Controllable preparation of wrapped Fe2O3@rGO composites and their lithium ion storage performance
-
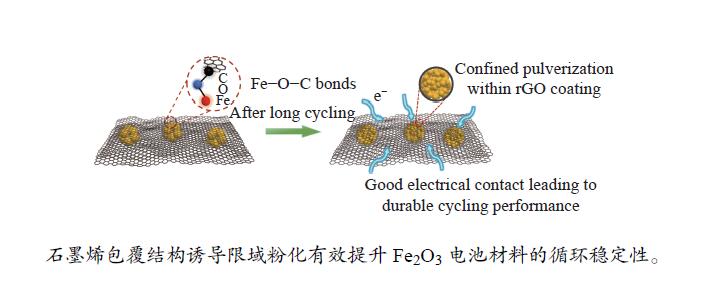
摘要: 本研究通过溶剂热法成功制备还原氧化石墨烯包覆Fe2O3空心球复合物(Fe2O3@rGO),并对其结构和性能进行了表征和测试。结果表明,包覆型Fe2O3@rGO负极材料由于内部存在大量的Fe−O−C键,明显提升了电子传输速率,同时石墨烯的物理限域显著降低了电极材料的粉化速率,因此,包覆型Fe2O3@rGO电极材料表现出优异的倍率性能(在大电流5 A/g下具有514 mA·h/g的可逆容量)和长循环寿命(在0.5 A/g下循环500圈后,容量保持987 mA·h/g,保持率为81.1%)。此项工作为制备高倍率、长寿命的石墨烯复合负极材料提供了一种有效策略。Abstract: In this paper, reduced graphene oxides wrapped hollow Fe2O3 spheres (Fe2O3@rGO) were successfully prepared by solvothermal method. Results show that plenty of Fe−O−C bonds between reduced graphene oxides and Fe2O3 significantly improved electron transfer rate of the composite anodes, and confinement effect of reduced graphene oxides slowed the pulverization rate of Fe2O3 during charge/discharge process. As expected, wrapped structured Fe2O3@rGO anode exhibited high rate capability of 514 mA·h/g at high current of 5.0 A/g and durable cycling life over 500 cycles with a capacity of 987 mA·h/g under 0.5 A/g with a capacity retention of 81.1%. This work provides an effective strategy for the preparation of high-rate and long-life graphene composite anode materials.
-
Key words:
- Fe2O3 /
- hollow sphere /
- RGO /
- anode /
- lithium-ion battery
-
图 4 样品的电化学性能图:(a)循环伏安曲线;(b)倍率性能;((c)、(d))不同电流密度下的充放电曲线;(e)循环性能;(f)库伦效率;(g)不同电极材料循环200圈后的电化学阻抗
Figure 4 (a) CV curves; (b) Rate performance; ((c), (d)) Charge-discharge profiles at different current density; (e) Cycling performance of the obtained samples; (f) Coulombic efficiency and (g) Electrochemical impedance spectra (EIS) of Fe2O3@rGO and Fe2O3/rGO anodes after 200 cycles
-
[1] 杨改秀, 王可欣, 张泽珍, 甄峰, 孙永明. 电沉积制备MnO2催化剂及其在微生物燃料电池中的应用[J]. 燃料化学学报,2020,48(7):889−896.YANG Gai-xiu, WANG Ke-xin, ZHANG Ze-zhen, ZHEN Feng, SUN Yong-ming. Preparation of MnO2 catalyst by electrochemical deposition and its application in the microbial fuel cells[J]. J Fuel Chem Technol,2020,48(7):889−896. [2] 马晓涛, 周娴娴, 李瑜, 刘晓晓, 郭倩, 段东红, 刘世斌. 纳米CoSe修饰氮掺杂多孔碳的可控制备及其锂硫电池多硫化物的催化转化效应[J]. 无机化学学报,2023,39(3):443−455.MA Xiao-tao, ZHOU Xian-xian, LI Yu, LIU Xiao-xiao, GUO Qian, DUAN Dong-hong, LIU Shi-bin. Controllable synthesis of N-doped porous carbon decorated with nano CoSe and catalytic effect on polysulfides conversion for Li-S battery[J]. Chin J Inorg Chem,2023,39(3):443−455. [3] MA J, GUO X, Yan Y, XUE H, PANG H. FeOx-based materials for electrochemical energy storage[J]. Adv Sci,2018,5:1700986. doi: 10.1002/advs.201700986 [4] 曹虎, 王帅, 吴沁宇, 宋广生, 马扬洲. 锂离子电池SiO2负极材料的改性研究进展[J]. 功能材料,2022,53(6):6067−6077. doi: 10.3969/j.issn.1001-9731.2022.06.010CAO Hu, WANG Shuai, WU Qin-yu, SONG Guang-sheng, MA Yang-zhou. Research progress on modification of SiO2 anode materials for lithium-ion batteries[J]. J Funct Mater,2022,53(6):6067−6077. doi: 10.3969/j.issn.1001-9731.2022.06.010 [5] HWANG J Y, MYUNG S T, SUN Y K. Sodium-ion batteries: present and future[J]. Chem Soc Rev,2017,46:3529−3614. doi: 10.1039/C6CS00776G [6] CHEN Y, YUAN X, YANG C, LIAN Y, RAZZAQ A. A, SHAH R, GUO J, ZHAO X, PENG Y, DENG Z. γ-Fe2O3 nanoparticles embedded in porous carbon fibers as binder-free anodes for high-performance lithium and sodium ion batteries[J]. J Alloys Compd,2019,777:127−134. doi: 10.1016/j.jallcom.2018.10.371 [7] GAO L, GU C, ZHAO J, SONG X, HUANG J. Preparation of manganese monoxide@reduced graphene oxide nanocomposites with superior electrochemical performances for lithium-ion batteries[J]. Ceram Int,2019,45(3):3425−3434. doi: 10.1016/j.ceramint.2018.10.257 [8] GUO L, SUN H, QIN C, LI W, WANG F, SONG W, DU J, ZHONG F, DING Y. Flexible Fe3O4 nanoparticles/N-doped carbon nanofibers hybrid film as binder-free anode materials for lithium-ion batteries[J]. Appl Surf Sci,2018,459:263−270. doi: 10.1016/j.apsusc.2018.08.001 [9] HE C, WU S, ZHAO N, SHI C, LIU E, LI J. Carbon-encapsulated Fe3O4 nanoparticles as a high-rate lithium ion battery anode material[J]. ACS Nano,2013,7(5):4459−4469. doi: 10.1021/nn401059h [10] LI W, YANG F, RUI Y, TANG B. Strong covalent interaction Fe2O3/nitrogen-doped porous carbon fiber hybrids as free-standing anodes for lithium-ion batteries[J]. J Mater Sci,2019,54:6500−6514. doi: 10.1007/s10853-019-03330-0 [11] FANG Y, CHEN Z, XIAO L, AI X, CAO Y, YANG H. Recent progress in iron-based electrode materials for grid-scale sodium-ion batteries[J]. Small,2018,14(9):1703116. doi: 10.1002/smll.201703116 [12] HUANG B, TAI K, ZHANG M, XIAO Y, DILLON S J. Comparative study of Li and Na electrochemical reactions with iron oxide nanowires[J]. Electrochim Acta,2014,118:143−149. doi: 10.1016/j.electacta.2013.12.007 [13] JIN Y, DANG L, ZHANG H, SONG C, LU Q, GAO F. Synthesis of unit-cell-thick α-Fe2O3 nanosheets and their transformation to γ-Fe2O3 nanosheets with enhanced LIB performances[J]. Chem Eng J,2017,326:292−297. doi: 10.1016/j.cej.2017.05.155 [14] LEE S H, SRIDHA V, JUNG J H, KARTHIKEYAN K, LEE Y, MUKHERJEE R, KORATKAR N, OH I. Graphene-nanotube-iron hierarchical nanostructure as lithium ion battery anode[J]. ACS Nano,2013,7(5):4242−4251. doi: 10.1021/nn4007253 [15] LIU J, WU Z, TIAN Q, WU W, XIAO X. Shape-controlled iron oxide nanocrystals: synthesis, magnetic properties and energy conversion applications[J]. CrystEngComm,2016,18:6303−6326. doi: 10.1039/C6CE01307D [16] ZHOU S, ZHOU Y, JIANG W, GUO H, WANG Z, LI X. Synthesis of Fe3O4 cluster microspheres/graphene aerogels composite as anode for high-performance lithium ion battery[J]. Appl Surf Sci,2018,439:927−933. doi: 10.1016/j.apsusc.2017.12.259 [17] PENKI T R, SHIVAKUMARA S, MINAKSHI M. Porous flower-like α-Fe2O3 Nanostructure: A high performance anode material for lithium-ion batteries[J]. Electrochim Acta,2015,167:330−339. doi: 10.1016/j.electacta.2015.03.146 [18] YANG S, SONG X, ZHANG P, GAO L. Heating-Rate-Induced Porous α-Fe2O3 with controllable pore size and crystallinity grown on graphene for supercapacitors[J]. ACS Appl Mater Interfaces,2015,7(1):75−79. doi: 10.1021/am507910f [19] PENG S, YU L, SUN M, CHENG G, LIN T, MO Y, LI Z. Bunched akaganeite nanorod arrays: Preparation and high-performance for flexible lithium-ion batteries[J]. J. Power Sources,2015,296(20):237−244. [20] YU X, YANG J, YUAN Z, GUO L, SUI Z, WANG M. Binder-free 3D porous Fe3O4-Fe2P-Fe@C films as high-performance anode materials for lithium-ion batteries[J]. Ceram Int,2020,46(11):17469−17477. doi: 10.1016/j.ceramint.2020.04.041 [21] LIANG H, CHEN W, WANG R, QI Z, MI J, WANG Z. X-shaped hollow α-FeOOH penetration twins and their conversion to α-Fe2O3 nanocrystals bound by high-index facets with enhanced photocatalytic activity[J]. Chem Eng J,2015,274:224−230. doi: 10.1016/j.cej.2015.03.125 [22] LIN M, TNG L, LIM T, CHOO M, ZHANG J, TAN H R, BAI S. Hydrothermal synthesis of octadecahedral hematite (α-Fe2O3) nanoparticles: An epitaxial growth from goethite (α-FeOOH)[J]. J Phys Chem C,2014,118(20):10903−10910. doi: 10.1021/jp502087h [23] LI F, LUO G, YU J, HUANG W, XU D, CHEN W, HUANG X, YANG S, FANG Y, YU X. Terminal hollowed Fe2O3@SnO2 heterojunction nanorods anode materials with enhanced performance for lithium-ion battery[J]. J Alloys Compd,2019,773:778−787. doi: 10.1016/j.jallcom.2018.09.159 [24] CAO H, ZHOU X, ZHENG C, LIU Z. Two-dimensional porous micro/nano metal oxides templated by graphene oxide[J]. ACS Appl Mater Interfaces,2015,7:11984−11990. doi: 10.1021/acsami.5b02014 [25] CHAI X, SHI C, LIU E, LI J, ZHAO N, HE C. Carbon-coated Fe2O3 nanocrystals with enhanced lithium storage capability[J]. Appl Surf Sci,2015,347:178−185. doi: 10.1016/j.apsusc.2015.04.074 [26] CHENG X, GUI X, LIN Z, ZHENG Y, LIU M, ZHAN R, ZHU Y, TANG Z. Three-dimensional α-Fe2O3/carbon nanotube sponges as flexible supercapacitor electrodes[J]. J Mater Chem A,2015,3(42):20927−20934. doi: 10.1039/C5TA03635F [27] FU Y, DONG C L, LEE W Y, CHEN J, GUO P, ZHAO L, SHEN S. Nb-doped hematite nanorods for efficient solar water splitting: Electronic structure evolution versus morphology alteration[J]. Chem Nano Mater,2016,2:704−711. [28] HAN T, WEI Y, JIN X, JIU H, ZHANG L, SUN Y, TIAN J, SHANG R, HANG D, ZHAO R. Hydrothermal self-assembly of α-Fe2O3 nanorings@graphene aerogel composites for enhanced Li storage performance[J]. J Mater Sci,2019,54:7119−7130. doi: 10.1007/s10853-019-03371-5 [29] LIU H, JIA M, ZHU Q, CAO B, CHEN R, WANG Y, WU F, XU B. 3D-0D graphene-Fe3O4 quantum dot hybrids as high-performance anode materials for sodium-ion batteries[J]. ACS Appl Mater Interfaces,2016,8:26878−26885. doi: 10.1021/acsami.6b09496 [30] FU Y, WEI Q, WANG X, ZHANG G, SHU H, YANG X, TAVARES A, SUN S. A facile synthesis of Fe3O4 nanoparticles/graphene for high-performance lithium/sodium-ion batteries[J]. RSC Adv,2016,6:16624−16633. doi: 10.1039/C5RA25835A [31] QI L, XIN Y, ZUO Z, YANG C, WU K, WU B, ZHOU H. Grape-like Fe3O4 agglomerates grown on graphene nanosheets for ultrafast and stable lithium storage[J]. ACS Appl Mater Interfaces,2016,8:17245−17252. doi: 10.1021/acsami.6b04274 [32] WU S, XU R, LU M, GE R, IOCOZZIA J, HAN C, JIANG B, LIN Z. Graphene-containing nanomaterials for lithium-ion batteries[J]. Adv Energy Mater,2015,5:1500400. doi: 10.1002/aenm.201500400 [33] YANG S, FENG X, WANG L, TANG K, MAIER J, MÜLLEN K. Graphene-based nanosheets with a sandwich structure[J]. Angew Chem Int Ed,2010,49:4795−4799. doi: 10.1002/anie.201001634 [34] QI H, CAO L, LI J, HUANG J, XU Z, CHENG Y, KONG Y, YANAGISAWA K. High pseudocapacitance in FeOOH/rGO composites with superior performance for high rate anode in Li-ion battery[J]. ACS Appl Mater Interfaces,2016,8(51):35253−35263. doi: 10.1021/acsami.6b11840 [35] QI H, CAO L, LI J, MA M, CHENG Y, WANG C, DANG H. Rice crust-like Fe3O4@C/rGO with improved extrinsic pseudocapacitance for high-rate and long-life Li-ion anode[J]. J Alloys Compd,2019,804:57−64. doi: 10.1016/j.jallcom.2019.06.284 [36] QI H, HUANG J, TANG L, MA M, DENG W, ZHANG C. Confined pulverization promoting durable pseudocapacitance for FeOOH@PEDOT anode in Li-ion battery[J]. J Electroanal Chem,2021,882:115005. doi: 10.1016/j.jelechem.2021.115005 [37] ETACHERI V, HONG C N, TANG J, POL V G. Cobalt nanoparticles chemically bonded to porous carbon nanosheets: A stable high-capacity anode for fast-charging lithium-ion batteries[J]. ACS Appl Mater Interfaces,2018,10:4652−4661. doi: 10.1021/acsami.7b15915 [38] HUANG Y, HU X, LI J, ZHANG J, CAI D, SA B, ZHAN H, WEN Z. Rational construction of heterostructured core-shell Bi2S3@Co9S8 complex hollow particles toward high-performance Li- and Na-ion storage[J]. Energy Storage Materials,2020,29:121−130. doi: 10.1016/j.ensm.2020.04.004 [39] BANG J, AHN J, ZHANG J, KO T H, Park B, LEE Y M, JUNG B K, LEE S Y, OK J, KIM B H, KIM T, CHOI J I, LEE C H, OH S J. Stretchable and directly patternable double-layer structure electrodes with complete coverage[J]. ACS Nano,2022,16:12134−12144. doi: 10.1021/acsnano.2c02664 [40] QI H, ZHAO C, HUANG J, HE C, TANG L, DENG W. Metastable FeCN2@nitrogen-doped carbon with high pseudocapacitance as an anode material for sodium ion batteries[J]. Nanoscale,2022,14:780−789. doi: 10.1039/D1NR06705B -
 2023-D024-SupportingInformation.docx
2023-D024-SupportingInformation.docx
-





 下载:
下载: