Promotion of Cu/Ce supported red mud for NO removal from low and medium temperature flue gas
-
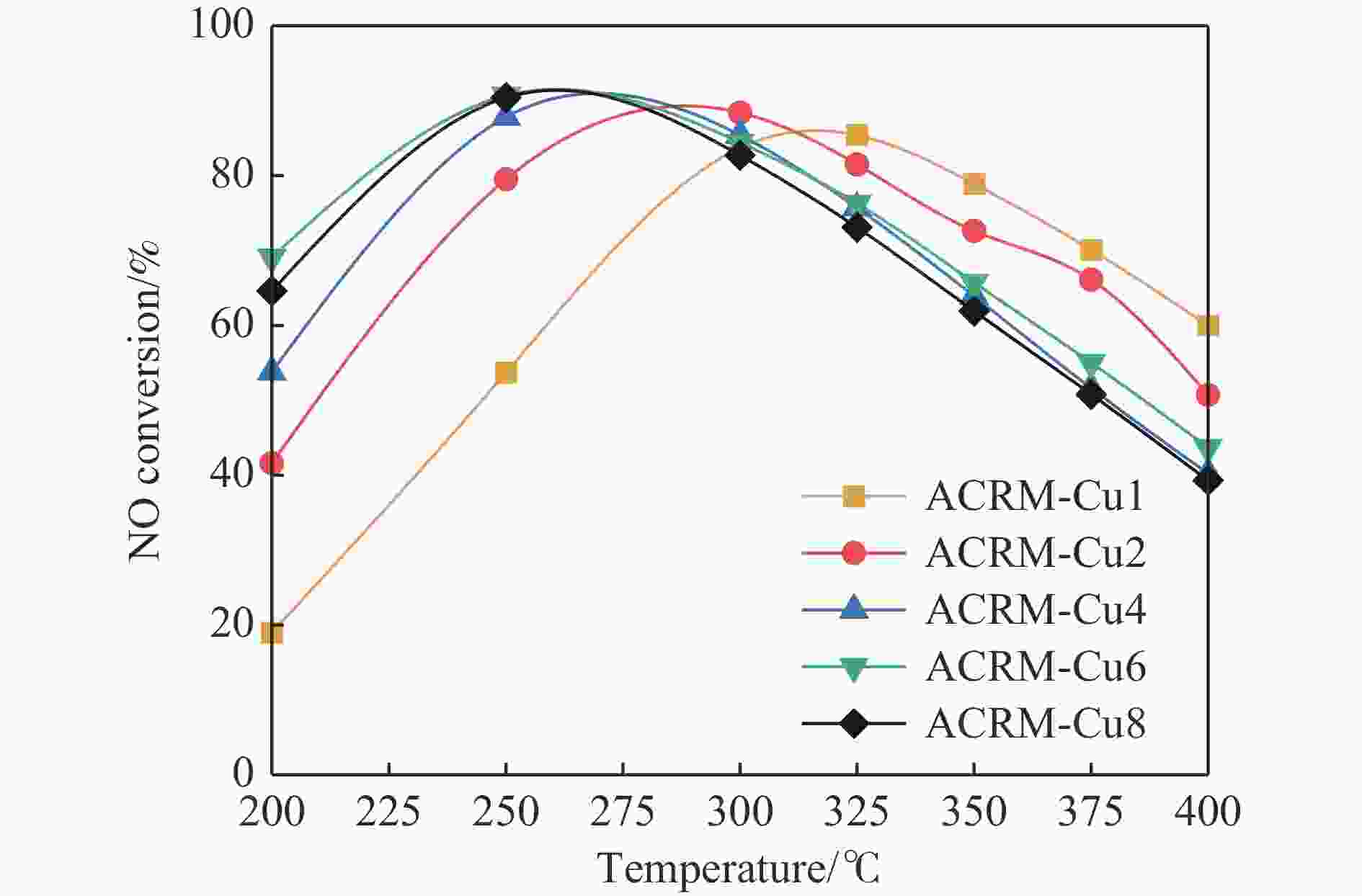
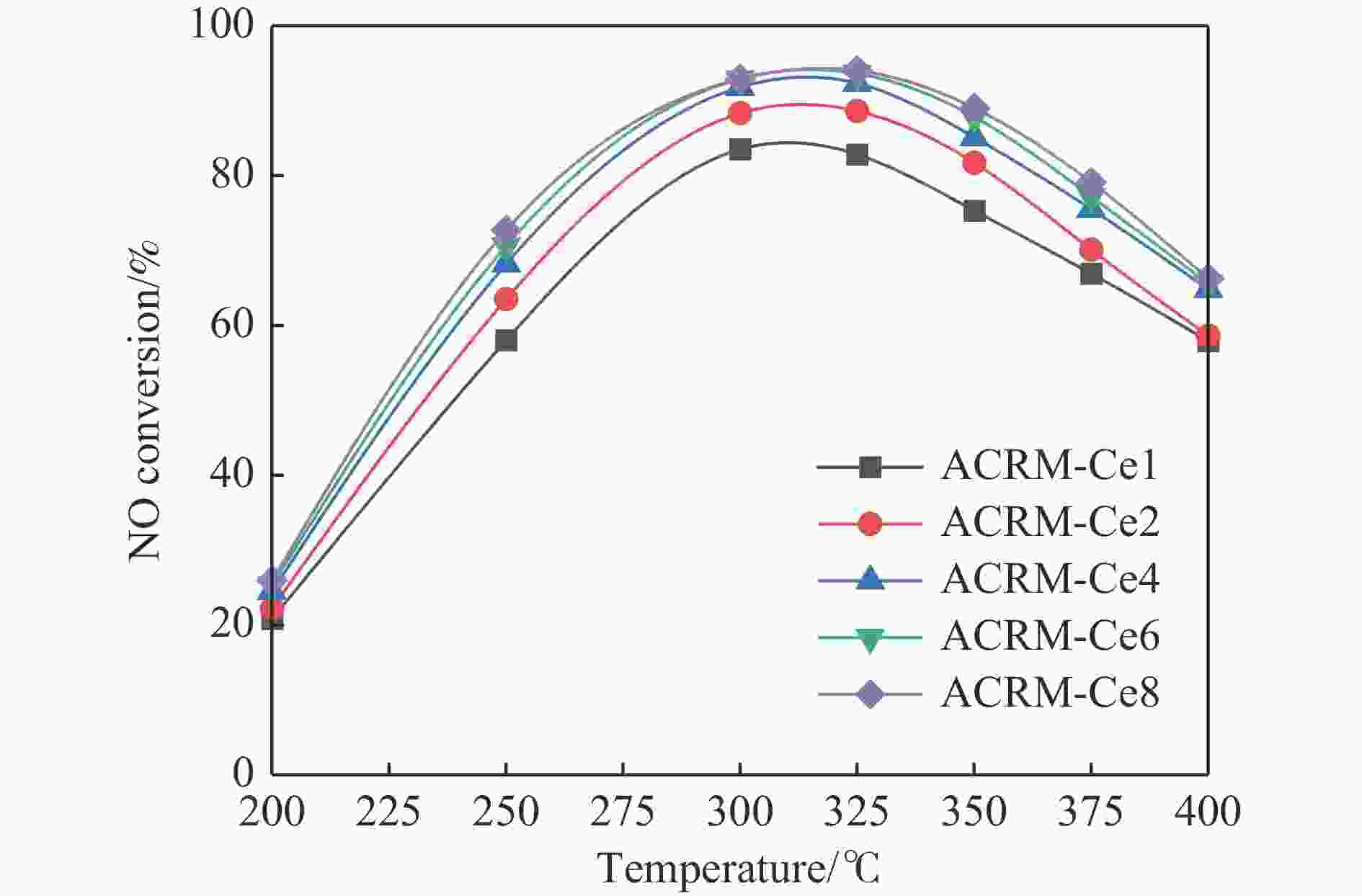
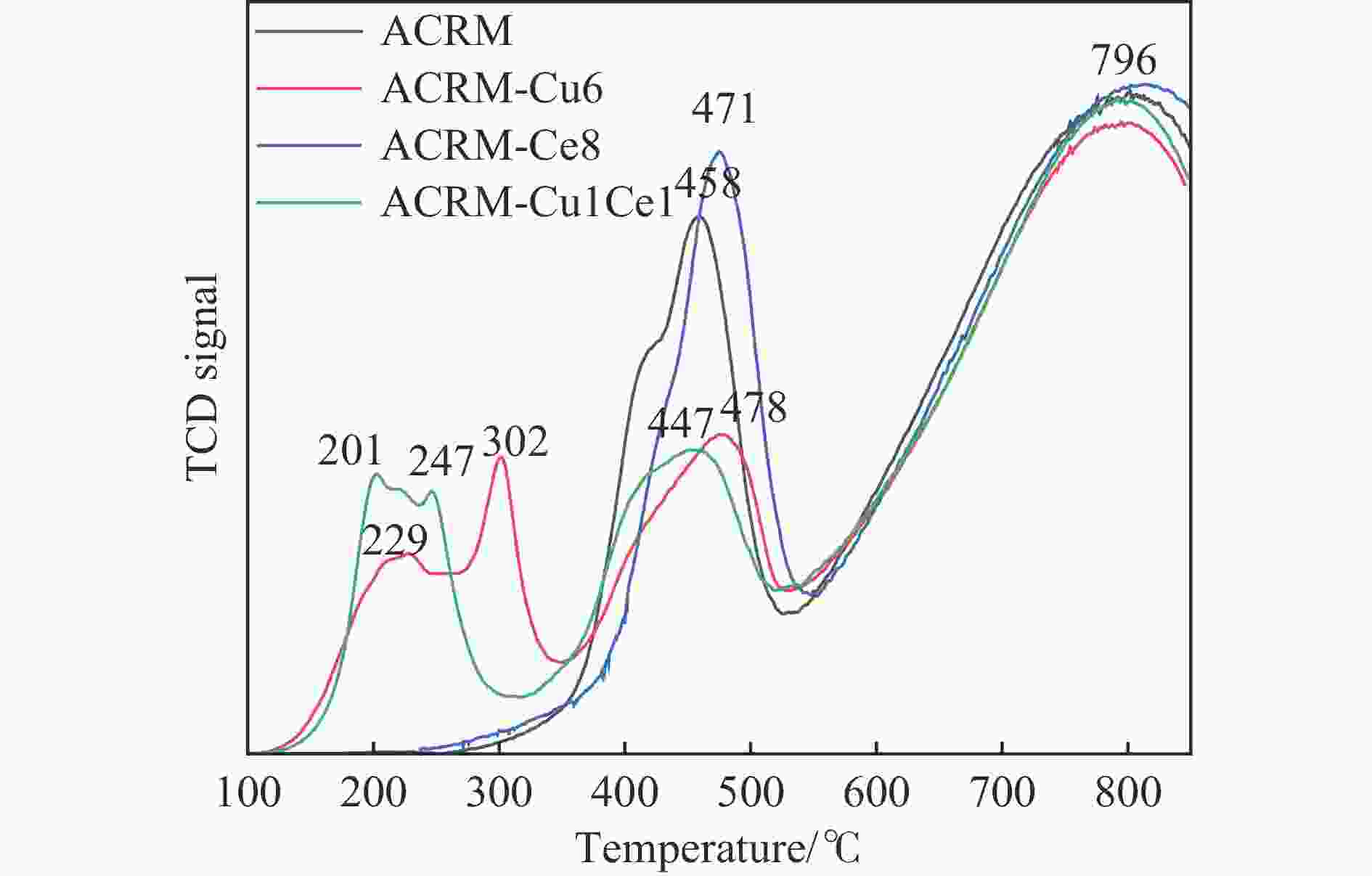
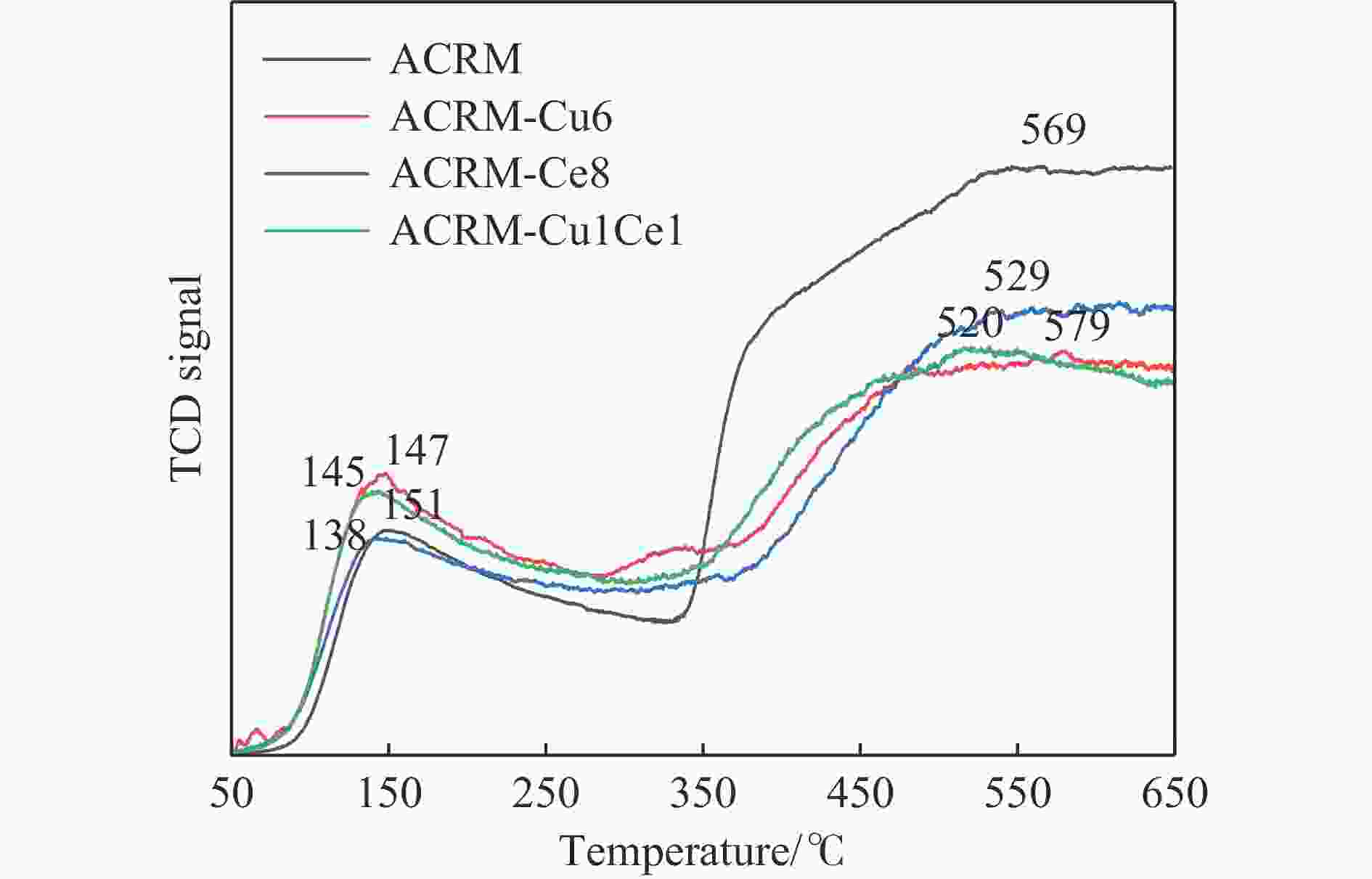
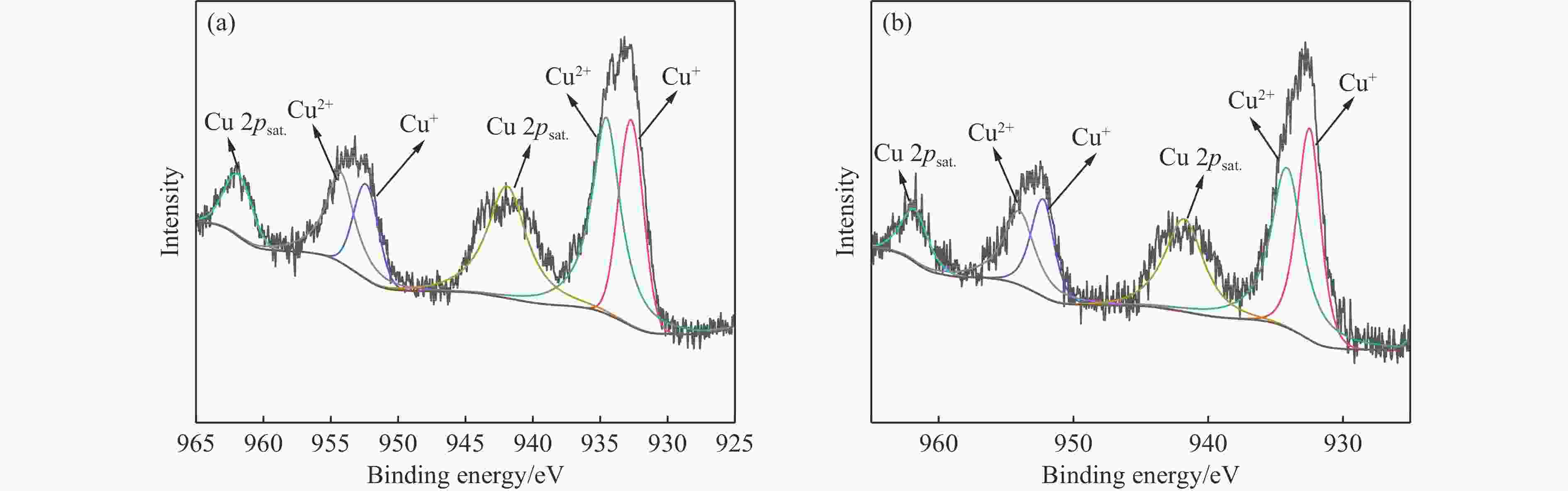
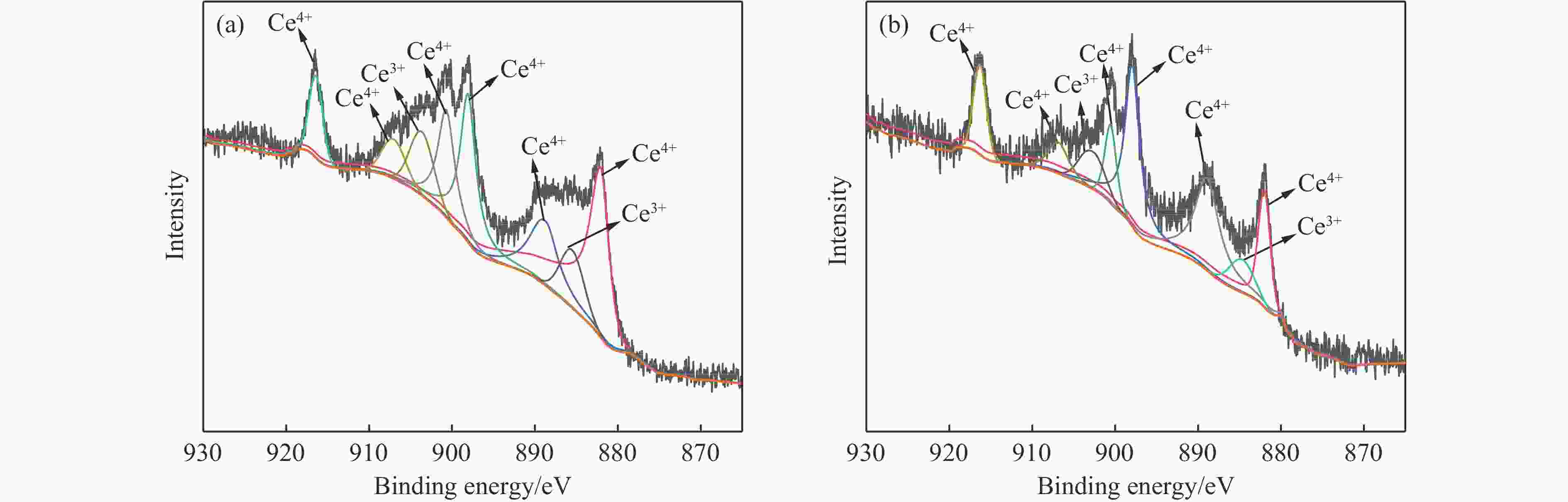
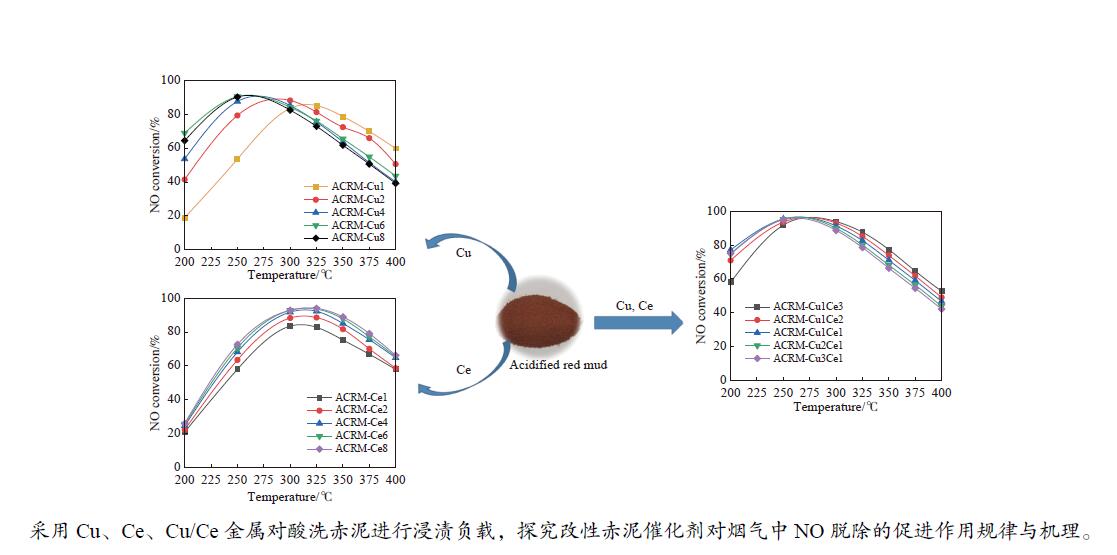
摘要: 本研究对酸洗赤泥催化剂进行Cu、Ce、Cu/Ce浸渍负载,并研究了金属改性赤泥对烟气中NOx的催化转化性能。研究结果表明,Cu负载催化剂中的Cu+与Cu2+,有效促进了赤泥对低温烟气(200–300 ℃)中的NO转化率,Cu的负载量达到6%时,赤泥的最高NO转化率达到了90.7%;而Ce负载催化剂中的Ce3+与Ce4+,有效促进了赤泥对中温烟气(200–400 ℃)中的NO转化率,Ce的负载量达到8%时,赤泥的最高NO转化率达到了94.0%;Cu/Ce负载催化剂表现出比单金属负载催化剂更好的低温NO转化率,最佳的负载Cu:Ce比例为1∶1,双金属负载催化剂表现出比Cu负载催化剂更好的中温(300–400 ℃)中的NO转化率,最高达到了95.5%。其原因是,在Cu/Ce协同作用下,Cu+以及Cu2+的还原过程分别从229、302 ℃降至201以及247 ℃,同时使发生Fe2O3→FeO的还原过程的温度降低,促使ACRM-Cu1Ce1具有更强的低温氧化还原能力,同时,双金属负载使催化剂具有更高的弱酸性峰,也使催化剂的强、弱酸性峰都向低温偏移,并使负载后的赤泥具有了较高的Fe离子平均氧化态以及较高的Cu+含量,促进了赤泥催化剂对低温NO的转化率。Abstract: Red mud is a solid waste in aluminum industry and has been proven to be an efficient alternative to NOx selective catalytic reduction (SCR) catalysts. Acid washing treatment to red mud can improve its alkalinity and surface properties, and increase the conversion rate of NOx. In this paper, Cu, Ce, and Cu/Ce was supported on acid washed red mud and NOx catalytic conversion performance on metal modified red mud catalysts was studied. The research results indicate that Cu+ and Cu2+ in the Cu supported catalyst effectively promote NO conversion rate of red mud in low-temperature (200−300 ℃) flue gas, reaching a maximum of 90.7%; Ce3+ and Ce4+ in Ce supported catalysts effectively promote the NO conversion rate of red mud in flue gas at 200–400 ℃, reaching a maximum of 94.0%; Cu/Ce supporting exhibits better NO conversion rate than single metal supported catalysts at low-temperatures, the optimal Cu∶Ce ratio for supporting is 1∶1; and also exhibits better NO conversion rate than Cu supported catalysts at high-temperature (300−400 ℃), reaching a maximum of 95.5%. The reason may be that under the synergistic effect of Cu/Ce, ACRM-Cu1Ce1 has stronger low-temperature redox ability, higher weak acidic peaks, higher average oxidation state of Fe ions, and higher Cu+ content.
-
Key words:
- red mud /
- SCR /
- Cu /
- Ce /
- supporting
-
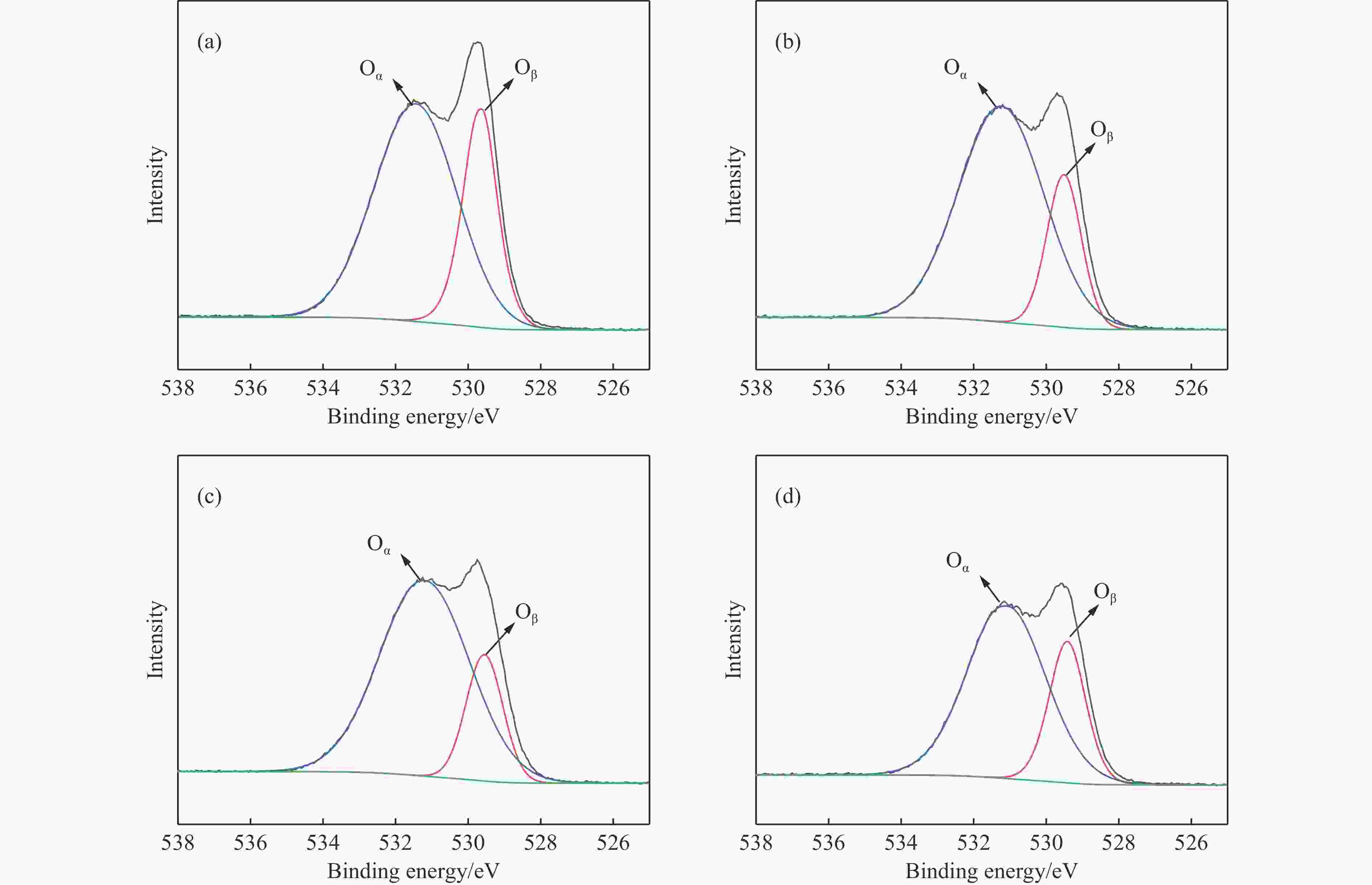
图 6 负载金属催化剂的等温吸附曲线与孔径分布
Figure 6 Isothermal adsorption curves and pore size distribution of the supported catalysts
(a), (b) and (c) are the isothermal adsorption curves of Cu supported, Ce supported and Cu/Ce supported catalysts, respectively; (d), (e) and (f) are the pore size distribution of the corresponding catalysts.
表 1 赤泥的组成分析
Table 1 Analysis of the composition of red mud
Sample Fe2O3 Al2O3 SiO2 TiO2 Na2O Others RM/% 42.0 21.3 22.5 3.7 6.9 3.6 表 2 催化剂的比表面积与孔径分布
Table 2 Surface area and pore size distribution of catalysts
Sample SBET/(m2·g−1) vt/(cm3·g−1) dave/nm ACRM-Cu1 51 0.155 9.3 ACRM-Cu2 53 0.168 9.8 ACRM-Cu4 45 0.149 9.7 ACRM-Cu6 44 0.156 10.3 ACRM-Cu8 42 0.149 14.2 ACRM-Ce1 50 0.172 9.8 ACRM-Ce2 54 0.162 9.6 ACRM-Ce4 49 0.148 9.4 ACRM-Ce6 49 0.147 9.6 ACRM-Ce8 50 0.137 9.2 ACRM-Cu3Ce1 42 0.146 10.7 ACRM-Cu2Ce1 41 0.154 11.5 ACRM-Cu1Ce1 43 0.137 10.1 ACRM-Cu1Ce2 45 0.139 10.0 ACRM-Cu1Ce3 48 0.143 9.4 -
[1] CHEN C, CAO Y, LIU S, et al. Review on the latest developments in modified vanadium-titanium-based SCR catalysts[J]. Chin J Catal,2018,39(8):1347−1365. doi: 10.1016/S1872-2067(18)63090-6 [2] KANG M, PARK E, KIM J, et al. Manganese oxide catalysts for NOx reduction with NH3 at low temperatures[J]. Appl Catal A: Gen,2007,327(2):261−269. doi: 10.1016/j.apcata.2007.05.024 [3] FAN J, NING P, SONG Z, et al. Mechanistic aspects of NH3-SCR reaction over CeO2/TiO2-ZrO2-SO42- catalyst: In situ DRIFTS investigation[J]. Chem Eng J,2018,334:855−863. doi: 10.1016/j.cej.2017.10.011 [4] Huang L, ZENG Y, CHANG Z, et al. Promotional effect of phosphorus modification on improving the Na resistance of V2O5-MoO3/TiO2 catalyst for selective catalytic reduction of NOx by NH3[J]. Mol Catal,2021,506:111565. doi: 10.1016/j.mcat.2021.111565 [5] ZHANG K, XU L, NIU S, et al. Iron-manganese-magnesium mixed oxides catalysts for selective catalytic reduction of NOx with NH3[J]. Korean J Chem Eng,2017,34(6):1858−1866. doi: 10.1007/s11814-017-0047-8 [6] XU L, NIU S, LU C, et al. NH3-SCR performance and characterization over magnetic iron-magnesium mixed oxide catalysts[J]. Korean J Chem Eng,2017,34(5):1576−1583. doi: 10.1007/s11814-017-0044-y [7] QIU Y, LIU B, DU J, et al. The monolithic cordierite supported V2O5-MoO3/TiO2 catalyst for NH3-SCR[J]. Chem Eng J,2016,294:264−272. doi: 10.1016/j.cej.2016.02.094 [8] WANG D, LUO J, YANG Q, et al. Deactivation mechanism of multipoisons in cement furnace flue gas on selective catalytic reduction catalysts[J]. Environ Sci Technol,2019,53(12):6937−6944. doi: 10.1021/acs.est.9b00337 [9] MACHIDA M, TOKUDOME Y, MAEDA A, et al. Nanometric platinum overlayer to catalyze NH3 oxidation with high turnover frequency[J]. ACS Catal,2020,10(8):4677−4685. doi: 10.1021/acscatal.0c00542 [10] MA H, SCHNEIDER W. Structure- and temperature-dependence of Pt-catalyzed ammonia oxidation rates and selectivities[J]. ACS Catal,2019,9(3):2407−2414. doi: 10.1021/acscatal.8b04251 [11] SUN M, LIU J, SONG C, et al. Different reaction mechanisms of ammonia oxidation reaction on Pt/Al2O3 and Pt/CeZrO2 with Various Pt States[J]. ACS Appl Mater Interfaces,2019,11(26):23102−23111. doi: 10.1021/acsami.9b02128 [12] LI P, ZHANG R, LIU N, et al. Efficiency of Cu and Pd substitution in Fe-based perovskites to promote N2 formation during NH3 selective catalytic oxidation (NH3-SCO)[J]. Appl Catal B: Environ,2017,203:174−188. doi: 10.1016/j.apcatb.2016.10.021 [13] SHIN J, KIM G, HONG S. Reaction properties of ruthenium over Ru/TiO2 for selective catalytic oxidation of ammonia to nitrogen[J]. Appl Surf Sci,2020,506:144906. doi: 10.1016/j.apsusc.2019.144906 [14] LIU Z, ZHU J, LI J, et al. Novel Mn-Ce-Ti mixed-oxide catalyst for the selective catalytic reduction of NOx with NH3[J]. ACS Appl Mater Interfaces,2014,6(16):14500−14508. doi: 10.1021/am5038164 [15] PAREDES J, ORDONEZ S, VEGA A, et al. Catalytic combustion of methane over red mud-based catalysts[J]. Appl Catal B: Environ,2004,47(1):37−45. doi: 10.1016/S0926-3373(03)00325-4 [16] LIU Z, SU H, CHEN B, et al. Activity enhancement of WO3 modified Fe2O3 catalyst for the selective catalytic reduction of NOx by NH3[J]. Chem Eng J, 2016, 299: 255−262: 119186. [17] LEE S, JANG J, LEE B, et al. The effect of binders on structure and chemical properties of Fe-K/gamma-Al2O3 catalysts for CO2 hydrogenation[J]. Appl Catal A: Gen,2003,253(1):293−304. doi: 10.1016/S0926-860X(03)00540-4 [18] SONG S, JIANG S. Selective catalytic oxidation of ammonia to nitrogen over CuO/CNTs: The promoting effect of the defects of CNTs on the catalytic activity and selectivity[J]. Appl Catal B: Environ,2012,117:346−350. [19] CHEN S, VASILIADES M, YAN Q, et al. Remarkable N2-selectivity enhancement of practical NH3-SCR over Co0.5Mn1Fe0.25Al0.75Ox-LDO: The role of Co investigated by transient kinetic and DFT mechanistic studies[J]. Appl Catal B: Environ,2020,277:119186. [20] RUTKOWSKA M, DUDA M, MACINA D, et al. Mesoporous beta zeolite functionalisation with FexCry oligocations; catalytic activity in the NH3-SCO process[J]. Microporous Mesoporous Mater,2019,278:1−13. doi: 10.1016/j.micromeso.2018.11.003 [21] CAMPISI S, PALLIGGIANO S, GERVASINI A, et al. Finely iron-dispersed particles on beta zeolite from solvated iron atoms: Promising catalysts for NH3-SCO[J]. J Phys Chem C,2019,123(18):11723−11733. doi: 10.1021/acs.jpcc.9b01474 [22] 郑足红, 童华, 童志权, 等. Mn-V-Ce/TiO2低温催化还原NO性能研究[J]. 燃料化学学报(中英文),2010,38(3):343−351.ZHENG Zuhong, TONG Hua, TONG Zhiquan, et al. Catalytic reduction of NO over Mn-V-Ce/TiO2 catalysts at low reaction temperature[J]. J Fuel Chem Technol,2010,38(3):343−351. [23] LIPPITS M, GLUHOI A, NIEUWENHUYS B. A comparative study of the selective oxidation of NH3 to N2 over gold, silver and copper catalysts and the effect of addition of Li2O and CeOx[J]. Catal Today,2008,137(2/4):446−452. doi: 10.1016/j.cattod.2007.11.021 [24] LIU W, LONG Y, LIU S, et al. Promotional effect of Ce in NH3-SCO and NH3-SCR reactions over Cu-Ce/SCR catalysts[J]. J Ind Eng Chem,2022,107:197−206. doi: 10.1016/j.jiec.2021.11.045 [25] 王继封, 王慧敏, 张亚青, 等. WO3的引入对MnOx-Fe2O3催化剂上NH3-SCR反应中N2选择性的促进作用[J]. 燃料化学学报(中英文),2019,47(7):814−822.WANG Jifeng, WANG Huimin, ZHANG Yaqing, et al. Promotion effect of tungsten addition on N2 selectivity of MnOx-Fe2O3 for NH3-SCR[J]. J Fuel Chem Technol,2019,47(7):814−822. [26] 王明洪, 王亮亮, 刘俊, 等. 过渡金属对选择性催化还原脱硝CeO2@TiO2催化剂低温活性的促进作用[J]. 燃料化学学报(中英文),2017,45(4):497−504.WANG Minghong, WANG Liangliang, LIU Jun, et al. Promoting effect of transition metal on low-temperature deNOx activity of CeO2@TiO2 catalyst for selective catalytic reduction[J]. J Fuel Chem Technol,2017,45(4):497−504. [27] DE RESENDE E, GISSANE C, NICOL R, et al. Synergistic co-processing of red mud waste from the bayer process and a crude untreated waste stream from bio-diesel production[J]. Green Chem,2013,15(2):496−510. doi: 10.1039/c2gc36714a [28] WU J, GONG Z, LU C, et al. Preparation and performance of modified red mud-based catalysts for selective catalytic reduction of NOx with NH3[J]. Catal,2018,8(1):35. [29] LYU F, HU Y, WANG L, et al. Dealkalization processes of bauxite residue: A comprehensive review[J]. J Hazard Mater,2021,403:123671. doi: 10.1016/j.jhazmat.2020.123671 [30] WANG D, PENG Y, XIONG S, et al. De-reducibility mechanism of titanium on maghemite catalysts for the SCR reaction: An in situ DRIFTS and quantitative kinetics study[J]. Appl Catal B: Environ,2018,221:556−564. doi: 10.1016/j.apcatb.2017.09.045 [31] GONG Z, MA J, WANG D, et al. Insights into modified red mud for the selective catalytic reduction of NOx: Activation mechanism of targeted leaching[J]. J Hazard Mater,2020,394:122536. doi: 10.1016/j.jhazmat.2020.122536 [32] MENG Y, LIU W, FIEDLER H, et al. Fate and risk assessment of emerging contaminants in reclaimed water production processes[J]. Front Environ Sci Eng,2021,15(5):104. doi: 10.1007/s11783-021-1392-8 [33] 康海彦, 莫杜娟, 张学军, 等. CeO2-WO3催化剂表面酸性和氧化还原性能在脱硝反应中的研究[J]. 燃料化学学报(中英文),2023,51(6):812−822. doi: 10.19906/j.cnki.JFCT.2023005KANG Haiyan, MO Dujuan, ZHANG Xuejun, et al. Investigation of the surface acidity and redox on the CeO2-WO3 catalyst for selective catalytic reduction with NH3[J]. J Fuel Chem Technol,2023,51(6):812−822. doi: 10.19906/j.cnki.JFCT.2023005 -
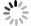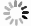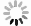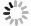



 下载:
下载: