TG-FTIR study on escape behavior of products from co-pyrolysis of coal and residuum
-
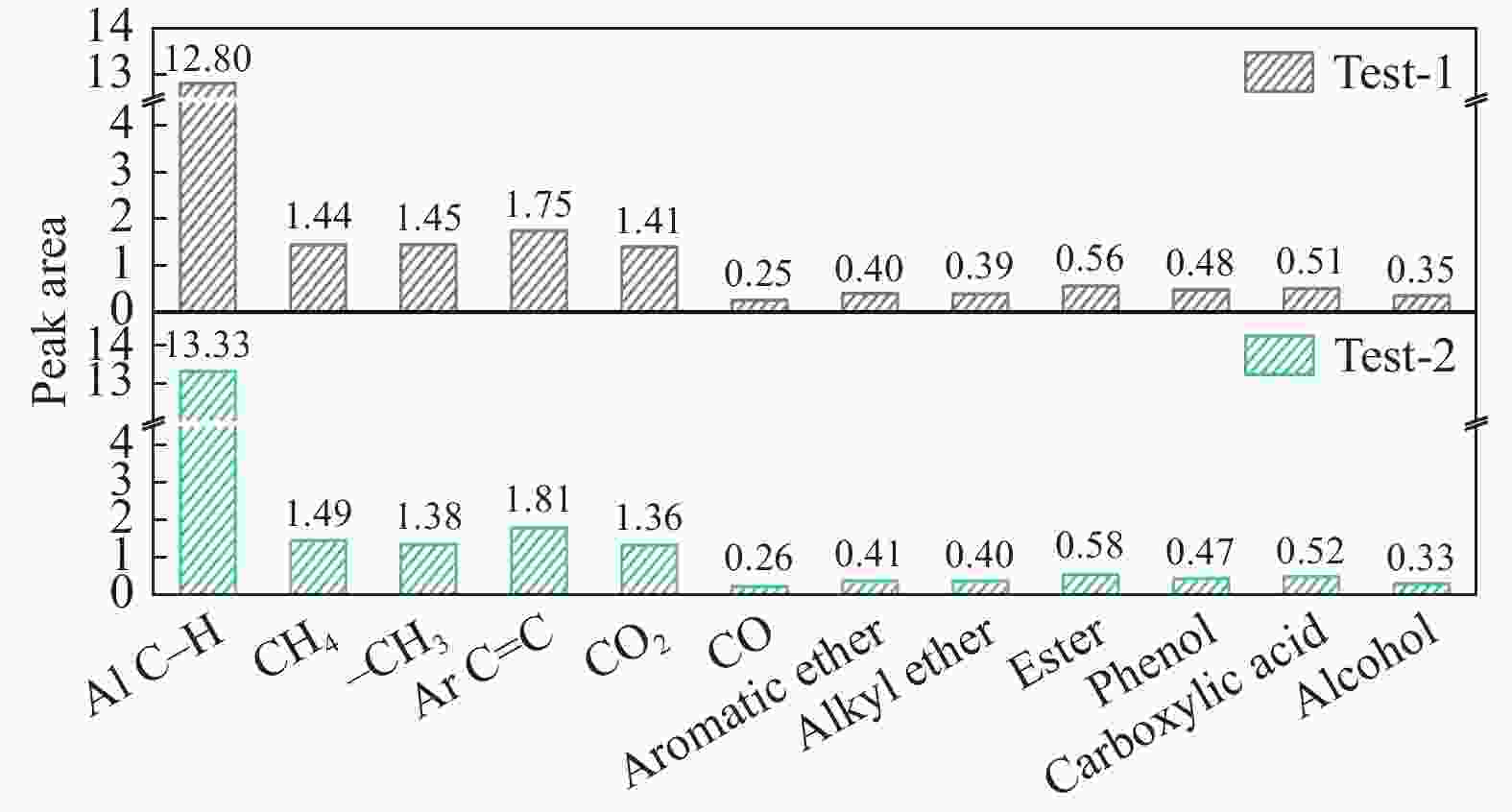
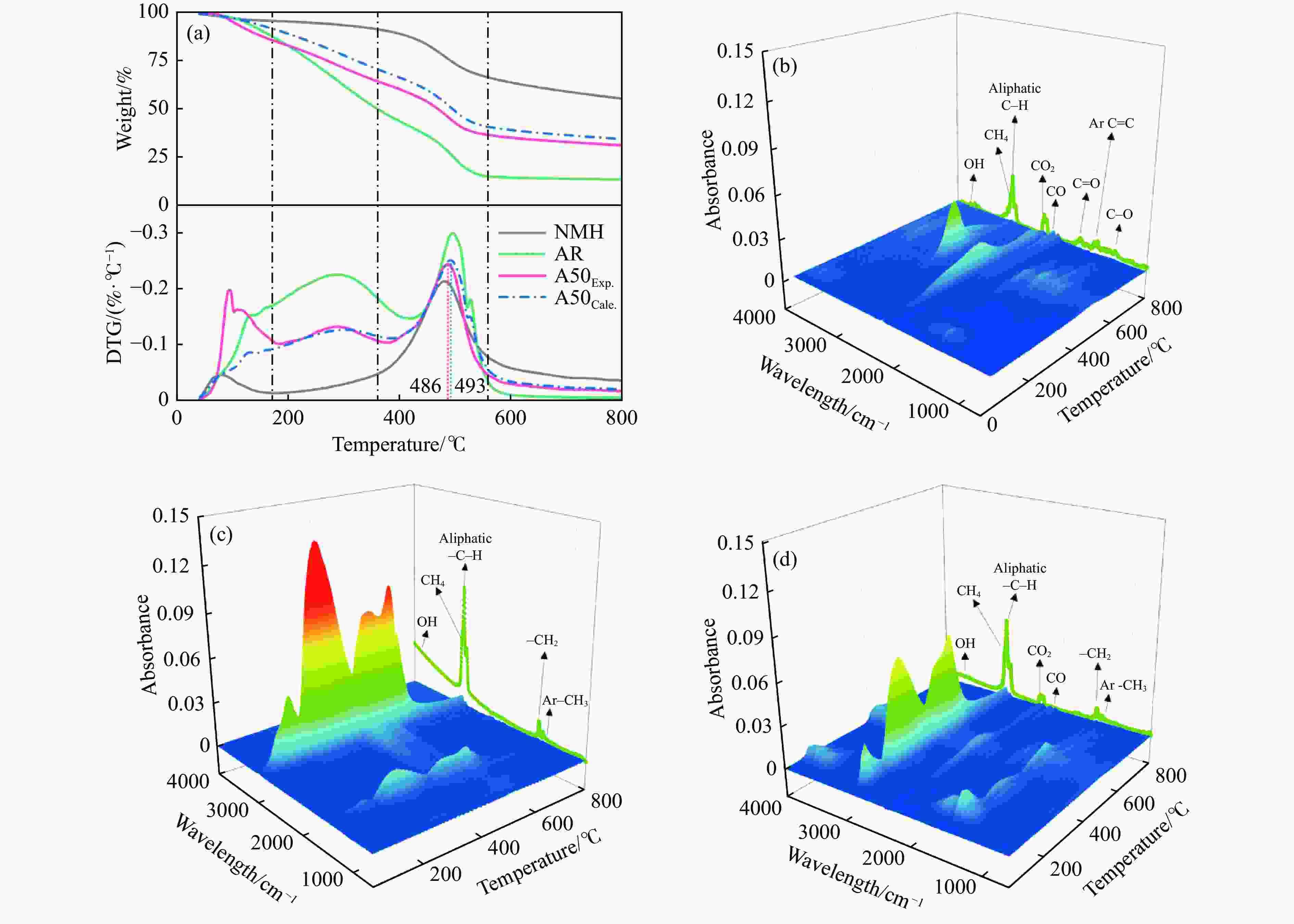
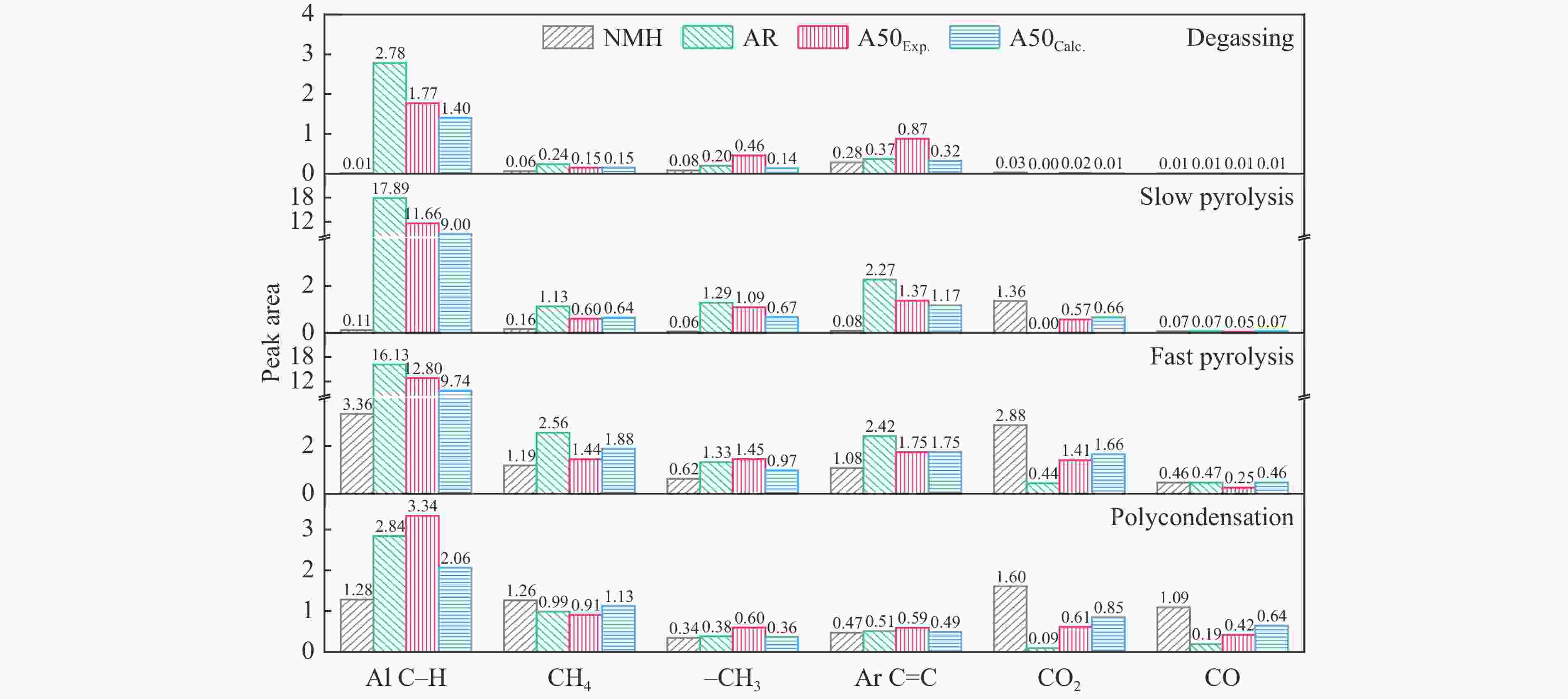
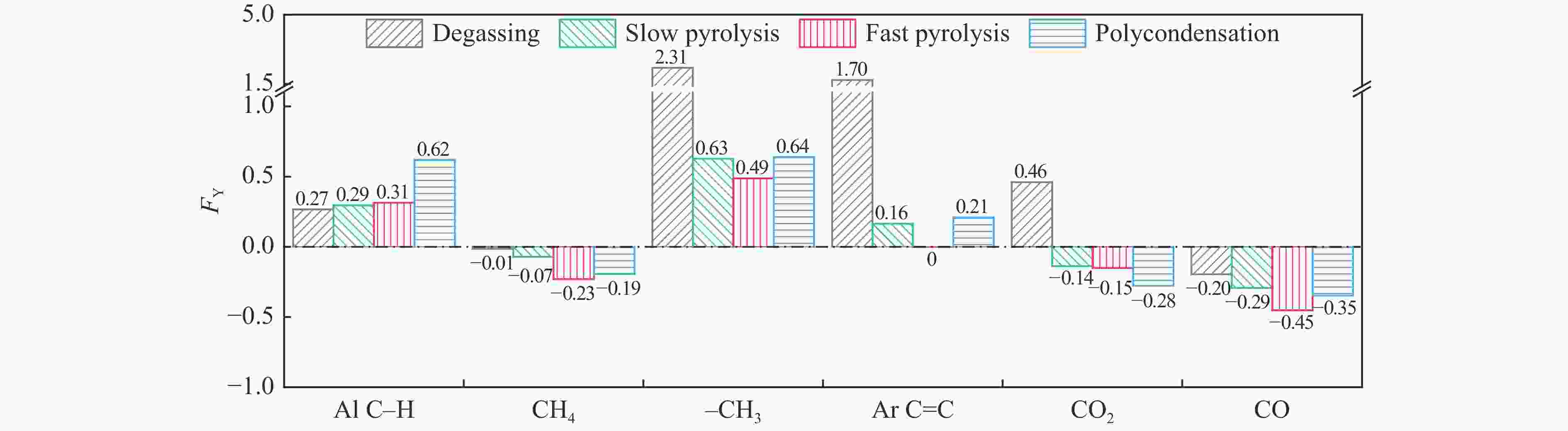
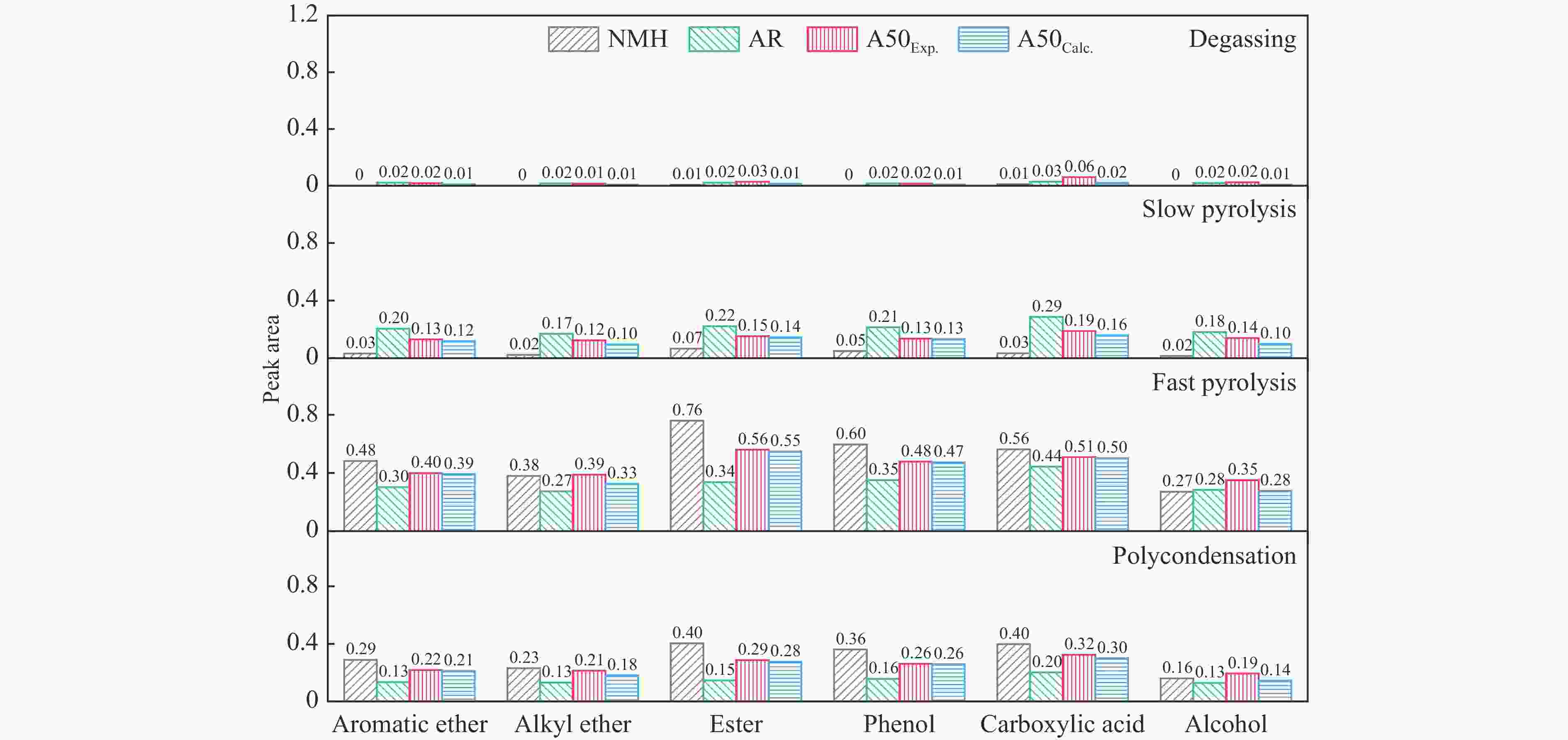
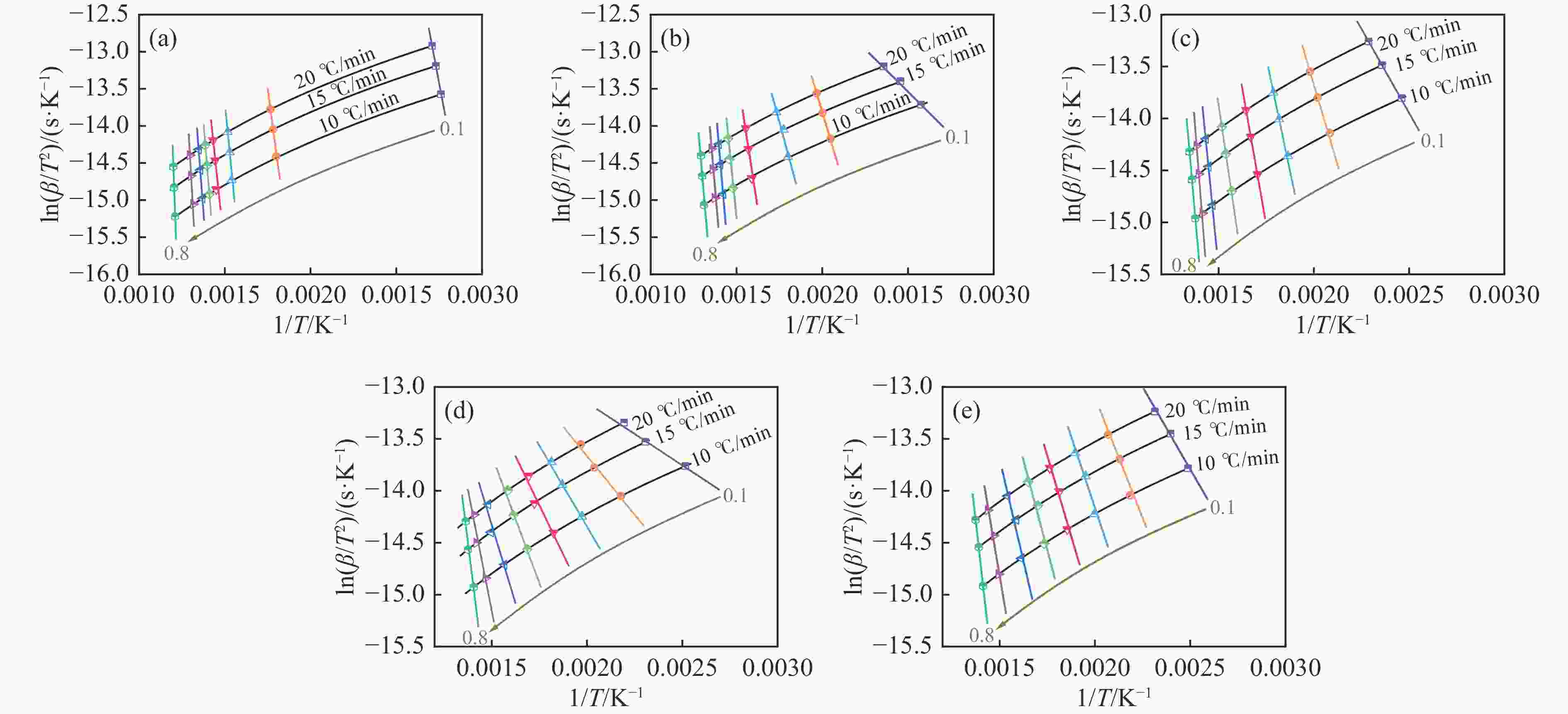
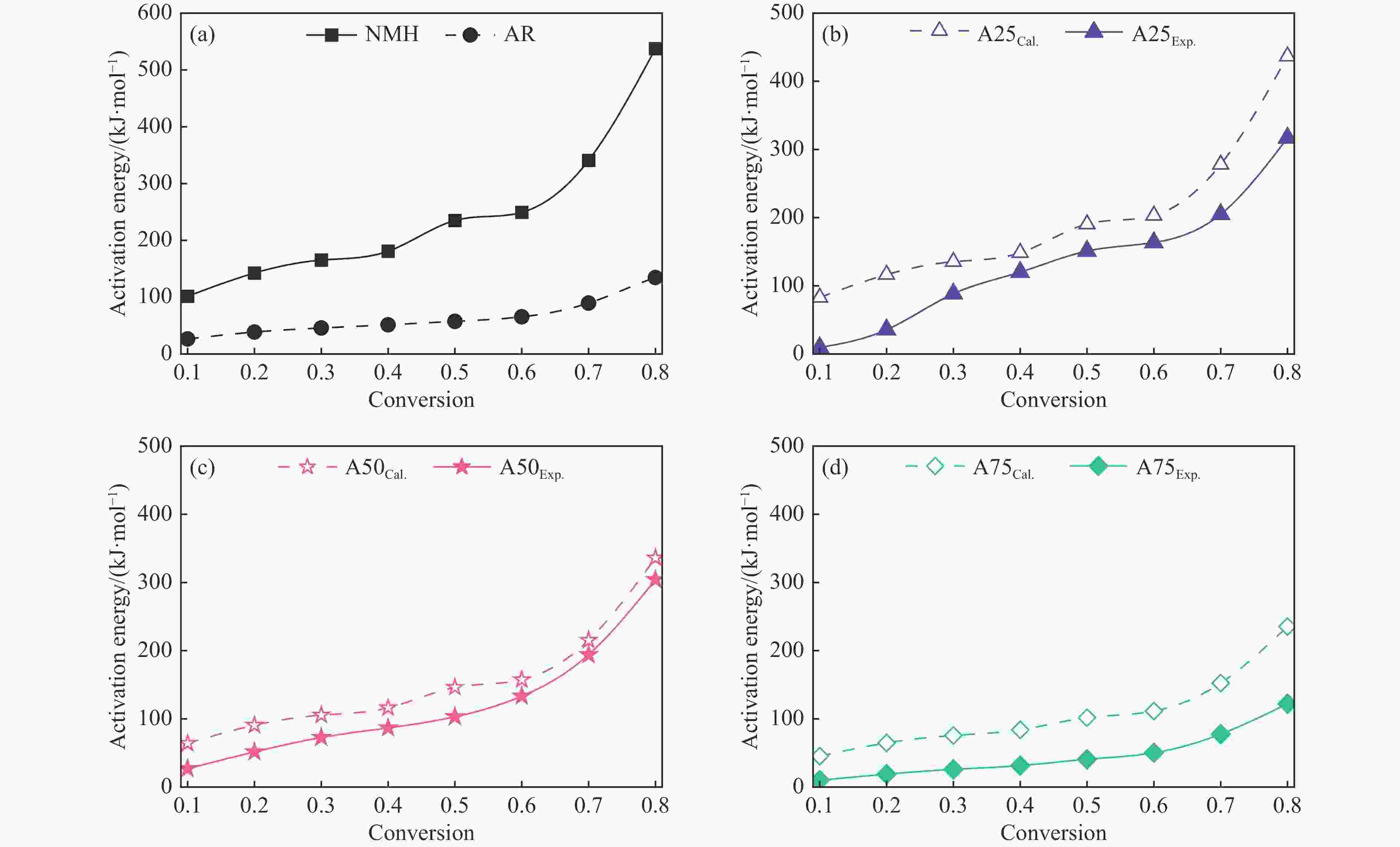
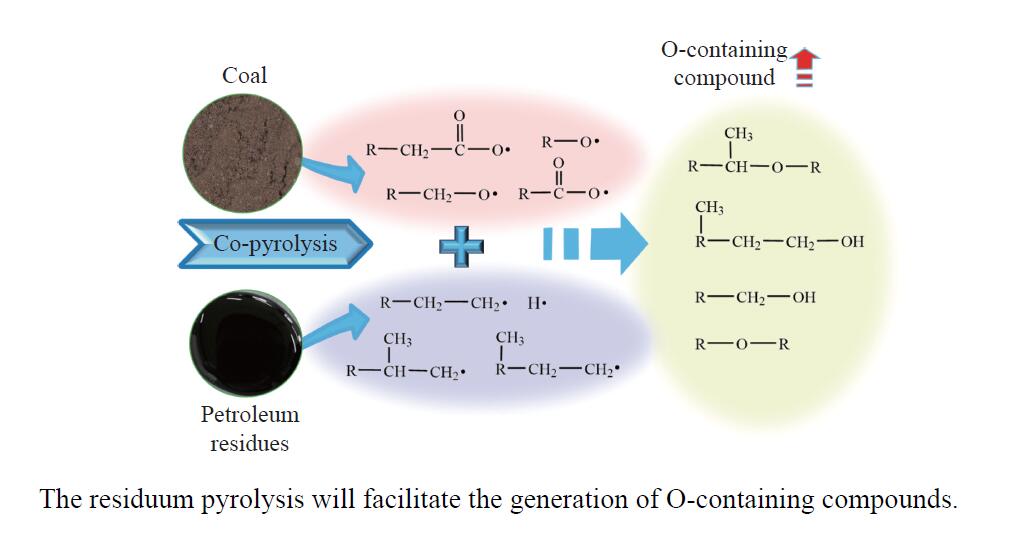
摘要: 煤油共液化过程中煤与重油先发生共热解,而后加氢转化为小分子产品。因此,阐明重油对煤热解逸出产物的影响规律是调控共液化产物组成的重要热化学基础。本研究采用TG-FTIR对比研究塔河渣油(AR)和淖毛湖煤(NMH)单独热解及其共热解过程,结合热解活化能计算,探索共热解过程中塔河渣油(AR)对淖毛湖煤(NMH)热解产物逸出产物的影响。结果表明,单独热解时AR先于NMH发生热解反应。两者1∶1(质量比)混合共热解时,相比于单独热解计算的理论值,最大失重峰温度前移7 ℃,失重率增加约3%,共热解平均活化能降低23.6 kJ/mol,表明AR率先热解会诱发NMH热解,降低热解反应能垒。TG-FTIR结果显示,AR产生的烷烃类自由基会与NMH热解产生的含氧自由基结合,形成醇、醚等烷基类含氧有机化合物,从而抑制煤中羧基转化为CO2的过程。研究结果有助于揭示共液化反应过程中重油对煤液化产物组成的影响。Abstract: Coal and residuum are first co-pyrolyzed, and then hydrogenated into small molecule products during co-liquefaction. Therefore, clarifying influence of residuum on coal pyrolysis performance is an important thermochemical basis for regulating the process. The co-pyrolysis behavior of atmospheric residuum (AR) and Naomaohu coal (NMH) were investigated by TG, TG-FTIR and distributed activation energy model. The results showed that the peak temperature of the maximum rate of weight loss for the co-pyrolysis process was reduced by 7 °C compared with the theoretical value calculated by weighted average of AR and NMH pyrolysis alone, while the weight loss increased by 3%, the average activation energy decreased by 23.6 kJ/mol. In addition, the peak area of alkyl O-containing functional groups such as alcohols and ethers increased, whereas those of CO and CO2 decreased, suggesting that AR had a positive effect on NMH pyrolysis. Meanwhile, alkyl radicals from AR decomposition would combine with O-containing radicals generated from coal pyrolysis, thus resulting in a decrease of CO and CO2 by inhibiting breakage of carboxyl groups. This work will provide a scientific evaluation basis for revealing the influence of residuum on composition of coal liquefaction product during co-liquefaction.
-
Key words:
- Tahe residuum /
- Naomaohu coal /
- co-pyrolysis /
- O-containing functional groups /
- promotion effect
-
表 1 NMH基本性质分析
Table 1 Proximate and ultimate analyses of NMH
Proximate analysis w/% Ultimate analysis wdaf/% H/C Petrographical analysis/% Mad Ad Vdaf C H N S Oa vitrinite inertinite exinite 10.36 9.45 52.12 74.65 5.96 1.29 0.35 17.75 0.96 67.7 2.9 29.4 a: by difference. 表 2 AR常规分析
Table 2 Basic properties of AR
Mechanical
impurities/%Viscosity
(mm2/s, 100 ℃)Carbon
residue/%Aromaticity SARA fraction/% Elemental analysis (%, daf) Sa Ar Re As C H N S Oa 0.03 154.4 13.70 0.27 45.36 17.79 21.45 15.40 85.86 11.34 0.53 2.09 0.18 SARA fractions: The saturates (Sa), aromatics (Ar), resins (Re), and asphaltenes (As) fractions. a: by difference. 表 3 五种样品的TG-DTG参数
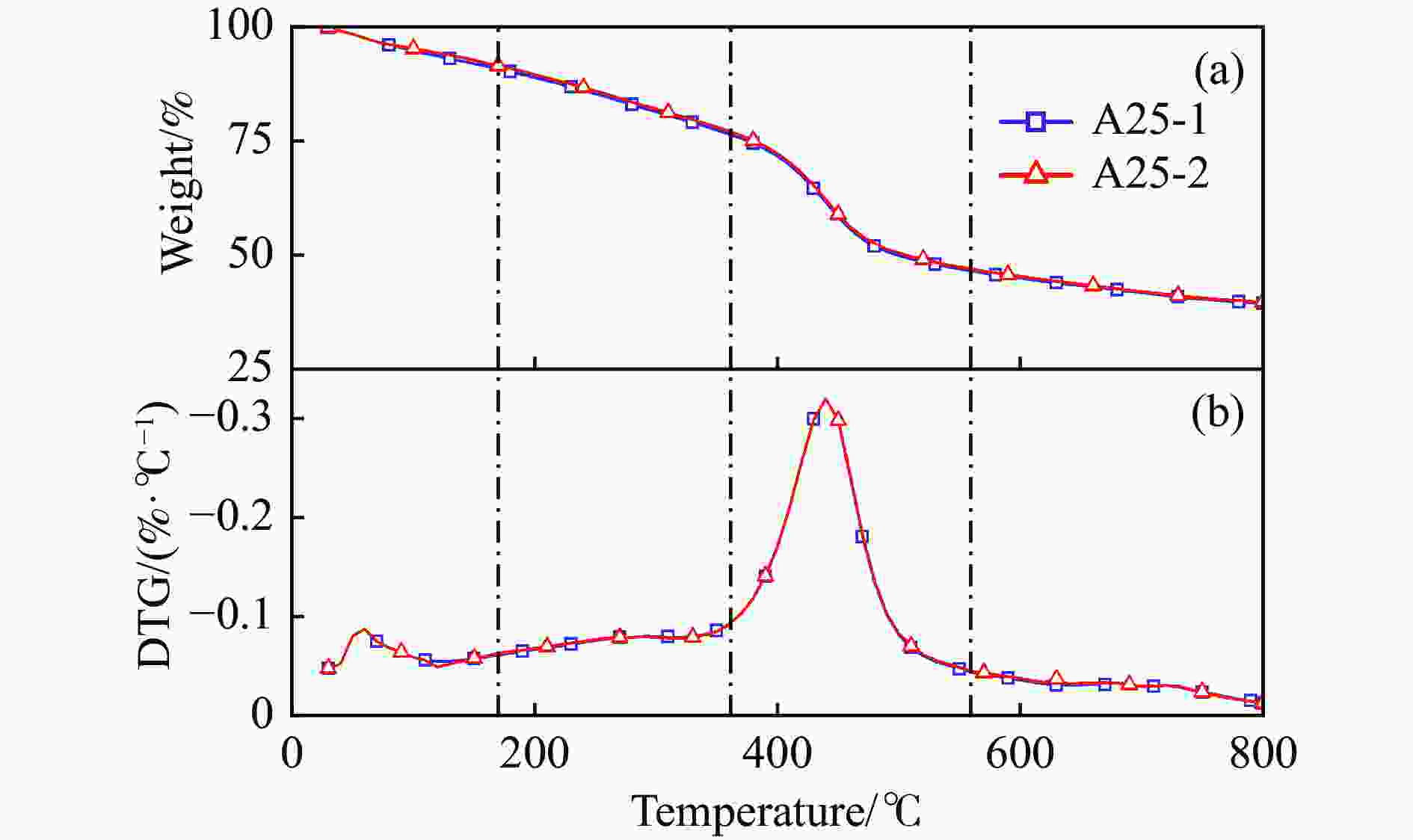
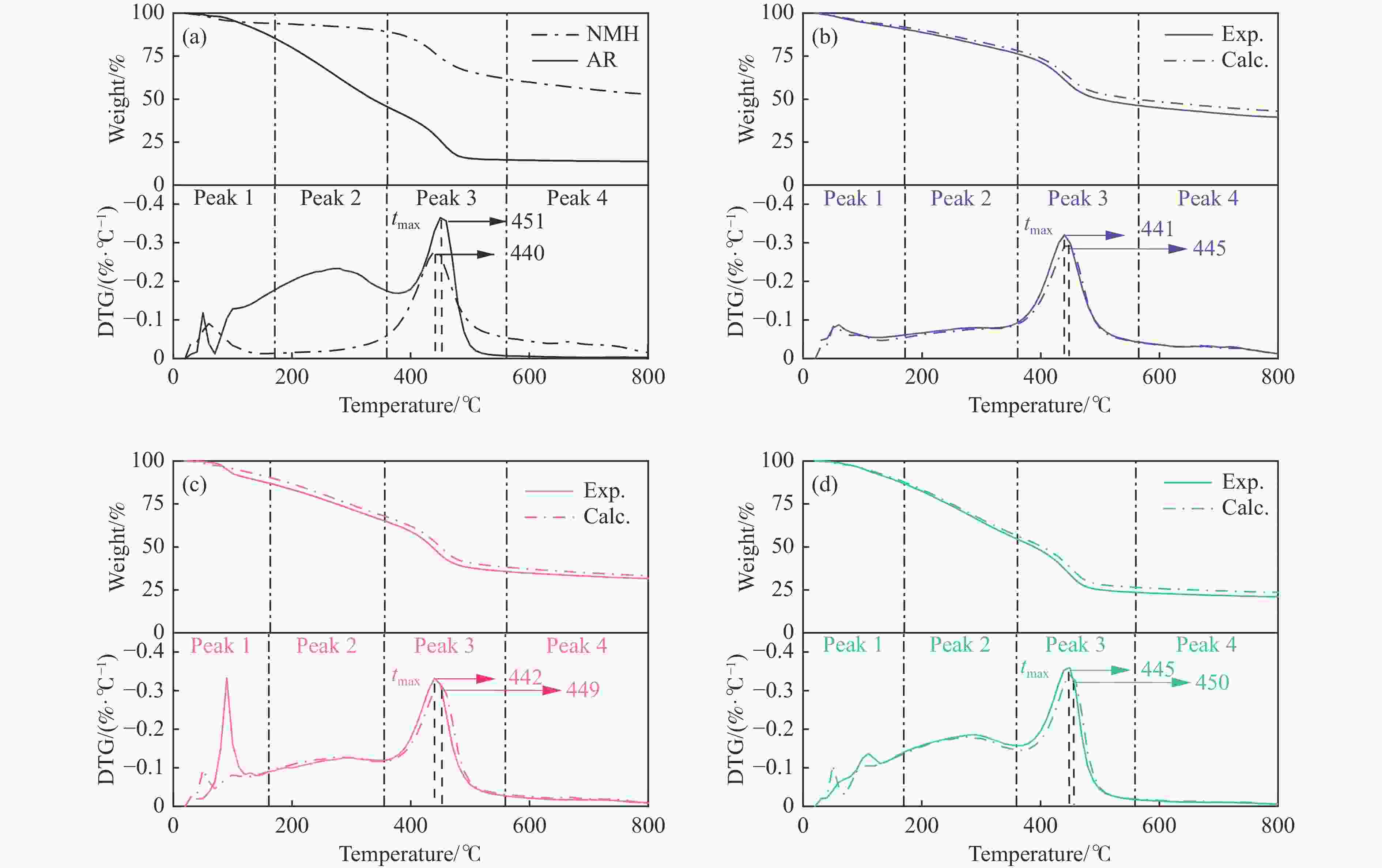
Table 3 TG-DTG parameters of five samples
Sample/
FYTotal weight
loss/
%Degassing
25−170 ℃Slow pyrolysis
170−360 ℃Fast pyrolysis
360−560 ℃Polycondensation
560−800 ℃peak
temp./
℃weight loss rate/
(%·℃−1)weight loss/
%peak
temp./
℃weight loss rate/
(%·℃−1)weight loss/
%peak
temp./
℃weight loss rate/
(%·℃−1)weight
loss/
%weight
loss/
%NMH 47.2 59.7 0.09 6.4 − − 4.6 440 0.28 26.8 9.4 A25Exp. 59.9 60.1 0.09 9.2 − − 14.6 441 0.32 29.5 6.6 A25Calc. 56.4 58.5 0.08 8.2 − − 13.6 445 0.29 28.2 6.4 FY 0.06 0.03 0.13 0.12 − − 0.07 −0.009 0.10 0.05 0.1 A50Exp. 69.2 91.2 0.33 13.0 − − 21.9 442 0.33 29.8 4.2 A50Calc. 66.6 50.2 0.09 9.4 − − 22.4 449 0.30 29.5 5.3 FY 0.04 0.82 2.67 0.38 − − −0.02 −0.02 0.1 0.01 −0.21 A75Exp. 78.4 108.0 0.14 14.0 286 0.19 31.2 445 0.36 30.5 2.7 A75Calc. 76.5 49.5 0.11 12.5 277 0.18 31.1 450 0.34 29.9 3.2 FY 0.02 1.18 0.27 0.12 0.03 0.06 0.00 −0.01 0.06 0.02 −0.16 AR 86.3 51.5 0.12 14.6 274.5 0.23 39.8 451 0.36 29.9 2.2 -
[1] 孙磊. 煤与重油共液化及其协同效应研究[D]. 马鞍山: 安徽工业大学, 2021.SUN Lei. Study on co-liquefaction of oil and coal by hydrogenation and its synergistic effects[D]. Ma'anshan: Anhui University of Technology, 2021. [2] ALI M F, AHMED S, QURESHI M S. Catalytic coprocessing of coal and petroleum residues with waste plastics to produce transportation fuels[J]. Fuel Process Technol,2011,92(5):1109−1120. doi: 10.1016/j.fuproc.2011.01.006 [3] 贾梦婷, 高山松, 张彦军, 等. 哈密煤与塔河重油共加氢反应性能的研究[J]. 燃料化学学报,2021,49(7):902−908. doi: 10.1016/S1872-5813(21)60037-3JIA Mengting, GAO Shansong, ZHANG Yanjun, et al. Co-hydrogenation behavior of Hami coal with Tahe residue[J]. J Fuel Chem Technol,2021,49(7):902−908. doi: 10.1016/S1872-5813(21)60037-3 [4] MALHOTRA R, MCMILLEN D F. Relevance of cleavage of strong bonds in coal liquefaction[J]. Energy Fuels,1993,7(2):227−233. doi: 10.1021/ef00038a012 [5] WU Z Q, WANG S Z, ZHAO J, et al. Synergistic effect on thermal behavior during co-pyrolysis of lignocellulosic biomass model components blend with bituminous coal[J]. Bioresour Technol,2014,169:220−228. doi: 10.1016/j.biortech.2014.06.105 [6] LI K, MA X X, HE R Y, et al. Co-pyrolysis characteristics and interaction route between low-rank coals and Shenhua coal direct liquefaction residue[J]. Chin J Chem Eng,2019,27(11):2815−2824. doi: 10.1016/j.cjche.2019.03.032 [7] QUAN C, XU S P, AN Y, et al. Co-pyrolysis of biomass and coal blend by TG and in a free fall reactor[J]. J Thermal Anal Calorim,2014,117(2):817−823. doi: 10.1007/s10973-014-3774-7 [8] 袁泉, 张乾, 梁丽彤, 等. 煤与催化裂化油浆共热解特性及气体逸出规律[J]. 煤炭学报,2021,46(8):2690−2698. doi: 10.13225/j.cnki.jccs.2020.0615YUAN Quan, ZHANG Qian, LIANG Litong, et al. Characteristics of co-pyrolysis of coal and FCC slurry and the evolution behavior of the produced gases[J]. J China Coal Soc,2021,46(8):2690−2698. doi: 10.13225/j.cnki.jccs.2020.0615 [9] OVALLES C, ROGEL E, HAJDU P, et al. Predicting coke morphology in Delayed Coking from feed characteristics[J]. Fuel,2020,263:116739. doi: 10.1016/j.fuel.2019.116739 [10] PRAJAPATI R, KOHLI K, MAITY S K. Residue upgradation with slurry phase catalyst: Effect of feedstock properties[J]. Fuel,2019,239:452−460. doi: 10.1016/j.fuel.2018.11.041 [11] MENG H Y, WANG S Z, CHEN L, et al. Investigation on synergistic effects and char morphology during co-pyrolysis of poly(vinyl chloride) blended with different rank coals from northern China[J]. Energy Fuels,2015,29(10):6645−6655. doi: 10.1021/acs.energyfuels.5b01437 [12] SONG Y H, YIN N, YAO D, et al. Co-pyrolysis characteristics and synergistic mechanism of low-rank coal and direct liquefaction residue[J]. Energy Sources, Part A,2019,41(21):2675−2689. doi: 10.1080/15567036.2019.1568639 [13] SONG Y H, LEI S M, LI J C, et al. In situ FTIR analysis of coke formation mechanism during co-pyrolysis of low-rank coal and direct coal liquefaction residue[J]. Renewable Energy,2021,179:2048−2062. doi: 10.1016/j.renene.2021.08.030 [14] WU Z Q, YANG W C, LI Y W, et al. Co-pyrolysis behavior of microalgae biomass and low-quality coal: Products distributions, char-surface morphology, and synergistic effects[J]. Bioresour Technol,2018,255:238−245. doi: 10.1016/j.biortech.2018.01.141 [15] 何清, 程晨, 龚岩, 等. 水热炭化生物质与煤共热解和共气化特性研究[J]. 燃料化学学报,2022,50(6):665−673.HE Qing, CHENG Chen, GONG Yan, et al. Study on co-pyrolysis and co-gasification of hydrothermal carbonized biomass and coal[J]. J Fuel Chem Technol,2022,50(6):665−673. [16] 张婷婷, 白宗庆, 侯冉冉, 等. 煤与废塑料共热解特性研究进展[J]. 化工进展,2021,40(5):2461−2470.ZHANG Tingting, BAI Zongqing, HOU Ranran, et al. Research progress on co-pyrolysis characteristics of coal and waste plastics[J]. Chem Ind Eng Progress,2021,40(5):2461−2470. [17] LU Y, WANG Y, ZHANG J, et al. Investigation on the characteristics of pyrolysates during co-pyrolysis of Zhundong coal and Changji oil shale and its kinetics[J]. Energy,2020,200:117529. doi: 10.1016/j.energy.2020.117529 [18] GUO F Q, LI X L, WANG Y, et al. Characterization of Zhundong lignite and biomass co-pyrolysis in a thermogravimetric analyzer and a fixed bed reactor[J]. Energy,2017,141:2154−2163. doi: 10.1016/j.energy.2017.11.141 [19] LI S D, CHEN X L, LIU A, et al. Co-pyrolysis characteristic of biomass and bituminous coal[J]. Bioresour Technol,2015,179:414−420. doi: 10.1016/j.biortech.2014.12.025 [20] 孙云娟. 生物质与煤共热解气化行为特性及动力学研究[D]. 北京: 中国林业科学研究院, 2013.SUN Yunjuan. Study on the characteristic and kinetic of biomass and coal co-pyrolysis[D]. Beijing: Chinese Academy of Forestry, 2013. [21] GYUL’MALIEV A M, GOLOVIN G S, GAGARIN S G. Classification of fossil fuels according to structural-chemical characteristics[J]. Solid Fuel Chem,2007,41(5):257−266. doi: 10.3103/S0361521907050011 [22] 孙志强. 基于煤化学结构指数法对新疆煤液化性能评价与西沟煤/渣油共液化强化研究[D]. 乌鲁木齐: 新疆大学, 2017.SUN Zhiqiang. Evaluation of Xinjiang low rank coal direct liquefaction based on coal chemistry structural index and improvement of the performance for co-liquefaction of Xigou coal and residue[D]. Urumqi: Xinjiang University, 2017. [23] 煤炭科学技术研究院有限公司. 煤油共炼原料技术条件[S]. 2018.Coal Science and Technology Research Institute Co. , Ltd. Technical conditions for coal oil co refining raw materials[S]. 2018. [24] LI S S, MA X Q, LIU G C, et al. A TG-FTIR investigation to the co-pyrolysis of oil shale with coal[J]. J Anal Appl Pyrolysis,2016,120:540−548. doi: 10.1016/j.jaap.2016.07.009 [25] 陈海翔, 刘乃安. 温度积分近似式研究[J]. 化学进展,2008,20(7/8):1015−1020.CHEN Haixiang, LIU Nai’an. Approximation Expressions for the Temperature Integral[J]. Prog Chem,2008,20(7/8):1015−1020. [26] MA Y Y, MA F Y, MO W L, et al. Five-stage sequential extraction of Hefeng coal and direct liquefaction performance of the extraction residue[J]. Fuel,2020,266(16):117039. [27] HOU R R, PANG K L, BAI Z Q, et al. Study on carboxyl groups in direct liquefaction of lignite: Conjoint analysis of theoretical calculations and experimental methods[J]. Fuel,2021,286:119298. doi: 10.1016/j.fuel.2020.119298 [28] LIU Q, WANG S R, ZHENG Y, et al. Mechanism study of wood lignin pyrolysis by using TG-FTIR analysis[J]. J Anal Appl Pyrolysis,2008,82(1):170−177. doi: 10.1016/j.jaap.2008.03.007 [29] LIN X C, WANG C H, IDETA K, et al. Insights into the functional group transformation of a Chinese brown coal during slow pyrolysis by combining various experiments[J]. Fuel,2014,118:257−264. doi: 10.1016/j.fuel.2013.10.081 [30] 陈泽洲. 煤加氢液化催化剂及相关条件下烃组分的反应研究[D]. 北京: 北京化工大学, 2018.CHEN Zezhou. Study on the reactions of catalysts and hydrocarbons in coal hydroliquefaction[D]. Beijing: Beijing University of Chemical Technology, 2018. [31] 何小强, 莫文龙, 王强, 等. 离子液体溶胀对煤直接液化残渣结构及热解性能的影响[J]. 燃料化学学报,2019,47(12):1417−1428.HE Xiaoqiang, MO Wenlong, WANG Qiang, et al. Effect of swelling treatment by ionic liquid on the structure and pyrolysis performance of the direct coal liquefaction residue[J]. J Fuel Chem Technol,2019,47(12):1417−1428. [32] 鲁阳. 准东煤与昌吉油页岩混合燃料热解/燃烧特性及其动力学研究[D]. 太原: 太原理工大学, 2020.LU Yang. Research on pyrolysis and combustion characteristics of Zhundong coal and Changji oil shale mixtures and their kinetics[D]. Taiyuan: Taiyuan University of Technology, 2020. [33] WANG Y G, ZHOU J L, BAI L, et al. Impacts of inherent O-containing functional groups on the surface properties of Shengli lignite[J]. Energy Fuels,2014,28(2):862−867. doi: 10.1021/ef402004j [34] GIROUX L, CHARLAND J P, MACPHEE J A. Application of thermogravimetric Fourier transform infrared spectroscopy (TG-FTIR) to the analysis of oxygen functional groups in coal[J]. Energy Fuels,2006,20(5):1988−1996. doi: 10.1021/ef0600917 [35] SCACCIA S. TG-FTIR and kinetics of devolatilization of Sulcis coal[J]. J Analy Appl Pyrolysis,2013,104:95−102. doi: 10.1016/j.jaap.2013.09.002 [36] FENG X B, CAO J P, ZHAO X Y, et al. Organic oxygen transformation during pyrolysis of Baiyinhua lignite[J]. J Anal Appl Pyrolysis,2016,117:106−115. doi: 10.1016/j.jaap.2015.12.010 [37] 杨伏生. 基于TG-MS方法的核桃壳/神府煤的凹凸棒土催化共热解动力学及机理研究[D]. 西安: 西安科技大学, 2019.YANG Fusheng. Kinetics and mechanism of copyrolysis of walnut shell and Shenfu coal using attapulgite as catalyst based on TG-MS method[D]. Xi’an: Xi’an University of Science and Technology, 2019. [38] CHEN X, LIU L, ZHANG L, et al. Thermogravimetric analysis and kinetics of the co-pyrolysis of coal blends with corn stalks[J]. Thermochim Acta,2018,659:59−65. doi: 10.1016/j.tca.2017.11.005 -
 2024-A034 支撑材料.docx
2024-A034 支撑材料.docx
-




 下载:
下载: