%)CeO2 − 82.7 7.1 5.454 3 N-Ti(5%)CeO2 − 87.1 6.7 5.466 4 N-Ti(12%)CeO2 − 90.7 6.2 5.472 5 Pd/N-CeO2 0.98 76.2 7.4 − 6 Pd/N-Ti(2%)CeO2 0.93 81.3 7.0 − 7 Pd/N-Ti(5%)CeO2 0.95 86.3 6.4 − 8 Pd/N-Ti(12%)CeO2 0.96 89.8 6.1 − a: Calculated as based on ICP-OES results; b: Obtained from N2 adsorption and desorption; c: Estimated from the broadening of CeO2 (111), (200), (220), and (113) diffraction peaks by using the Scherrer formula from the XRD patterns of corresponding CeO2 samples and catalysts; d: Calculated from the CeO2 planes (111), (200), (220), and (113) by the MDI Jade software 表 2 不同Pd/N-CeO2的Pd的K边EXAFS拟合数据
Table 2 Results of Pd K-edge EXAFS spectra fitted for various Pd/N-CeO2
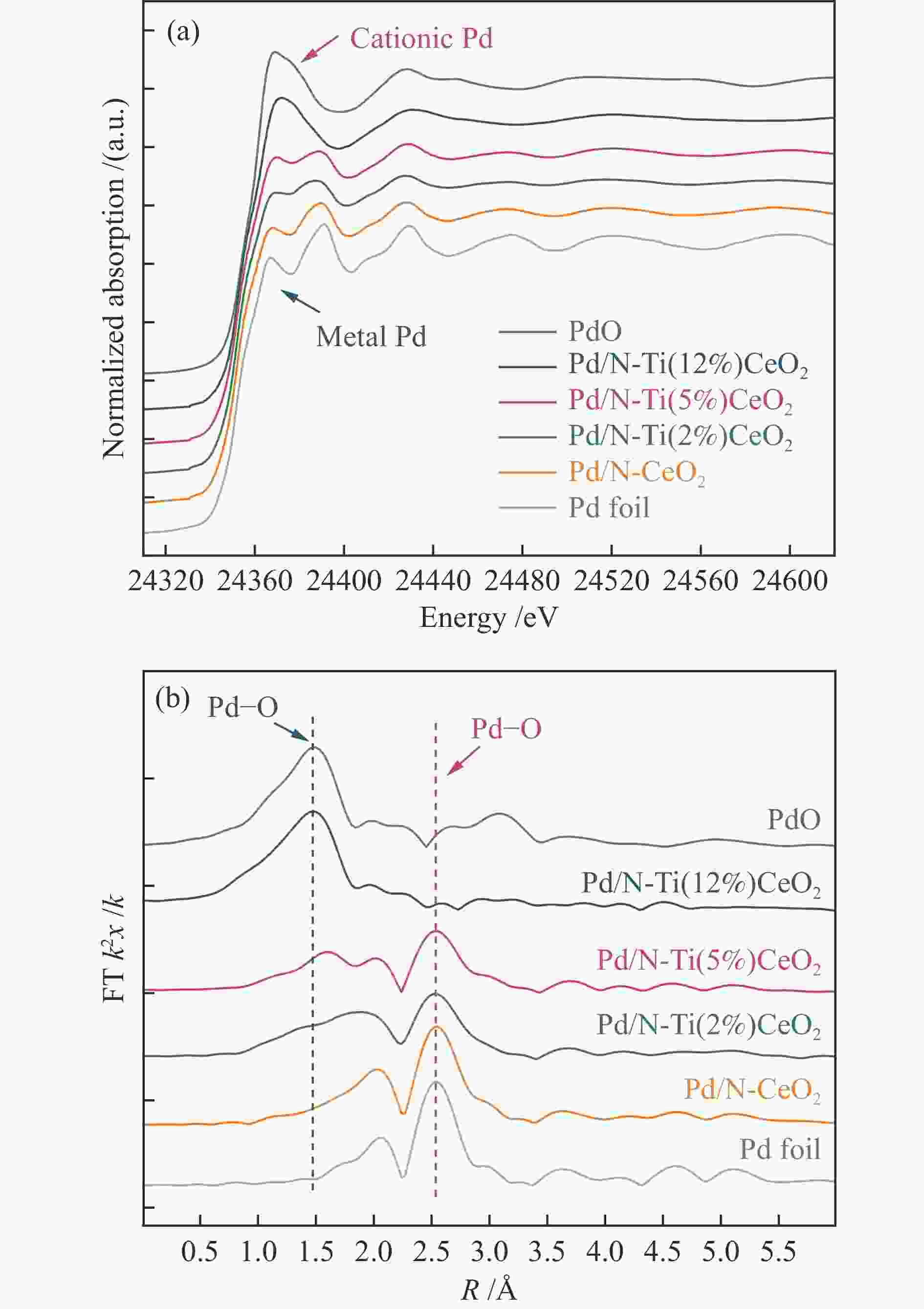
Sample Shell CN Ntotal R /Å ∆E /eV σ2 /Å2 R-factor Pd/N-Ti(12%)CeO2 Pd–O 2.2 (±0.3) 3.3 1.99 (±0.02) 5.38 0.005 0.0173 Pd–Pd 0.9 (±0.4) 2.71 (±0.03) 0.14 0.002 Pd/N-Ti(5%)CeO2 Pd–O 1.9 (±0.2) 4.0 1.99 (±0.02) 8.21 0.004 0.0062 Pd–Pd 2.0 (±0.3) 2.72 (±0.02) 0.05 0.003 Pd/N-Ti(2%)CeO2 Pd–O 1.7 (±0.3) 4.2 2.00 (±0.04) 9.23 0.004 0.0084 Pd–Pd 2.3 (±0.3) 2.73 (±0.02) 0.16 0.003 Pd/N-CeO2 Pd–O 1.2 (±0.4) 4.6 2.02 (±0.03) 8.72 0.004 0.0043 Pd–Pd 3.5 (±0.2) 2.74 (±0.01) 0.19 0.003 Note: CN, coordination number; ∆E, inner core correction; R, distances; σ2, Debye-Waller Factor (Fit range 3 < k < 11; 1.2 < R < 3.2; number of independent points = 9.5) 表 3 不同催化剂在醇类氧化反应中的评价
Table 3 Performances of various catalysts in the aerobic oxidation of alcohols
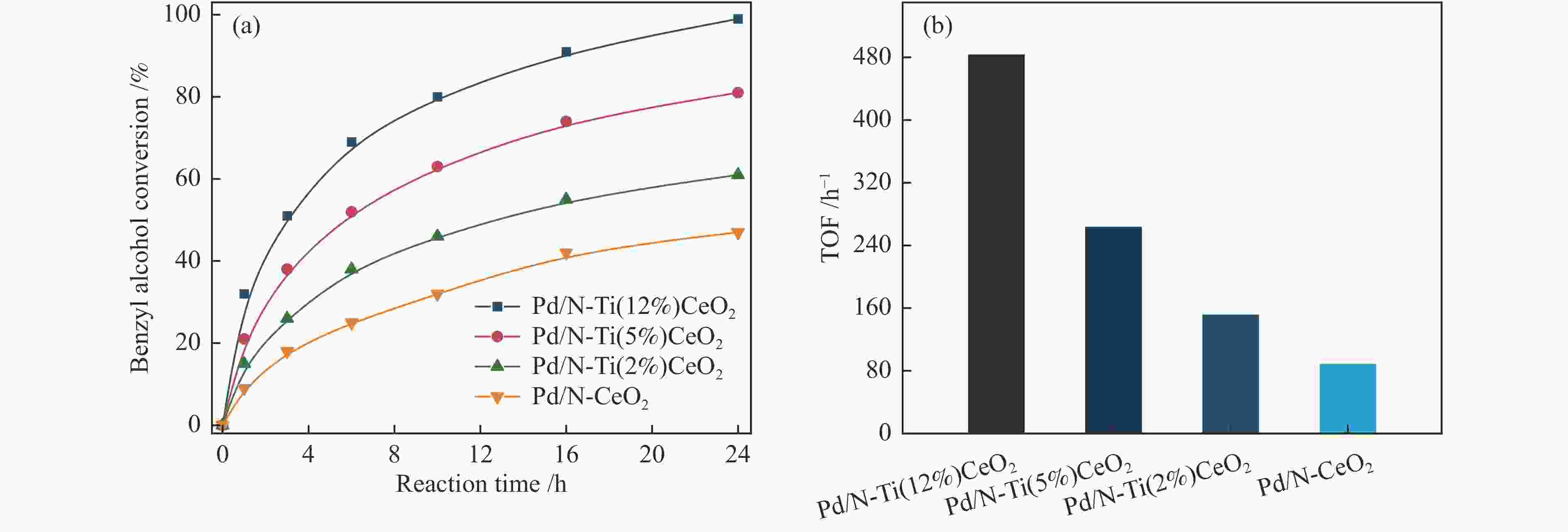
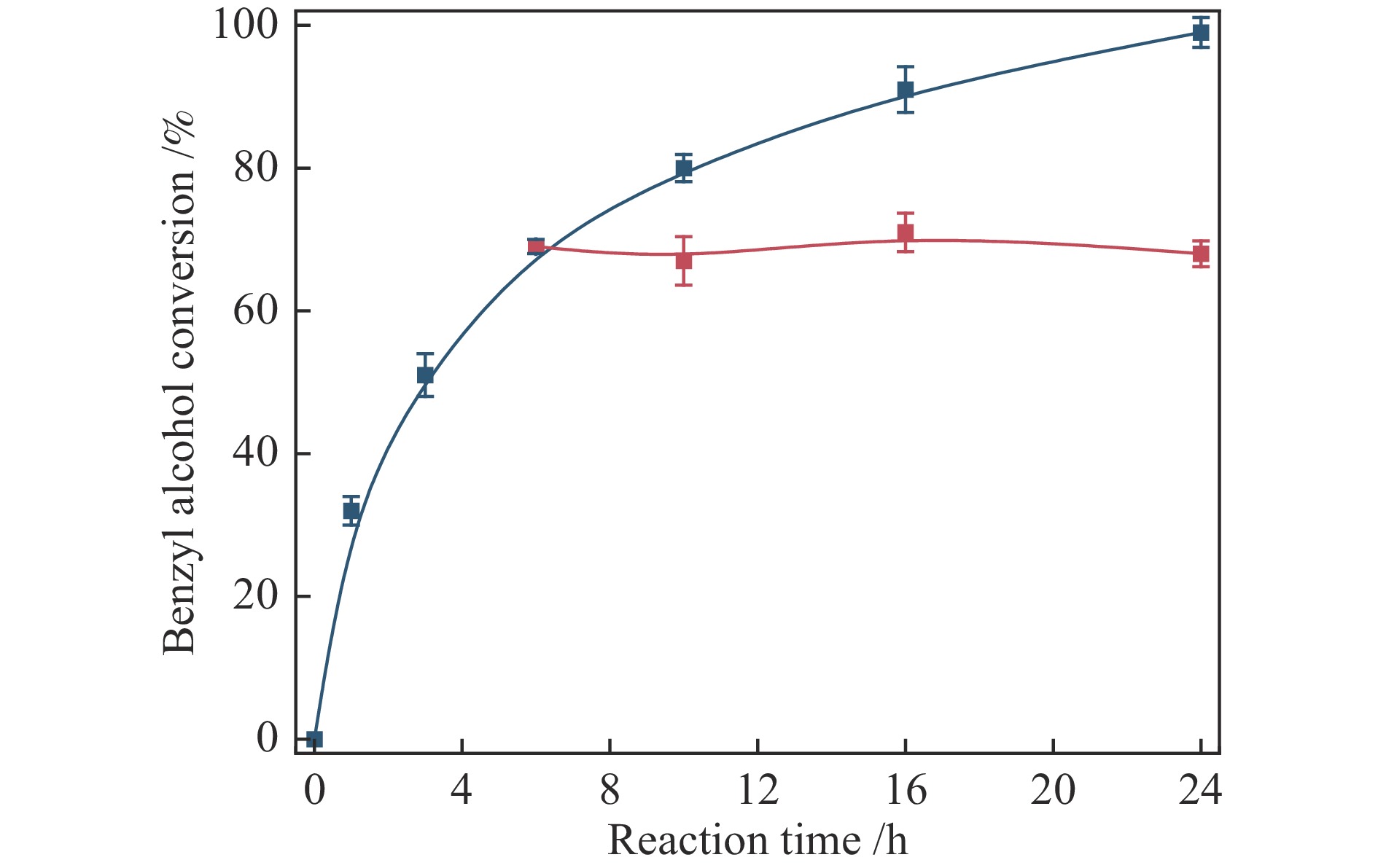
Entry Substrate Catalyst Conversion /% Selectivitye /% TOFf /h−1 Carbon balance /% 1a Benzyl alcohol N-Ti(12%)CeO2 0.8 99.2 − 98.1 2a Benzyl alcohol Pd/N-Ti(12%)CeO2 98.6 98.4 480 97.5 3a Benzyl alcohol Pd/N-Ti(5%)CeO2 81.3 97.6 265 99 4a Benzyl alcohol Pd/N-Ti(2%)CeO2 60.7 95 153 98.3 5a Benzyl alcohol Pd/N-CeO2 46.7 93.7 90 97.8 6a p-Chlorobenzyl alcohol Pd/N-Ti(12%)CeO2 90.7 99.1 376 94.2 7a p-Nitrobenzyl alcohol Pd/N-Ti(12%)CeO2 84.2 98.5 309 95.6 8a o-Methylbenzyl alcohol Pd/N-Ti(12%)CeO2 99.3 98.1 513 96.4 9a p-Methylbenzyl alcohol Pd/N-Ti(12%)CeO2 98.9 97.2 498 98.7 10a p-Methoxylbenzyl alcohol Pd/N-Ti(12%)CeO2 99.5 95.1 486 94.8 11a Cinnamyl alcohol Pd/N-Ti(12%)CeO2 96.7 99.6 457 98.5 12b Cyclohexanol Pd/N-Ti(12%)CeO2 87.5 97.1 235 94.6 13c Butanol Pd/N-Ti(12%)CeO2 81.4 90.1 164 95.3 14d Ethanol Pd/N-Ti(12%)CeO2 80.3 95.7 138 93.8 a: Entries 1–11: 10 mL deionized water, 6.0 mmol substrate, certain amount catalyst (the molar ratio of substrate/Pd was about 2000), 0.5 MPa O2, 90 ℃ for 24 h; b: Entries 12: 2.0 mmol cyclohexanol, the molar ratio of cyclohexanol/Pd was about 600, 90 ℃ for 36 h;
c: Entries 13: 1.0 mmol butanol, the molar ratio of butanol/Pd was about 300, 90 ℃ for 40 h; d: Entries 14: 1.0 mmol ethanol, the molar ratio of ethanol/Pd was about 300, 90 ℃ for 40 h; e: The selectivity refers to corresponding acids for entries 12–14 and to the corresponding aldehydes for other entries; f: The TOF values based on the reaction for initial 15 min -
[1] GUO Z, LIU B, ZHANG Q, DENG W, WANG Y, YANG Y. Recent advances in heterogeneous selective oxidation catalysis for sustainable chemistry[J]. Chem Soc Rev,2014,43:3480−3524. doi: 10.1039/c3cs60282f [2] NAJAFISHIRTARI S, FRIEDEL ORTEGA K, DOUTHWAITE M, PATTISSON S, HUTCHINGS G J, BONDUE C J, TSCHULIK K, WAFFEL D, PENG B, DEITERMANN M, BUSSER G W, MUHLER M, BEHRENS M. A perspective on heterogeneous catalysts for the selective oxidation of alcohols[J]. Chem - Eur J,2021,27:1−26. doi: 10.1002/chem.202004683 [3] 吴超龙, 喻敏, 姚小泉. 醇的选择性催化氧化研究进展[J]. 化工时刊,2017,31:27−35.WU Chao-long, YU Min, YAO Xiao-quan. Research progress in selective catalytic oxidation of alcohols[J]. Chem Ind Times,2017,31:27−35. [4] 刘晓, 王涛, 张晨, 宋宪根, 宁丽丽, 丁云杰. Co(Ⅱ)-席夫碱配合物在水溶液中催化苯甲醇氧化制苯甲醛的研究[J]. 石油化工,2015,44:1467−1474.LIU Xiao, WANG Tao, ZHANG Chen, SONG Xian-gen, NING Li-li, DING Yun-jie. Aerobic oxidation of benzyl alcohol to benzaldehyde with Co(Ⅱ)-schiff base complex catalysts in aqueous solution[J]. Petrochem Technol,2015,44:1467−1474. [5] LEI L, WU Z, WANG R, QIN Z, CHEN C, LIU Y, WANG G, FAN W, WANG J. Controllable decoration of palladium sub-nanoclusters on reduced graphene oxide with superior catalytic performance in selective oxidation of alcohols[J]. Catal Sci Technol,2017,7:5650−5661. doi: 10.1039/C7CY01732D [6] LIU J, ZOU S, WANG H, XIAO L, ZHAO H, FAN J. Synergistic effect between Pt0 and Bi2O3-x for efficient room-temperature alcohol oxidation under base-free aqueous conditions[J]. Catal Sci Technol,2017,7:1203−1210. doi: 10.1039/C6CY02596J [7] LI T, LIU F, TANG Y, LI L, MIAO S, SU Y, ZHANG J, HUANG J, SUN H, HARUTA M, WANG A, QIAO B, LI J, ZHANG T. Maximizing the number of interfacial sites in single-atom catalysts for the highly selective, solvent-free oxidation of primary alcohols[J]. Angew Chem, Int Ed,2018,57:7795−7799. doi: 10.1002/anie.201803272 [8] TAN H T, CHEN Y, ZHOU C, JIA X, ZHU J, CHEN J, RUI X, YAN Q, YANG Y. Palladium nanoparticles supported on manganese oxide-CNT composites for solvent-free aerobic oxidation of alcohols: Tuning the properties of Pd active sites using MnOx[J]. Appl Catal B: Environ,2012,119−120:166−174. [9] LIU J, ZOU S, WU J, KOBAYASHI H, ZHAO H, FAN J. Green catalytic oxidation of benzyl alcohol over Pt/ZnO in base-free aqueous medium at room temperature[J]. Chin J Catal,2018,39:1081−1089. doi: 10.1016/S1872-2067(18)63022-0 [10] ZHANG P, GONG Y, LI H, CHEN Z, WANG Y. Solvent-free aerobic oxidation of hydrocarbons and alcohols with Pd@N-doped carbon from glucose[J]. Nat Commun,2013,4:1593−1603. doi: 10.1038/ncomms2586 [11] LEI L, LIU H, WU Z, QIN Z, WANG G, MA J, LUO L, FAN W, WANG J. Aerobic oxidation of alcohols over isolated single Au atoms supported on CeO2 nanorods: Catalysis of interfacial [O–Ov–Ce–O–Au] sites[J]. ACS Appl Nano Mater,2019,2:5214−5223. doi: 10.1021/acsanm.9b01091 [12] ZHENG H, WEI Z H, HU X Q, XU J, XUE B. Atmospheric selective oxidation of benzyl alcohol catalyzed by Pd nanoparticles supported on CeO2 with various morphologies[J]. ChemistrySelect,2019,4:5470−5475. doi: 10.1002/slct.201900757 [13] XIN P, LI J, XIONG Y, WU X, DONG J, CHEN W, WANG Y, GU L, LUO J, RONG H, CHEN C, PENG Q, WANG D, LI Y. Revealing the active species for aerobic alcohol oxidation by using uniform supported palladium catalysts[J]. Angew Chem, Int Ed,2018,57:4642−4646. doi: 10.1002/anie.201801103 [14] OLMOS C M, CHINCHILLA L E, VILLA A, DELGADO J J, HUNGRíA A B, BLANCO G, PRATI L, CALVINO J J, CHEN X. Size, nanostructure, and composition dependence of bimetallic Au-Pd supported on ceria-zirconia mixed oxide catalysts for selective oxidation of benzyl alcohol[J]. J Catal,2019,375:44−55. doi: 10.1016/j.jcat.2019.05.002 [15] MIAO Z, WU T, LI J, YI T, ZHANG Y, YANG X. Aerobic oxidation of 5-hydroxymethylfurfural (HMF) effectively catalyzed by a Ce0.8Bi0.2O2-δ supported Pt catalyst at room temperature[J]. RSC Adv,2015,5:19823−19829. doi: 10.1039/C4RA16968A [16] LEI L, WU Z, LIU H, QIN Z, CHEN C, LUO L, WANG G, FAN W, WANG J. A facile method for the synthesis of graphene-like 2D metal oxides and their excellent catalytic application in the hydrogenation of nitroarenes[J]. J Mater Chem A,2018,6:9948−9961. doi: 10.1039/C8TA02338G [17] ZHANG S, CHANG C-R, HUANG Z-Q, LI J, WU Z, MA Y, ZHANG Z, WANG Y, QU Y. High catalytic activity and chemoselectivity of sub-nanometric Pd clusters on porous nanorods of CeO2 for hydrogenation of nitroarenes[J]. J Am Chem Soc,2016,138:2629−2637. doi: 10.1021/jacs.5b11413 [18] INFANTES-MOLINA A, VILLANOVA A, TALON A, KOHAN M G, GRADONE A, MAZZARO R, MORANDI V, VOMIERO A, MORETTI E. Au-decorated Ce-Ti mixed oxides for efficient CO preferential photooxidation[J]. ACS Appl Mater Interfaces,2020,12:38019−38030. doi: 10.1021/acsami.0c08258 [19] GHORBANLOO M, NADA A A, EL-MAGHRABI H H, BEKHEET M F, RIEDEL W, DJAMEL B, VITER R, ROUALDES S, SOLIMAN F S, MOUSTAFA Y M, MIELE P, BECHELANY M. Superior efficiency of BN/Ce2O3/TiO2 nanofibers for photocatalytic hydrogen generation reactions[J]. Appl Surf Sci,2022,594:153438−153451. doi: 10.1016/j.apsusc.2022.153438 [20] LI S, ZHU H, QIN Z, WANG G, ZHANG Y, WU Z, LI Z, CHEN G, DONG W, WU Z, ZHENG L, ZHANG J, HU T, WANG J. Morphologic effects of nano CeO2-TiO2 on the performance of Au/CeO2-TiO2 catalysts in low-temperature CO oxidation[J]. Appl Catal B: Environ,2014,144:498−506. doi: 10.1016/j.apcatb.2013.07.049 [21] DUTTA P, PAL S, SEEHRA M S, SHI Y, EYRING E M, ERNST R D. Concentration of Ce3 + and oxygen vacancies in cerium oxide nanoparticles[J]. Chem Mater,2006,18:5144−5146. doi: 10.1021/cm061580n [22] ESCH F, FABRIS S, ZHOU L, MONTINI T, AFRICH C, FORNASIERO P, COMELLI G, ROSEI R. Electron localization determines defect formation on ceria substrates[J]. Science,2005,309:752−755. doi: 10.1126/science.1111568 [23] LAGUNA O H, SARRIA F R, CENTENO M A, ODRIOZOLA J A. Gold supported on metal-doped ceria catalysts (M = Zr, Zn and Fe) for the preferential oxidation of CO (PROX)[J]. J Catal,2010,276:360−370. doi: 10.1016/j.jcat.2010.09.027 [24] WANG X, WU G, GUAN N, LI L. Supported Pd catalysts for solvent-free benzyl alcohol selective oxidation: Effects of calcination pretreatments and reconstruction of Pd sites[J]. Appl Catal B: Environ,2012,115−116:7−15. doi: 10.1016/j.apcatb.2011.12.011 [25] MILLER H A, LAVACCHI A, VIZZA F, MARELLI M, DI BENEDETTO F, D'ACAPITO F, PASKA Y, PAGE M, DEKEL D R. A Pd/C-CeO2 Anode catalyst for high-performance platinum-free anion exchange membrane fuel cells[J]. Angew Chem, Int Ed,2016,55:6004−6007. doi: 10.1002/anie.201600647 [26] WANG M, WANG F, MA J, LI M, ZHANG Z, WANG Y, ZHANG X, XU J. Investigations on the crystal plane effect of ceria on gold catalysis in the oxidative dehydrogenation of alcohols and amines in the liquid phase[J]. Chem Commun,2014,50:292−294. doi: 10.1039/C3CC46180G [27] MURAVEV V, SPEZZATI G, SU Y-Q, PARASTAEV A, CHIANG F-K, LONGO A, ESCUDERO C, KOSINOV N, HENSEN E J M. Interface dynamics of Pd-CeO2 single-atom catalysts during CO oxidation[J]. Nat Catal,2021,4:469−478. doi: 10.1038/s41929-021-00621-1 [28] WANG H, FAN W, HE Y, WANG J, KONDO J N, TATSUMI T. Selective oxidation of alcohols to aldehydes/ketones over copper oxide-supported gold catalysts[J]. J Catal,2013,299:10−19. doi: 10.1016/j.jcat.2012.11.018 -




 下载:
下载: