Preparation of layered porous carbon supported ruthenium catalyst and its performance for ammonia borane hydrolyzing to hydrogen
-
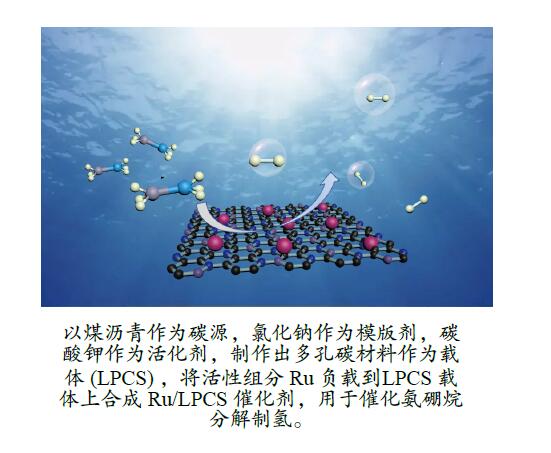
摘要: 本研究以煤沥青为炭材料,氯化钠为模板剂,碳酸钾为活化剂,在氩气气氛下高温煅烧得到载体层状多孔炭片(LPCS),通过浸渍法向其中加入RuCl3金属溶液,将活性组分Ru负载到LPCS载体上合成Ru/LPCS催化剂,并对其催化氨硼烷水解制氢的性能进行了研究。结果表明,Ru/LPCS催化剂中,当煅烧温度为1123 K时,Ru的负载量为2%时,催化剂的反应转化频率(TOF)值最大,此时有光下催化剂的TOF为334.8 min−1,是无光照时TOF的1.38倍。在光照下,催化剂的活化能(Ea)从90.60 kJ/mol下降到70.33 kJ/mol。氨硼烷水解制氢速率相对于其浓度的级数为0.75,而相对于催化剂的用量满足于一级动力学关系。Abstract: In this paper, layered porous carbon sheet (LPCS) was obtained through high temperature calcination under argon atmosphere by using coal pitch as carbon material, sodium chloride as template agent and potassium carbonate as activator. Then, the active component Ru was loaded onto the LPCS support by impregnation to synthesize Ru/LPCS catalyst whose catalytic performance for hydrogen production by hydrolysis of ammonia borane was studied. The results showed that in the presence of light, the maximum value of the turnover frequency (TOF), 334.8 min−1, was obtained at a calcination temperature of 1123 K and a loading of 2% of Ru which is 1.38 times higher than that in the absence of light. The activation energy (Ea) of the catalyst decreased from 90.60 to 70.33 kJ/mol in the presence of light. The hydrogen production rate order with respect to ammonia borane concentration was 0.75, which is first-order relation to the amount of the catalyst.
-
表 1 催化剂的ICP和BET的测试
Table 1 ICP and BET Results of the catalysts
Catalyst SBET/
(m2·g−1)Theoretical
Ru/mmolReality
Ru/mmol3%Ru/LPCS1123 1042.1 0.06 0.041 2.5%Ru/LPCS1123 − 0.05 0.037 2%Ru/LPCS1123 980.6 0.04 0.027 1.5%Ru/LPCS1123 − 0.03 0.021 1%Ru/LPCS1123 964.5 0.02 0.013 LPCS1123 990.2 0 0 -
[1] ALPAYDIN C Y, GÜLBAY S K, COLPAN C O. A review on the catalysts used for hydrogen production from ammonia borane[J]. Int J Hydrogen Energy,2020,45(5):3414−3434. doi: 10.1016/j.ijhydene.2019.02.181 [2] EPPINGER J, HUANG K W. Formic acid as a hydrogen energy carrier[J]. ACS Energy Lett,2017,2(1):188−195. doi: 10.1021/acsenergylett.6b00574 [3] MEHDI S, LIU Y Y, WEI H J, et al. P-induced Co-based interfacial catalysis on Ni foam for hydrogen generation from ammonia borane[J]. Appl Catal B: Environ,2023,325:122317. doi: 10.1016/j.apcatb.2022.122317 [4] SEMIZ L. Hydrogen generation from ammonia borane by polymer supported platinum films[J]. Chem Phys Lett,2021,767:138365. doi: 10.1016/j.cplett.2021.138365 [5] SONG H Q, WU M, TANG Z Y, et al. Single atom ruthenium-doped CoP/CDs nanosheets via splicing of carbon-dots for robust hydrogen production[J]. Angew Chem Int Ed,2021,60(13):7234−7244. doi: 10.1002/anie.202017102 [6] YANG J G, YUAN Q, LIU Y, et al. Low-cost ternary Ni-Fe-P catalysts supported on Ni foam for hydrolysis of ammonia borane[J]. Inorg Chem Front,2019,6(5):1189−1194. doi: 10.1039/C9QI00048H [7] ZHANG H, GU X J, LIU P L, et al. Highly efficient visible-light-driven catalytic hydrogen evolution from ammonia borane using non-precious metal nanoparticles supported by graphitic carbon nitride[J]. J Mater Chem A,2017,5(5):2288−2296. doi: 10.1039/C6TA08987A [8] AKBAYRAK S, TONBUL Y, ÖZKAR S. Tungsten (VI) oxide supported rhodium nanoparticles: Highly active catalysts in hydrogen generation from ammonia borane[J]. Int J Hydrogen Energy,2021,46(27):14259−14269. doi: 10.1016/j.ijhydene.2021.01.156 [9] YAO Q L, DING Y Y, LU Z H. Noble-metal-free nanocatalysts for hydrogen generation from boron-and nitrogen-based hydrides[J]. Inorg Chem Front,2020,7(20):3837−3874. doi: 10.1039/D0QI00766H [10] 曹云钟, 郑君宁, 吴慧, 等. Pt基催化剂催化氨硼烷水解产氢的研究进展[J]. 稀有金属,2023,47(8):1122−1131.CAO Yunzhong, ZHENG Junning, WU Hui, et al. Advances in hydrogen production by ammonia borane hydrolysis over Pt-based catalysts[J]. Chin J Rare Met,2023,47(8):1122−1131. [11] ÖZKAR S. A review on platinum (0) nanocatalysts for hydrogen generation from the hydrolysis of ammonia borane[J]. Dalton Trans,2021,50(36):12349−12364. doi: 10.1039/D1DT01709H [12] ZHOU L M, MENG J, LI P, et al. Ultrasmall cobalt nanoparticles supported on nitrogen-doped porous carbon nanowiresfor hydrogen evolution from ammonia borane[J]. Mater Horiz,2017,4(2):268−273. doi: 10.1039/C6MH00534A [13] CHEN W Y, LI D L, WANG Z J, et al. Reaction mechanism and kinetics for hydrolytic dehydrogenation of ammonia borane on a Pt/CNT catalyst[J]. AIChE J,2017,63(1):60−65. doi: 10.1002/aic.15389 [14] TONBUL Y, AKBAYRAK S, ÖZKAR S. Magnetically separable rhodium nanoparticles as catalysts for releasing hydrogen from the hydrolysis of ammonia borane[J]. J Colloid Interface Sci,2019,553:581−587. doi: 10.1016/j.jcis.2019.06.038 [15] 马明超, 臧甲忠, 于海斌, 等. 贵金属催化剂理性设计在选择性加氢反应中的研究进展[J]. 无机盐工业,2021,53(7):23−29. doi: 10.19964/j.issn.1006-4990.2021-0008MA Mingchao, ZANG Jiazhong, YU Haibin, et al. Research progress rational design of noble metal catalysts for selective hydrogenation[J]. Inorg Chem Ind,2021,53(7):23−29. doi: 10.19964/j.issn.1006-4990.2021-0008 [16] OIU P Y, DESCHAMPS F, CALDARELLA G, et al. Investigation of platinum and palladium as potential anodic catalysts for direct borohydride and ammonia borane fuel cells[J]. J Power Sources,2015,297:492−503. doi: 10.1016/j.jpowsour.2015.08.022 [17] PENG S G, LIU J C, ZHANG J, et al. An improved preparation of graphene supported ultrafine ruthenium (0) NPs: Very active and durable catalysts for H2 generation from methanolysis of ammonia borane[J]. Int J Hydrogen Energy,2015,40(34):10856−10866. doi: 10.1016/j.ijhydene.2015.06.113 [18] 席楠, 程春晖, 李红伟, 等. 苯选择加氢制环己烯在钌基催化体系中的技术进展[J]. 精细化工,2020,37(12):2457−2466. doi: 10.13550/j.jxhg.20200474XI Nan, CHENG Chunhui, LI Hongwei, et al. Technical progress of selective hydrogenation of benzene to cyclohexene in ruthenium-based catalytic system[J]. Fine Chem,2020,37(12):2457−2466. doi: 10.13550/j.jxhg.20200474 [19] AKBAYRAK S, ÖZKAR S. Ruthenium (0) nanoparticles supported on multiwalled carbon nanotube as highly active catalyst for hydrogen generation from ammonia-borane[J]. ACS Appl Mater Interfaces,2012,4(11):6302−6310. doi: 10.1021/am3019146 [20] FU L, CAI L. Ru nanoparticles loaded on tannin immobilized collagen fibers for catalytic hydrolysis of ammonia borane[J]. Int J Hydrogen Energy,2021,46(18):10749−10762. doi: 10.1016/j.ijhydene.2020.12.152 [21] LI S H, SONG X R, LI Y T, et al. Efficient hydrolytic dehydrogenation of ammonia borane over ultrafine Ru nanoparticles supported on biomass-derived porous carbon[J]. Int J Hydrogen Energy,2021,46(54):27555−27566. doi: 10.1016/j.ijhydene.2021.06.029 [22] GIL-HERRERA L K, PARIENTE J A, GALLEGO-GÓMEZ F, et al. Hierarchically porous carbon photonic structures[J]. Adv Funct Mater,2018,28(27):1703885. doi: 10.1002/adfm.201703885 [23] ZANG X N, DONG Y, JIAN C Y, et al. Upgrading carbonaceous materials: Coal, tar, pitch, and beyond[J]. Matter,2022,5(2):430−447. doi: 10.1016/j.matt.2021.11.022 [24] WANG H, XU C, CHEN Q, et al. Nitrogen-doped carbon-stabilized Ru nanoclusters as excellent catalysts for hydrogen production[J]. ACS Sustainable Chem Eng,2018,7(1):1178−1184. [25] DING X Y, GU R, SHI P H, et al. A three-dimensional hierarchical porous carbon network decorated with MnO2 nanoparticles (HPCM) as an efficient sulfur host for high-performance lithium-sulfur batteries (LSBs)[J]. J Alloys Compd,2020,835:155206. doi: 10.1016/j.jallcom.2020.155206 [26] FENG Y L, ZHANG S F, ZHU L H, et al. Reduced graphene oxide-supported ruthenium nanocatalysts for highly efficient electrocatalytic hydrogen evolution reaction[J]. Int J Hydrogen Energy,2022,47(94):39853−39863. doi: 10.1016/j.ijhydene.2022.09.154 [27] LI W D, ZHAO Y X, LIU Y, et al. Exploiting Ru-induced lattice strain in CoRu nanoalloys for robust bifunctional hydrogen production[J]. Angew Chem Int Ed,2021,133(6):3327−3335. doi: 10.1002/ange.202013985 [28] ZHANG F F, ZHU Y L, CHEN Y, et al. RuCo alloy bimodal nanoparticles embedded in N-doped carbon: A superior pH-universal electrocatalyst outperforms benchmark Pt for the hydrogen evolution reaction[J]. J Mater Chem A,2020,8(25):12810−12820. doi: 10.1039/D0TA04491A [29] 吴慧, 郑君宁, 李蓉, 等. NiPt/CeO2催化剂的制备及其催化水合肼分解制氢性能研究[J/OL]. 中国稀土学报. https://kns.cnki.net/kcms2/detail/11.2365.TG.20230725.1716.008.html.WU Hui, ZHENG Junning, LI Rong, et al. Synthesis of NiPt/CeO2 catalyst and its catalytic performance for hydrogen production via hydrazine hydrate decomposition[J/OL]. J Chin Soc Rare Earths. https://kns.cnki.net/kcms2/detail/11.2365.TG.20230725.1716.008.html. [30] MA J W, WANG J, LIU J, et al. Electron-rich ruthenium encapsulated in nitrogen-doped carbon for efficient hydrogen evolution reaction over the whole pH[J]. J Colloid Interface Sci,2022,620:242−252. doi: 10.1016/j.jcis.2022.03.149 [31] BARMAN B K, SARKAR B, GHOSH P, et al. In situ decoration of ultrafine Ru nanocrystals on N-doped graphene tube and their applications as oxygen reduction and hydrogen evolution catalyst[J]. ACS Appl Energy Mater,2019,2(10):7330−7339. doi: 10.1021/acsaem.9b01318 [32] HAN W W, CHEN L L, MA B, et al. Ultra-small Mo2C nanodots encapsulated in nitrogen-doped porous carbon for pH-universal hydrogen evolution: Insights into the synergistic enhancement of HER activity by nitrogen doping and structural defects[J]. J Mater Chem A,2019,7(9):4734−4743. doi: 10.1039/C8TA11098K [33] SUN S N, YU Q F, LI M, et al. Surface modification of porous carbon nanomaterials for water vapor adsorption[J]. ACS Appl Nano Mater,2023,6(4):2822−2834. doi: 10.1021/acsanm.2c05205 [34] 李贵, 梁雨, 郑君宁, 等. Rh/N-GMCs纳米催化剂的制备及其催化氨硼烷水解产氢性能研究[J]. 燃料化学学报(中英文),2023,51(4):528−537.LI Gui, LIANG Yu, ZHENG Junning, et al. Preparation of Rh/N-GMCs nanocatalyst and its catalytic performance for the Hydrolytic dehydrogenation of ammonia borane[J]. J Fuel Chem Technol,2023,51(4):528−537. [35] RAKAP M, KALU E E, ÖZKAR S. Polymer-immobilized palladium supported on TiO2 (Pd-PVB-TiO2) as highly active and reusable catalyst for hydrogen generation from the hydrolysis of unstirred ammonia-borane solution[J]. Int J Hydrogen Energy,2011,36(2):1448−1455. doi: 10.1016/j.ijhydene.2010.10.097 [36] WEN L, ZHENG Z, LUO W, et al. Ruthenium deposited on MCM-41 as efficient catalyst for hydrolytic dehydrogenation of ammonia borane and methylamine borane[J]. Chin Chem Lett,2015,26(11):1345−1350. doi: 10.1016/j.cclet.2015.06.019 [37] 王小燕, 张若凡, 司航, 等. 椰壳炭负载钌催化剂的制备及其催化氨硼烷水解制氢性能[J]. 石油炼制与化工,2023,54(7):64−70. doi: 10.3969/j.issn.1005-2399.2023.07.012WANG Xiaoyan, ZHANG Ru-fan, SI Hang, et al. Preparation of coconut shell carbon supported ruthenium as a catalyst for the hydrolytic dehydrogenation of ammonia borane[J]. Pet Process Petrochem,2023,54(7):64−70. doi: 10.3969/j.issn.1005-2399.2023.07.012 [38] 查达伟. 椰壳纳米炭粉的电催化与光催化性能研究[D]. 合肥: 合肥工业大学, 2016.ZHA Dawei. Study of electrocatalytic and photocatalytic performance of coconut shell nano-carbon[D]. Hefei: Hefei University of Technology, 2016. [39] ZHAI Q G, XIE S J, FAN W Q, et al. Photocatalytic conversion of carbon dioxide with water into methane: platinum and copper (I) oxide Co-catalysts with a core-shell structure[J]. Angew Chem Int Ed,2013,125(22):5888−5891. doi: 10.1002/ange.201301473 [40] XIAO J D, HAN L L, LUO J, Et al. Integration of plasmonic effects and schottky junctions into metal-organic framework composites: Steering charge flow for enhanced visible-light photocatalysis[J]. Angew Chem Int Ed,2018,57(4):1118−1118. doi: 10.1002/anie.201713194 -




 下载:
下载: