Theoretical calculation study on the reaction mechanism of methanol/dimethyl ether carbonylation catalyzed by the B/Al/Ga-MOR zeolites
-
摘要: 采用DFT计算比较分析了B、Al和Ga分别同晶取代MOR分子筛八元环侧袋T3位点及十二元环孔道T4位点时甲醇及二甲醚羰基化反应机制的共性及差异。研究发现,CO插入甲氧基生成乙酰基的反应遵循SN2机制,且为羰基化反应过程中的决速步;473 K下,无论甲醇或二甲醚为原料,生成的乙酰基更倾向于与甲醇中的CH3O作用生成乙酸甲酯;T3位点具有更好的羰基化择形性,而T4位点上更倾向于发生由三甲基氧鎓离子生成芳烃导致催化剂失活的副反应。与Al-MOR相比,在T3位点引入B和Ga会导致羰基化反应能垒的升高,降低其催化性能;而在T4位点引入B和Ga(尤其是B)则可大幅提升其生成三甲基氧鎓离子的能垒,抑制芳烃生成过程,提升催化剂稳定性。本工作有助于认识MOR分子筛不同孔道内酸性位点发生同晶取代时催化羰基化反应机制的差异,为调控设计高效MOR沸石催化剂提供一定的理论支撑。Abstract: The reaction mechanism of methanol/dimethyl ether (DME) carbonylation catalyzed by isomorphously substituted B-, Al-, and Ga-MOR zeolites (B/Al/Ga-MOR) was comparatively investigated by the density functional theory (DFT) calculations. The commonalities and differences between methanol and dimethyl ether as the reactant as well as among various MOR zeolites in the catalytic reaction pathways were disclosed, where one Si atom was substituted by B, Al or Ga at the 8-ring side pockets T3 sites or the 12-ring channels T4 sites of MOR. The results indicate that the insertion of CO into methoxy group to form acetyl groups follows the SN2 mechanism and is the rate-determining step in the carbonylation reactions. Under 473 K, either methanol or dimethyl ether is used as feedstock, the formed acetyl group prefers to interact with CH3O in methanol to form methyl acetate. The T3 sites show better carbonylation selectivity, whereas T4 sites display better trimethoxonium ions selectivity which favors the generation of aromatics and leads to the catalyst deactivation. Comparing with Al-MOR, the introduction of Ga and B at the T3 sites increases the free energy barriers of carbonylation, whereas the introduction of Ga and B in particular at the T4 sites can substantially increase the energy barriers of generating trimethyloxonium ions, which can effectively suppress the side reaction and improve the catalyst stability. This work contributes to the understanding of the catalytic roles of various acidic sites in different channels of the MOR zeolites and provides certain theoretical support for tailoring and designing efficient MOR zeolite catalysts for methanol/dimethyl ether carbonylation.
-
Key words:
- carbonylation /
- methanol /
- dimethyl ether /
- B/Al/Ga-MOR zeolites /
- DFT calculation /
- reaction mechanism
-
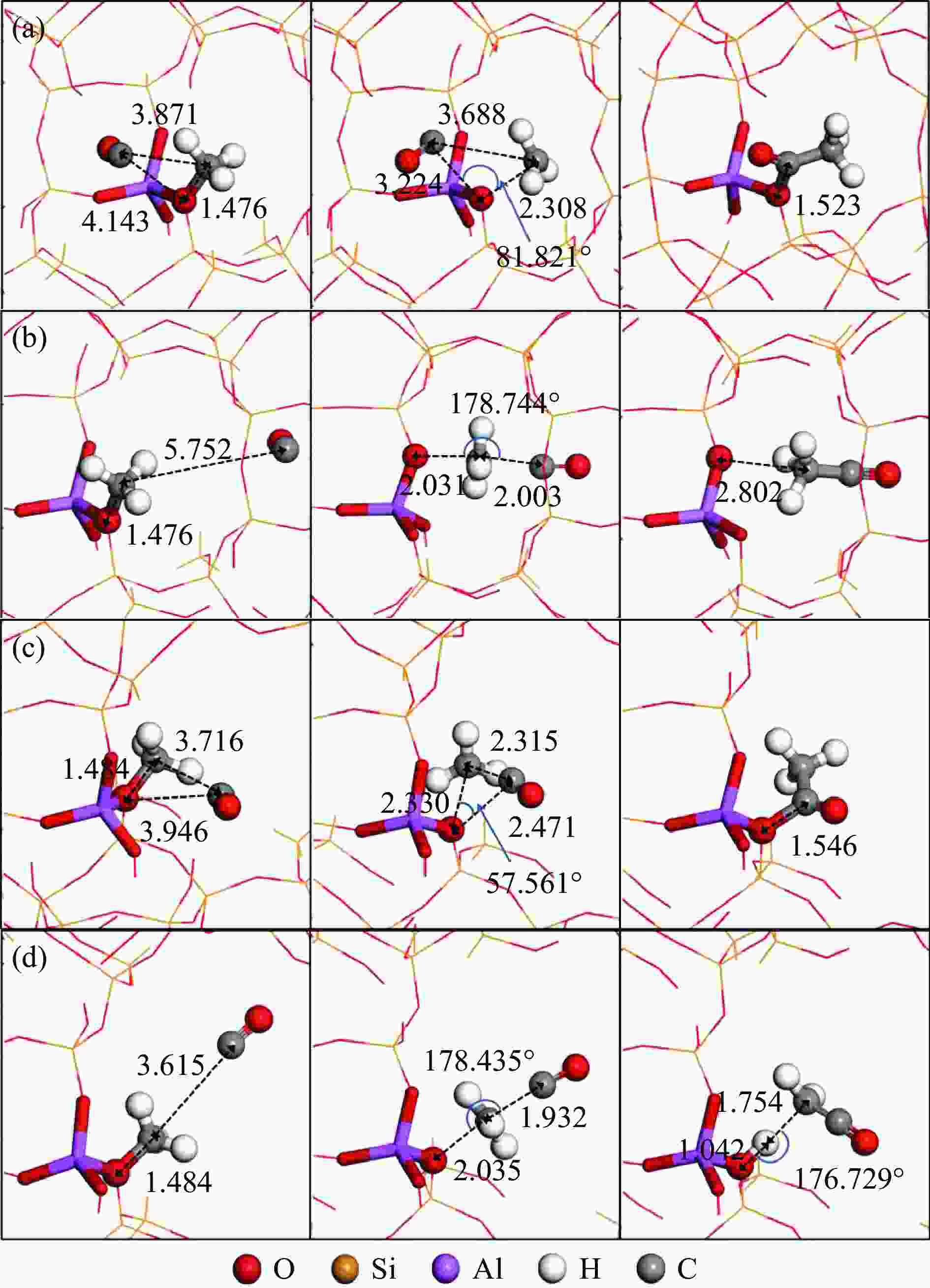
图 3 甲氧基与CO反应的反应物、过渡态和产物的结构
Figure 3 Structures of ISs, TSs and FSs for the reaction of methoxy group with CO: the insertion mechanism (a) and SN2 mechanism (b) at T3 sites of the 8-ring side pockets, and the insertion mechanism (c) and SN2 mechanism (d) at T4 sites of the 12-ring channels
(Red, yellow, purple, white and gray spheres represent O, Si, Al, H and C atoms, respectively; ball-and-stick parts represent the reaction center atoms, stick parts represent Brönsted acid sites, and the lines represent the outer frame atoms)
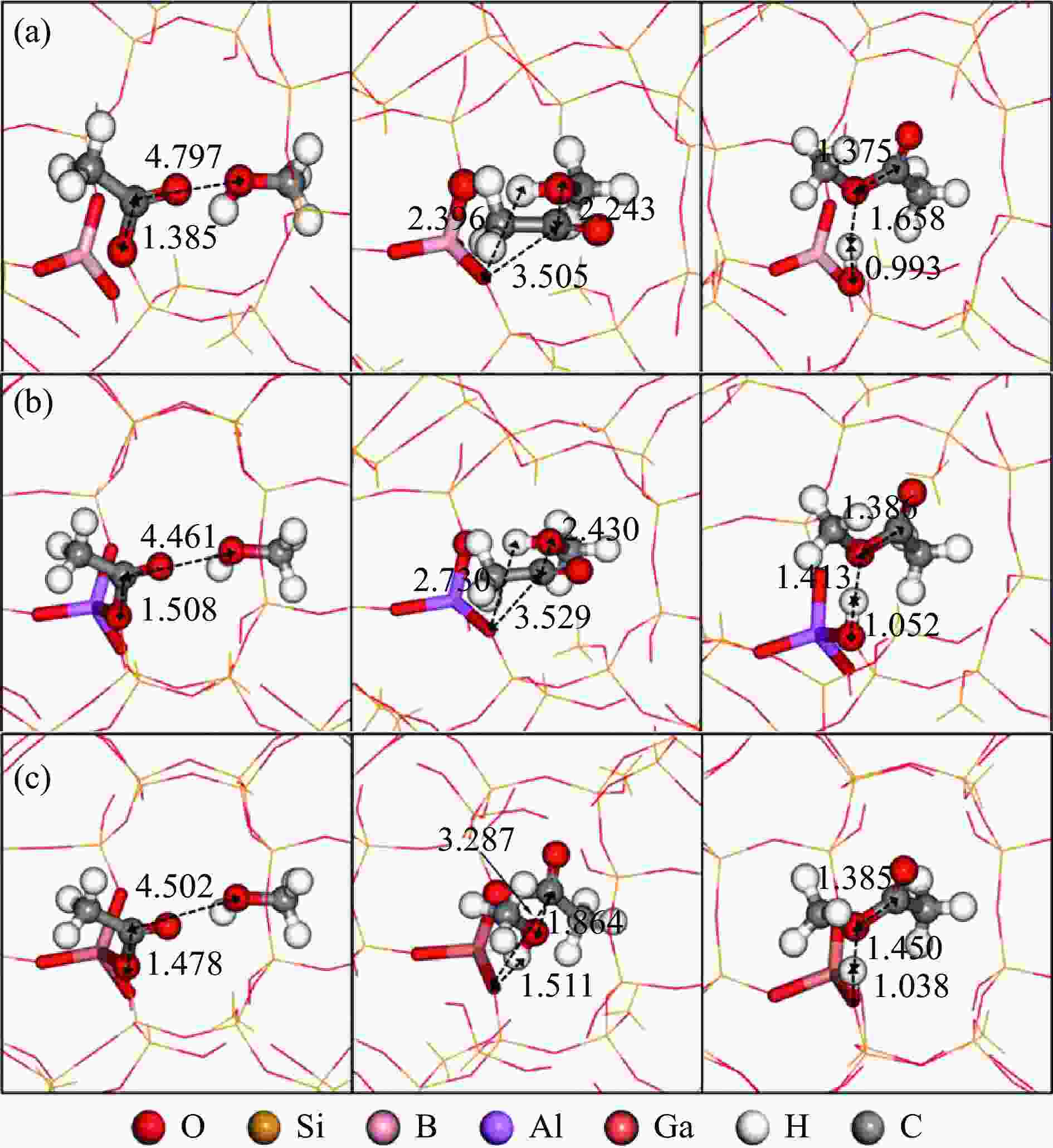
图 6 乙酰基物种与MeOH反应(TS3(M2))的反应物、过渡态和产物的结构
Figure 6 Structures of ISs, TSs and FSs for the reaction of acetyl species with MeOH (TS3(M2)): (a), (b) and (c) are located at the T3 sites of the 8-ring side pockets of B/Al/Ga-MOR, respectively
(Red, yellow, pink, purple, orange, white and gray spheres represent O, Si, B, Al, Ga, H and C atoms, respectively; ball-and-stick parts represent the reaction center atoms, stick parts represent Brönsted acid sites, and the lines represent the outer frame atoms)
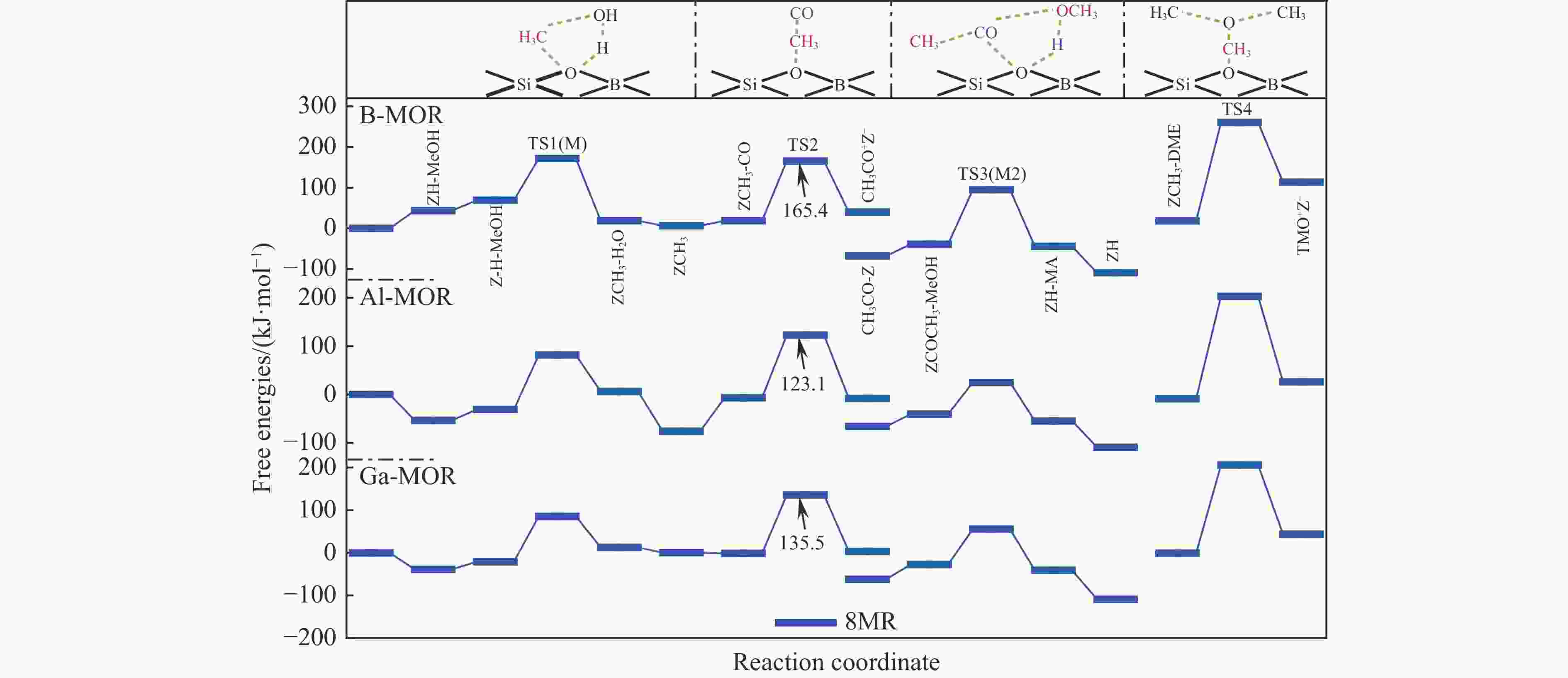
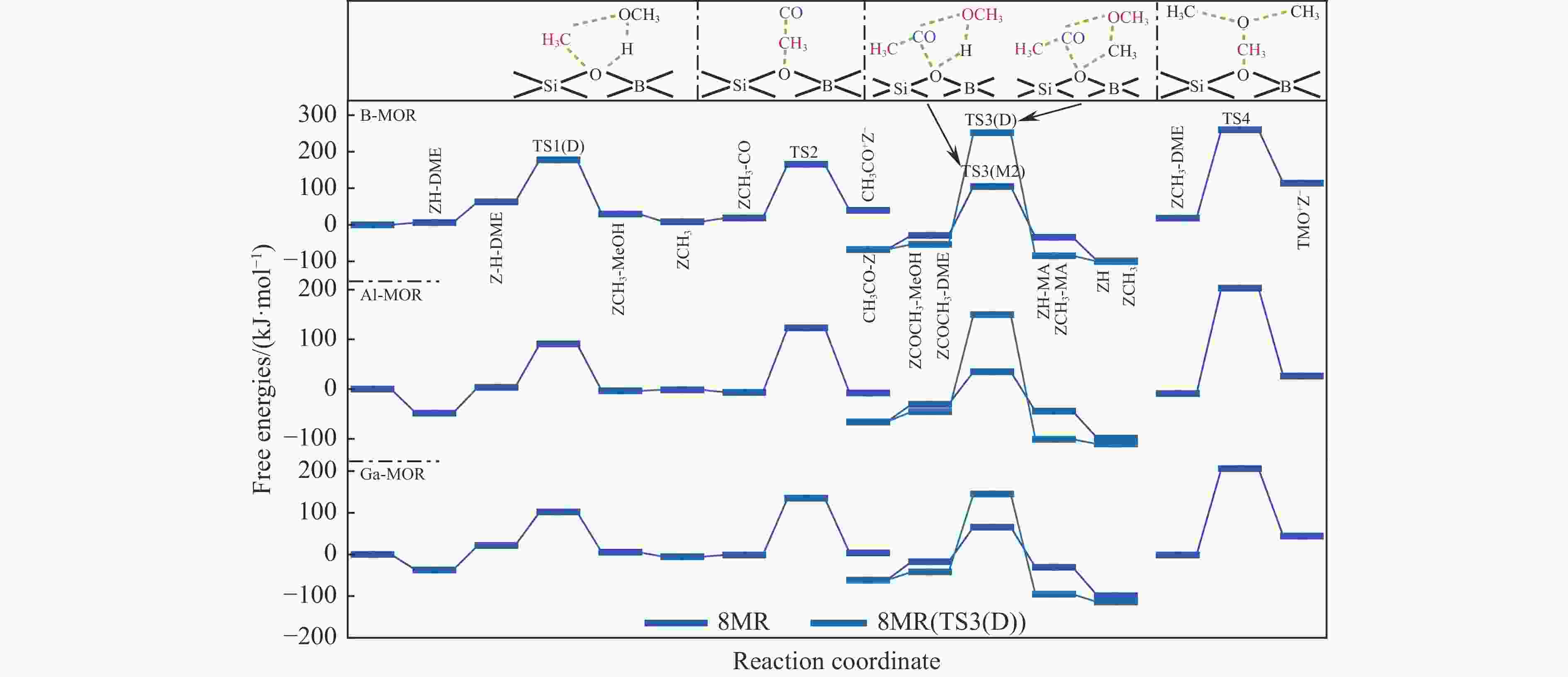
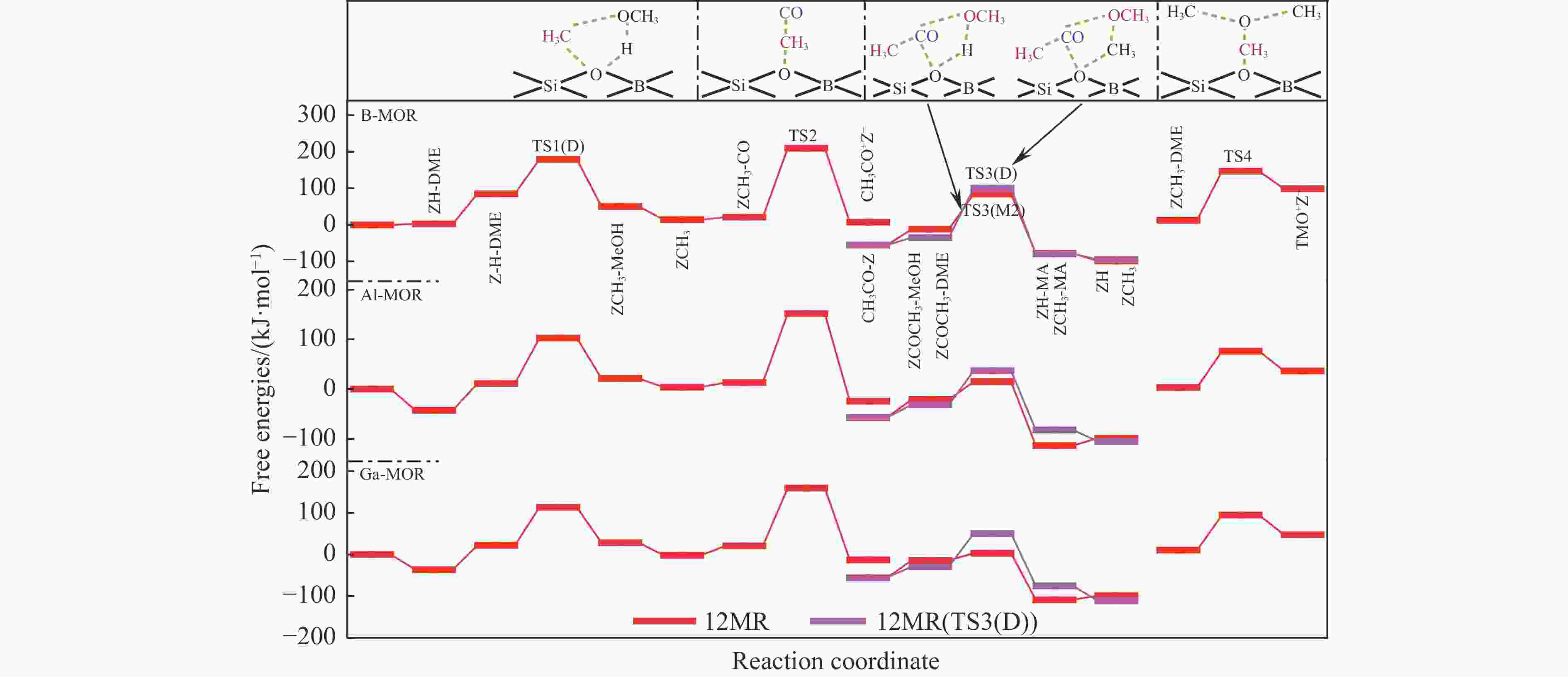
表 1 473 K下甲氧基与 CO 反应形成乙酰基的本征吉布斯自由能垒($ {\Delta {G}}_{\mathrm{i}\mathrm{n}\mathrm{t}}^{\ne } $)
Table 1 Calculated intrinsic Gibbs free energy barriers ($ {\Delta {G}}_{\mathrm{i}\mathrm{n}\mathrm{t}}^{\ne } $) for the reaction of methoxy group with CO to form an acetyl group at 473 K
Mechanism $ {\Delta {G}}_{\mathrm{i}\mathrm{n}\mathrm{t}}^{\ne } $/ (kJ·mol−1) T3 T4 DFT DFT-D DFT DFT-D Insertion 220 174 220 214 SN2 132 130 128 139 表 3 B/Al/Ga-MOR分子筛八元环侧袋和十二元环孔道内的质子亲和势和氨气吸附能
Table 3 Proton affinities (PA) and NH3 adsorption energies (${\Delta E}_{{\rm{ads\cdot NH_3}}}$) in the 8-ring side pockets and 12-ring channels of B/Al/Ga-MOR
Acid sites PA/
(kJ·mol−1)${\Delta E}_{ {\rm{ads\cdot NH_3} } }$/
(kJ·mol−1)T3 B-MOR 6.17 −1.09 Al-MOR 5.89 −1.74 Ga-MOR 6.12 −1.59 T4 B-MOR 6.28 −1.09 Al-MOR 5.90 −1.81 Ga-MOR 6.13 −1.75 表 2 473 K下B/Al/Ga-MOR分子筛八元环侧袋中甲醇及二甲醚羰基化反应的自由能垒($ {\Delta{G}}_{\mathrm{i}\mathrm{n}\mathrm{t}}^{\ne } $)、速率常数($ k $)、焓势垒($ {\Delta H}_{{\rm{int}}}^{\ne } $)、熵损失($ {-T\Delta S}_{{\rm{int}}}^{\ne } $)和反应自由能($ {\Delta G}_{{\rm{R}}} $)
Table 2 Calculated kinetic results of free energy barriers ($ {\Delta{G}}_{\mathrm{i}\mathrm{n}\mathrm{t}}^{\ne } $), relative rate constants ($ k $), enthalpy barriers ($ {\Delta H}_{{\rm{int}}}^{\ne } $) and entropy losses ($ {-T\Delta S}_{{\rm{int}}}^{\ne } $), and thermodynamic results of reaction free energies ($ {\Delta G}_{{\rm{R}}} $) of each reaction step for MeOH and DME carbonylation in the 8-ring side pockets of B/Al/Ga-MOR at 473 K
Reaction step
8MR$ {\Delta{G}}_{\mathrm{i}\mathrm{n}\mathrm{t}}^{\ne } $
/(kJ·mol−1)$ k $
/s−1$ {\Delta H}_{{\rm{int}}}^{\ne } $
/(kJ·mol−1)$ {-T\Delta S}_{{\rm{int}}}^{\ne } $
/(kJ·mol−1)${\Delta G}_{{\rm{R}}}$
/(kJ·mol−1)B-MOR TS1(M) 103 4.16×101 97 6 −50 TS1(D) 115 1.97×100 116 −1 −33 TS2 146 7.41×10−4 136 10 21 TS3(M1) 171 1.29×10−6 157 14 −35 TS3(M2) 133 2.02×10−2 114 19 −6 TS3(D) 306 1.59×10−21 271 35 −31 TS4 242 1.85×10−14 220 22 96 Al-MOR TS1(M) 113 3.27×100 114 −1 37 TS1(D) 87 2.43×103 87 0 −7 TS2 130 4.33×10−2 113 17 −1 TS3(M1) 97 1.91×102 86 11 −80 TS3(M2) 65 6.54×105 41 24 −14 TS3(D) 195 2.87×10−9 186 9 −55 TS4 212 3.81×10−11 187 25 35 Ga-MOR TS1(M) 107 1.50×101 108 −1 33 TS1(D) 80 1.44×104 81 −1 −16 TS2 137 7.31×10−3 117 20 5 TS3(M1) 123 2.57×10−1 123 0 −69 TS3(M2) 83 6.72×103 50 33 −13 TS3(D) 187 2.20×10−8 157 30 −54 TS4 207 1.36×10−10 183 24 45 表 4 473 K下B/Al/Ga-MOR分子筛十二元环孔道内甲醇及二甲醚羰基化反应的自由能垒($ {\Delta{G}}_{\mathrm{i}\mathrm{n}\mathrm{t}}^{\ne } $ )、速率常数($ k $)、焓势垒($ {\Delta H}_{{\rm{int}}}^{\ne } $)、熵损失($ {-T\Delta S}_{{\rm{int}}}^{\ne } $ )和反应自由能(${\Delta G}_{{\rm{R}}}$)
Table 4 Calculated kinetic results of free energy barriers ($ {\Delta{G}}_{\mathrm{i}\mathrm{n}\mathrm{t}}^{\ne } $), relative rate constants ($ k $), enthalpy barriers ($ {\Delta H}_{{\rm{int}}}^{\ne } $) and entropy losses ($ {-T\Delta S}_{{\rm{int}}}^{\ne } $), and thermodynamic results of reaction free energies (${\Delta G}_{{\rm{R}}}$) of each reaction step for MeOH and DME carbonylation in the 12-ring channels of B/Al/Ga-MOR at 473 K
Reaction step 12MR $ {\Delta{G}}_{\mathrm{i}\mathrm{n}\mathrm{t}}^{\ne } $ (kJ·mol−1) $ k $
/s−1$ {\Delta H}_{{\rm{int}}}^{\ne } $
/(kJ·mol−1)$ {-T\Delta S}_{{\rm{int}}}^{\ne } $
/(kJ·mol−1)$ {\Delta G}_{{\rm{R}}} $
/(kJ·mol−1)B-MOR TS1(M) 77 3.09×104 75 2 −53 TS1(D) 94 4.10×102 86 8 −35 TS2 188 1.70×10−8 147 41 −14 TS3(M1) 128 7.21×10−2 112 16 −37 TS3(M2) 96 2.46×102 85 11 −67 TS3(D) 136 9.43×10−3 124 12 −45 TS4 134 1.57×10−2 115 19 86 Al-MOR TS1(M) 93 5.29×102 89 4 22 TS1(D) 91 8.79×102 95 −4 10 TS2 139 4.40×10−3 106 33 −38 TS3(M1) 39 4.86×108 30 9 −53 TS3(M2) 35 1.34×109 10 25 −93 TS3(D) 68 3.05×105 45 23 −51 TS4 73 8.55×104 69 4 −34 Ga-MOR TS1(M) 126 1.20×10−1 121 5 51 TS1(D) 92 6.82×102 93 −1 6 TS2 139 4.40×10−3 107 32 −33 TS3(M1) 57 5.00×106 27 30 −40 TS3(M2) 17 1.31×1011 8 9 −94 TS3(D) 80 1.44×104 75 5 −46 TS4 84 5.21×103 69 15 37 -
[1] LI Y, HE D W, NIU D J, et al. Acetic acid production from food wastes using yeast and acetic acid bacteria micro-aerobic fermentation[J]. Bioprocess Biosyst Eng,2015,38(5):863−869. doi: 10.1007/s00449-014-1329-8 [2] SANTIAGO M A N, SÁNCHEZ-CASTILLO M A, CORTRIGHT R D, et al. Catalytic reduction of acetic acid, methyl acetate, and ethyl acetate over silica-supported copper[J]. J Catal,2000,193(1):16−28. doi: 10.1006/jcat.2000.2883 [3] KALCK P, LE BERRE C, SERP P. Recent advances in the methanol carbonylation reaction into acetic acid[J]. Coord Chem Rev,2020,402(1):213078. [4] CHENG Z Z, HUANG S Y, LI Y, et al. Deactivation kinetics for the carbonylation of dimethyl ether to methyl acetate on H-MOR[J]. Ind Eng Chem Res,2017,56(46):13618−13627. doi: 10.1021/acs.iecr.7b03500 [5] FAN B H, ZHANG W N, GAO P, et al. Quantitatively mapping the distribution of intrinsic acid sites in mordenite zeolite by high-field 23Na solid-state nuclear magnetic resonance[J]. J Phys Chem Lett,2022,13(23):5186−5194. doi: 10.1021/acs.jpclett.2c00932 [6] LIU S P, FANG X D, LIU Y, et al. Dimethyl ether Carbonylation over mordenite zeolite modified by Alkyimidazolium ions[J]. Catal Commun,2020,147:106161. doi: 10.1016/j.catcom.2020.106161 [7] HAM H, JUNG H S, KIM H S, et al. Gas-phase carbonylation of dimethyl ether on the stable seed-derived ferrierite[J]. ACS Catal,2020,10(9):5135−5146. doi: 10.1021/acscatal.9b05144 [8] FENG X B, YAO J, LI H J, et al. A brand new zeolite catalyst for carbonylation reaction[J]. Chem Commun,2019,55(8):1048−1051. doi: 10.1039/C8CC08411D [9] FUJIMOTO K, SHIKADA T, OMATA K, et al. Vapor phase carbonylation of methanol with solid acid catalysts[J]. Chem Lett,1984,13(12):2047−2050. doi: 10.1246/cl.1984.2047 [10] CHEUNG P, BHAN A, SUNLEY G J, et al. Selective carbonylation of dimethyl ether to methyl acetate catalyzed by acidic zeolites[J]. Angew Chem,2006,118(10):1647−1650. doi: 10.1002/ange.200503898 [11] CHEUNG P, BHAN A, SUNLEY G J, et al. Site requirements and elementary steps in dimethyl ether carbonylation catalyzed by acidic zeolites[J]. J Catal,2007,245(1):110−123. doi: 10.1016/j.jcat.2006.09.020 [12] NARSIMHAN K, MICHAELIS V K, MATHIES G, et al. Methane to acetic acid over Cu-exchanged zeolites: Mechanistic insights from a site-specific carbonylation reaction[J]. J Am Chem Soc,2015,137(5):1825−1832. doi: 10.1021/ja5106927 [13] BLASCO T, BORONAT M, CONCEPCIÓN P, et al. Carbonylation of methanol on metal-acid zeolites: Evidence for a mechanism involving a multisite active center[J]. Angew Chem,2007,119(21):4012−4015. doi: 10.1002/ange.200700029 [14] BORONAT M, MARTINEZ-SANCHEZ C, LAW D, et al. Enzyme-like specificity in zeolites: A unique site position in mordenite for selective carbonylation of methanol and dimethyl ether with CO[J]. J Am Chem Soc,2008,130(48):16316−16323. doi: 10.1021/ja805607m [15] LI B J, XU J, HAN B, et al. Insight into dimethyl ether carbonylation reaction over mordenite zeolite from in-situ solid-state NMR spectroscopy[J]. J Phys Chem C,2013,117(11):5840−5847. doi: 10.1021/jp400331m [16] CHU Y Y, LO A Y, WANG C, et al. Origin of high selectivity of dimethyl ether carbonylation in the 8-membered ring channel of mordenite zeolite[J]. J Phys Chem C,2019,123(25):15503−15512. doi: 10.1021/acs.jpcc.9b01874 [17] WANG S R, GUO W W, ZHU L J, et al. Methyl acetate synthesis from dimethyl ether carbonylation over mordenite modified by cation exchange[J]. J Phys Chem C,2014,119(1):524−533. [18] REULE ALLEN A C, SEMAGINA N. Zinc hinders deactivation of copper-mordenite: Dimethyl ether carbonylation[J]. ACS Catal,2016,6(8):4972−4975. doi: 10.1021/acscatal.6b01464 [19] ALBERTI A. Location of Brønsted sites in mordenite[J]. Zeolites,1997,19(5/6):411−415. doi: 10.1016/S0144-2449(97)00114-0 [20] LI Y, SUN Q, HUANG S Y, et al. Dimethyl ether carbonylation over pyridine-modified MOR: Enhanced stability influenced by acidity[J]. Cataly Today,2018,311(1):81−88. [21] NI Y M, SHI L, LIU H C, et al. A green route for methanol carbonylation[J]. Catal Sci Technol,2017,7(20):4818−4822. doi: 10.1039/C7CY01621B [22] HE T, LIU X C, XU S T, et al. Role of 12-ring channels of mordenite in DME carbonylation investigated by solid-state NMR[J]. J Phys Chem C,2016,120(39):22526−22531. doi: 10.1021/acs.jpcc.6b07958 [23] LU P, YANG G H, TANAKA Y, et al. Ethanol direct synthesis from dimethyl ether and syngas on the combination of noble metal impregnated zeolite with Cu/ZnO catalyst[J]. Cataly Today,2014,232(1):22−26. [24] MA M, ZHAN E S, HUANG X M, et al. Carbonylation of dimethyl ether over Co-HMOR[J]. Catal Sci Technol,2018,8(8):2124−2130. doi: 10.1039/C8CY00407B [25] LI Y, HUANG S Y, CHENG Z Z, et al. Promoting the activity of Ce-incorporated MOR in dimethyl ether carbonylation through tailoring the distribution of Brønsted acids[J]. Appl Catal B: Environ,2019,256(5):117777. [26] KRESSE G, HAFNER J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium[J]. Phys Rev B: Condens Matter Mater Phys,1994,49(20):14251−14269. doi: 10.1103/PhysRevB.49.14251 [27] GRIMME S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction[J]. J Comput Chem,2006,27(15):1787−1799. doi: 10.1002/jcc.20495 [28] WANG V, XU N, LIU J C, et al. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code[J]. Comput Phys Commun,2021,267:108033. doi: 10.1016/j.cpc.2021.108033 [29] EYRING H. The Activated complex in chemical reactions[J]. J Chem Phys,1935,3:107−115. doi: 10.1063/1.1749604 [30] YUAN S P, WANG J G, LI Y M, et al. Theoretical studies on the properties of acid site in isomorphously substituted ZSM-5[J]. Mol Catal A: Chem,2002,178(1/2):267. doi: 10.1016/S1381-1169(01)00335-1 [31] JONES A J, IGLESIA E. The strength of Brønsted acid sites in microporous Aluminosilicates[J]. ACS Catal,2015,5:5741−5755. doi: 10.1021/acscatal.5b01133 [32] WANG S, LI S Y, ZHANG L, et al. Mechanistic insights into the catalytic role of various acid sites on ZSM-5 zeolite in the carbonylation of methanol and dimethyl ether[J]. Catal Sci Technol,2018,8(12):3193−3204. doi: 10.1039/C8CY00296G [33] BORONAT M, MARTINEZ C, CORMA A. Mechanistic differences between methanol and dimethyl ether carbonylation in side pockets and large channels of mordenite[J]. Phys Chem Chem Phys,2011,13(7):2603−2612. doi: 10.1039/c0cp01996h [34] RASMUSSEN D B, CHRISTENSEN J M, TEMEL B, et al. Ketene as a reaction intermediate in the carbonylation of dimethyl ether to methyl acetate over mordenite[J]. Angew Chem Int Ed,2015,54(25):7261−7264. doi: 10.1002/anie.201410974 [35] RASMUSSEN D B, CHRISTENSEN J M, TEMEL B, et al. Reaction mechanism of dimethyl ether carbonylation to methyl acetate over mordenite-a combined DFT/experimental study[J]. Catal Sci Technol,2017,7(5):1141−1152. doi: 10.1039/C6CY01904H [36] LIU Z Q, YI X F, WANG G R, et al. Roles of 8-ring and 12-ring channels in mordenite for carbonylation reaction: From the perspective of molecular adsorption and diffusion[J]. J Catal,2019,369:335−344. doi: 10.1016/j.jcat.2018.11.024 [37] KING S T. Reaction mechanism of oxidative carbonylation of methanol to dimethyl carbonate in Cu-Y zeolite[J]. J Catal,1996,161(2):530−538. doi: 10.1006/jcat.1996.0215 [38] CALDERAZZO F, COTTON F A. Carbon monoxide insertion reactions. I. the carbonylation of methyl manganese pentacarbonyl and decarbonylation of acetyl manganese pentacarbonyl[J]. Inorg Chem,1962,1(1):30−36. doi: 10.1021/ic50001a008 [39] MA H, LIAO J, WEI Z H, et al. Trimethyloxonium ion - a zeolite confined mobile and efficient methyl carrier at low temperatures-a DFT study coupled with microkinetic analysis[J]. Catal Sci Technol,2022,12:3328. doi: 10.1039/D2CY00207H [40] ZHOU Z Q, LIU H C, NI Y M, et al. Direct conversion of dimethyl ether and CO to acetone via coupling carbonylation and ketonization[J]. J Catal,2021,396:360−373. doi: 10.1016/j.jcat.2021.03.006 [41] BHAN A, ALLIAN A D, SUNLEY G J, et al. Specificity of sites within eight-membered ring zeolite channels for carbonylation of methyls to acetyls[J]. J Am Chem Soc,2007,129(16):4919−4924. doi: 10.1021/ja070094d [42] YUAN S P, WANG J G, LI Y W, et al. Density functional investigations into the siting of Fe and the acidic properties of isomorphously substituted mordenite by B, Al, Ga and Fe[J]. J Mol Struct: Theochem,2004,674(1/3):267−274. doi: 10.1016/S0166-1280(03)00463-9 [43] CAI K, HUANG S Y, LI Y, et al. Influence of acid strength on the reactivity of dimethyl ether carbonylation over H-MOR[J]. ACS Sustainable Chem Eng,2018,7(2):2027−2034. -





 下载:
下载: