Progress in design and application research of nitrogen carrier in chemical looping ammonia synthesis technology
-
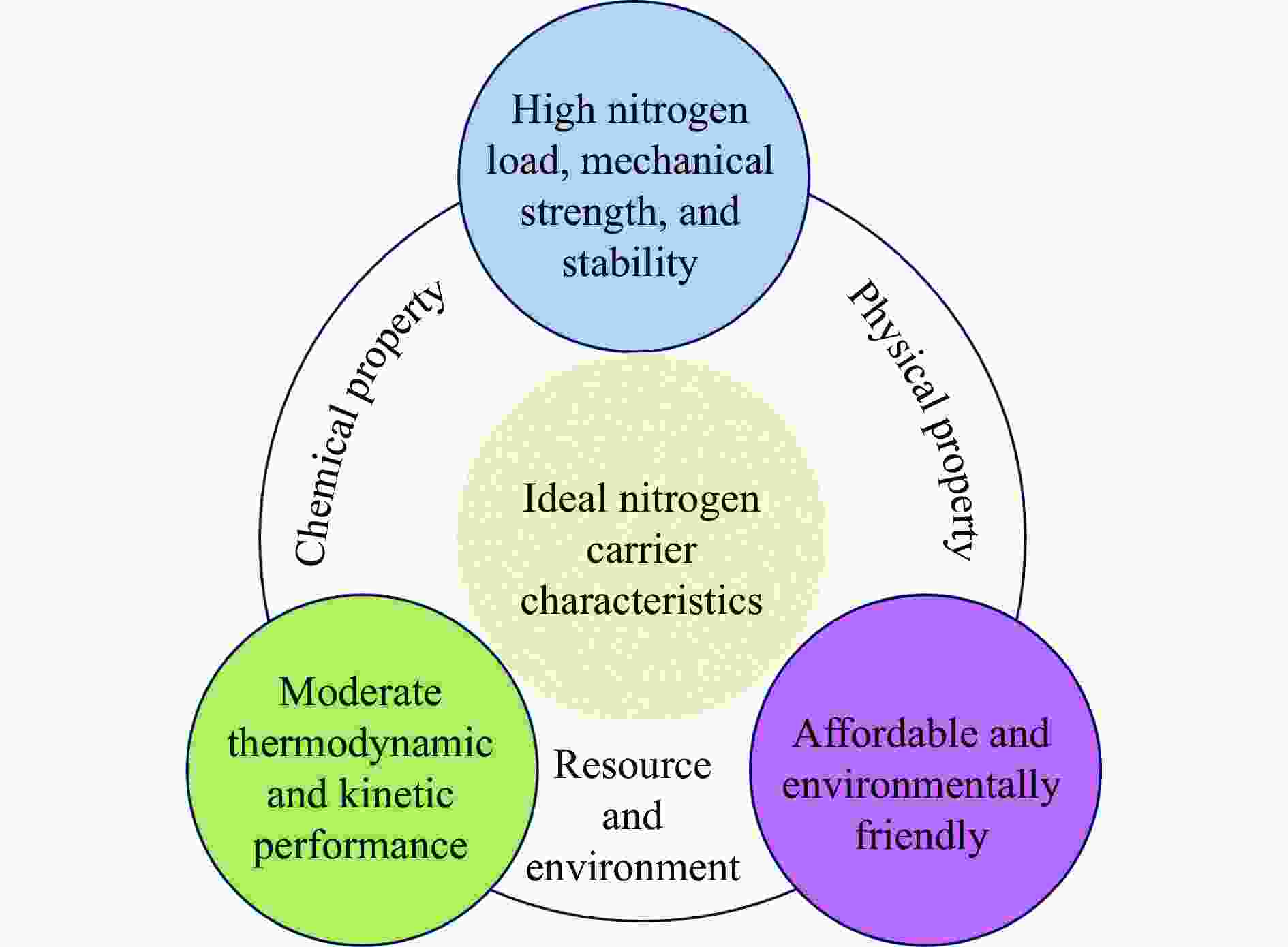
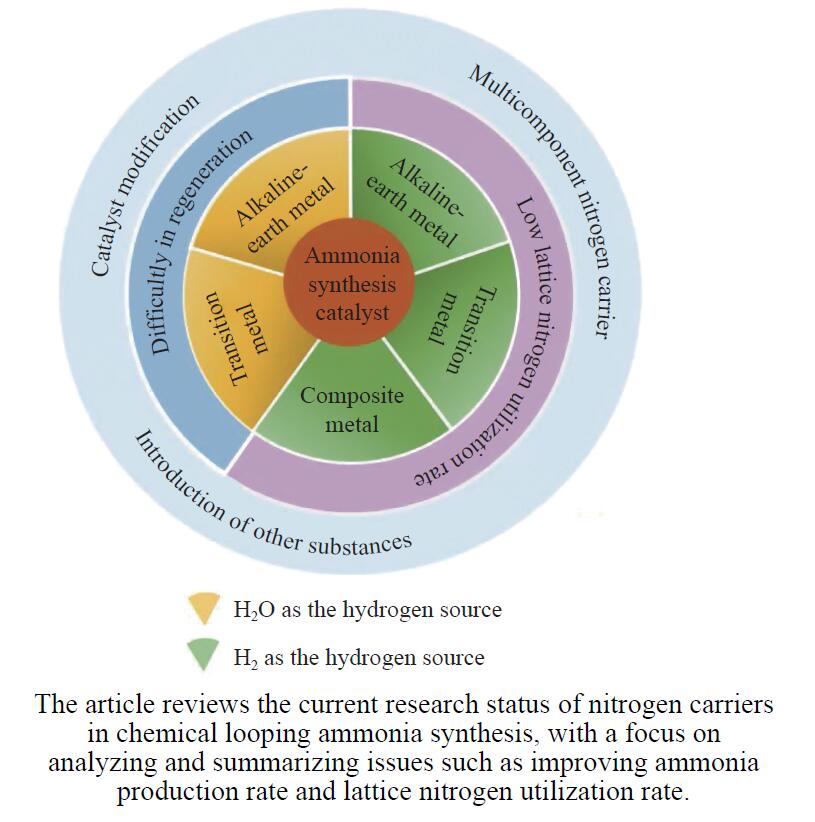
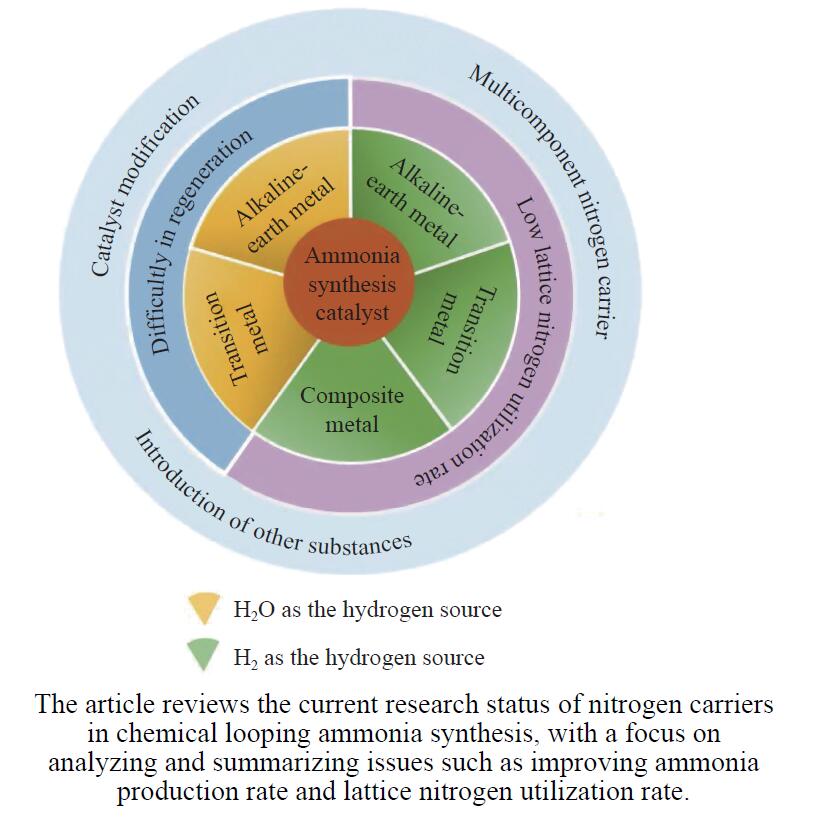
摘要: 氨不仅是氮肥生产的主要原料,也是可再生能源储存与转化过程中的能源载体之一。因此,开发温和条件下的合成氨技术是近年来重要的研究课题。化学链合成氨技术通过载氮体的传递作用,将合成氨反应解耦为固氮与释氨等多步反应,具有操作简便、反应温和、能耗低等优点。载氮体作为化学链合成氨的关键,起到传递能量及氮物种的作用,目前,载氮体固氮效率低,极大地限制了化学链合成氨技术的发展。基于此,本工作对化学链合成氨中载氮体的设计与应用研究进行综述。首先,对载氮体的设计理论进行了归纳与总结;其次,介绍了载氮体的研究现状,重点对如何提高载氮体的产氨速率以及如何提升晶格氮利用率等问题进行了综述;最后,对化学链合成氨技术所面临的机遇与挑战进行了研究,为今后载氮体的设计与开发提供了参考依据。Abstract: Ammonia is not only the main raw material of nitrogen fertilizer production, but also one of the energy carriers for the storage and conversion process of renewable energy. Therefore, the development of a mild ammonia synthesis technology has become an important research topic in recent years. The chemical looping ammonia synthesis technology decouples the ammonia synthesis reaction into several steps, including the nitrogen fixation and the ammonia release, which has the advantages of easy operation, mild reaction, and low energy consumption. As the key to the chemical looping ammonia synthesis, nitrogen carriers play the role of transferring energy and nitrogen species. However, the current low nitrogen fixation efficiency of nitrogen carriers severely limits the development of the chemical looping ammonia synthesis technology. Therefore, this article reviews the research on the design, preparation and application of nitrogen carriers for the chemical looping ammonia synthesis. Firstly, the design theory of nitrogen carrier is summarized; secondly, the current research status of nitrogen carrier is introduced, with a focus on how to improve the ammonia production rate of nitrogen carrier and the utilization rate of lattice nitrogen; finally, the opportunities and challenges of chemical looping ammonia synthesis technology are discussed, which provide a reference for the design and development of nitrogen carrier in the future.
-
Key words:
- ammonia synthesis /
- chemical looping /
- catalyst /
- reaction kinetics /
- transition metals /
- metal nitride
1) # These authors contributed to the work equally -
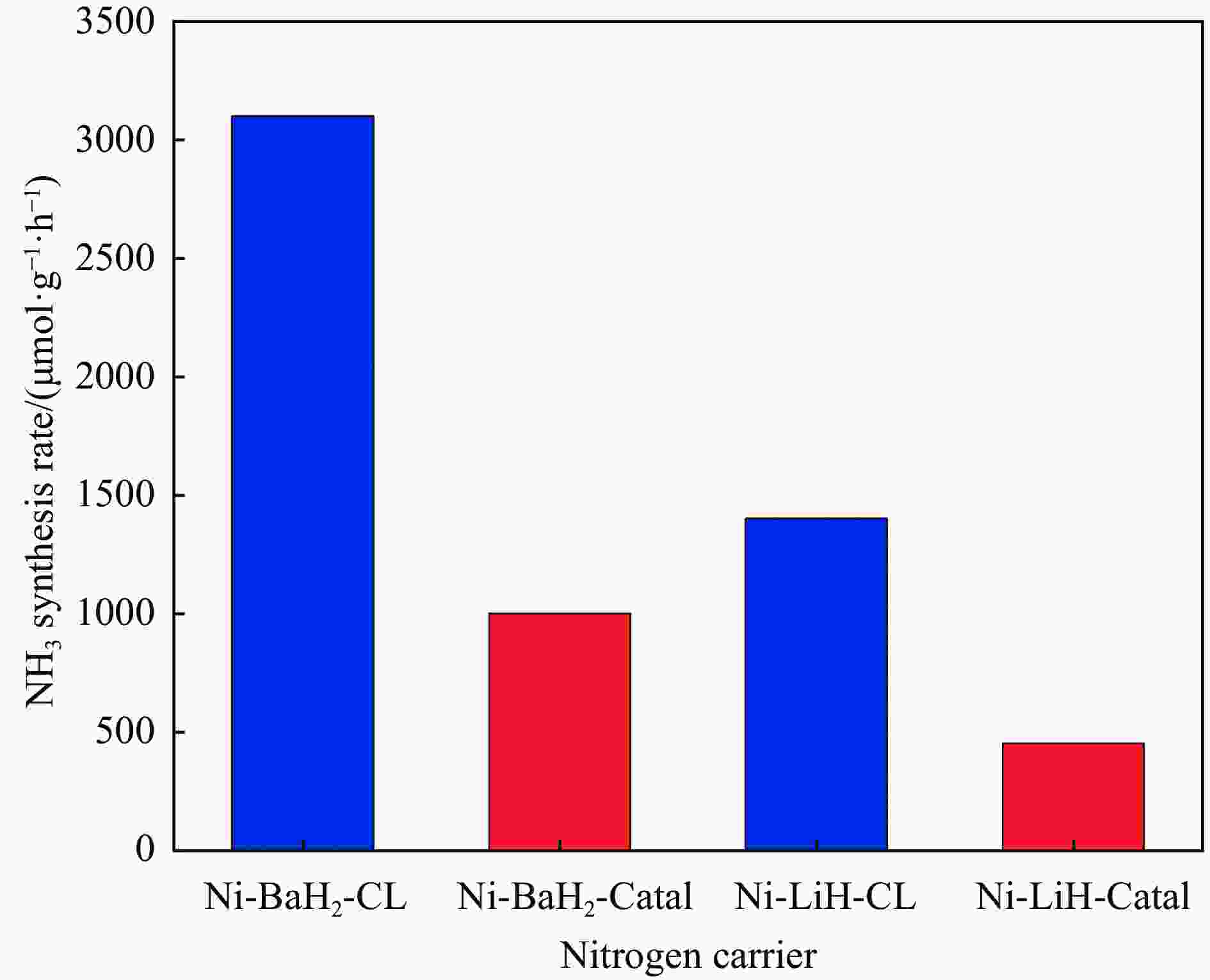
表 1 Ni催化剂对BaH2固氮加氢的影响
Table 1 Effect of Ni catalyst on nitrogen fixation and hydrogenation of BaH2
N2 fixation rate/
(μmol·g−1·h−1)NH3 production rate/
(μmol·g−1·h−1)BaH2 ≈3000 − 50%Ni-BaH2 ≈5800 − BaNH − ≈9000 Nitridized 50%Ni-BaH2 − ≈29000 −: Data not found in the literature. 表 2 常见载氮体涉及的化学链合成氨过程
Table 2 Common nitride-mediated CLAS
No. Nitrogen carrier
pairsNitrogen carrier
typeHydrogen source Reaction conditions Reaction rate Ref. 1 Li-Li3N-LiOH ionic nitride H2O electrolysis: 450 ℃
nitrogen fixation: 22−100 ℃
hydrolysis: 22−100 ℃, 0.5 h/12 h− [56] 2 Mg-Mg3N2-MgO ionic nitride H2O light source heating
reduction & hydrolysis:
pressure<100 mtorr
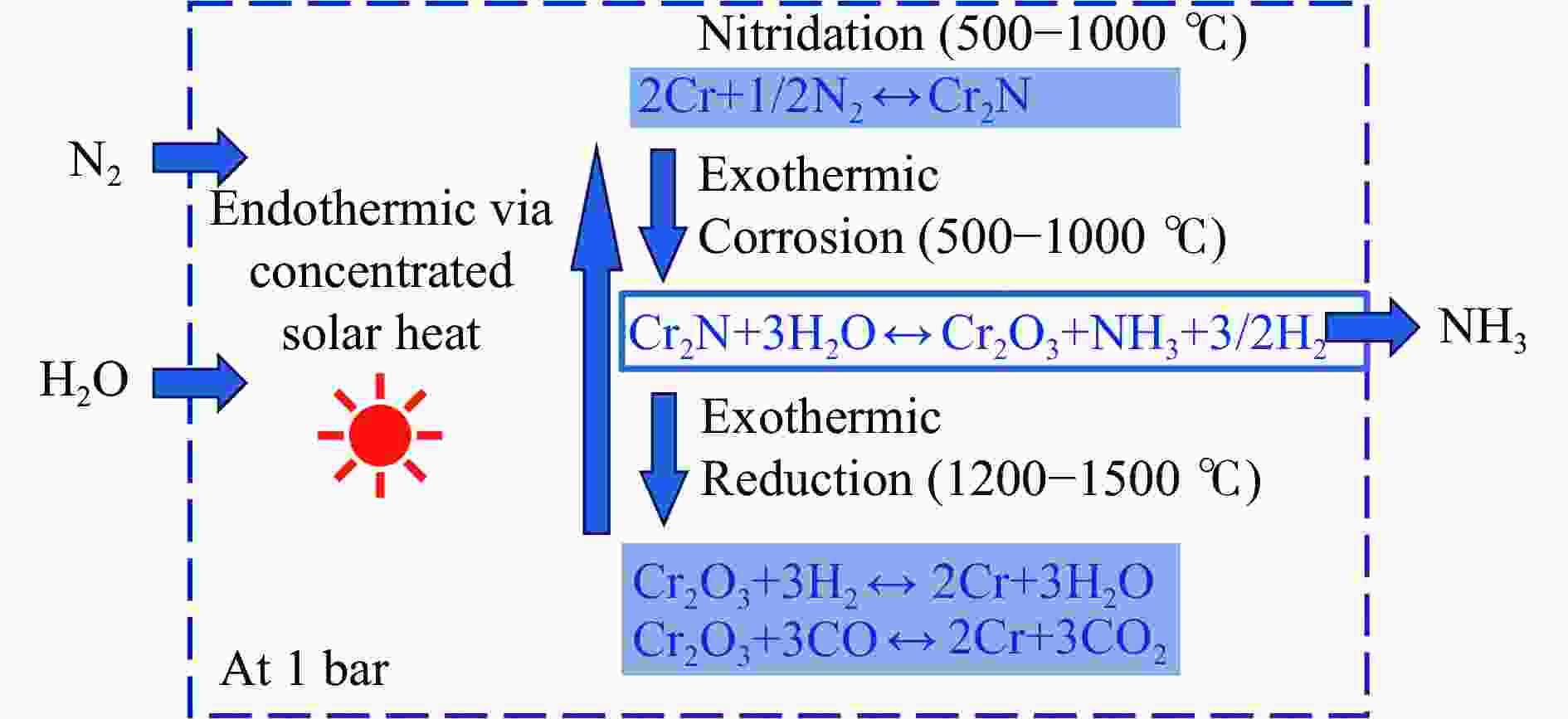
nitrogen fixation: 0.1 MPa1.67 μmol/(g·h) [60] 3 Cr2N-Cr2O3 transition metal nitride H2O nitrogen fixation: 983±40 ℃, 360 min
hydrolysis: 500−1600 ℃, 60 min
reduction: 800−1600 ℃, 30 min108 μmol/(g·h) [64] 4 MaNb-MaNb-d (Mn) transition metal nitride H2 nitrogen fixation: 700 ℃, 120 min
750 ℃, 240 min
hydrogenation: 300−1000 ℃, 60 min55.3 μmol/(g·h) [51] 5 Mo-Mo2N transition metal nitride H2 nitrogen fixation: 600 ℃, 60 min
hydrogenation: 400−600 ℃, 60 min4576 μmol/(g·h) [102] 6 MH2-MaNb (Ca, Sr) ionic nitride H2 nitrogen fixation: 200−1000 ℃,
240 min/420 min
hydrogenation: 300−1000 ℃, 60 minCa3N2: 98 μmol/(g·h)
Sr2N: 81 μmol/(g·h)[51] 7 BaH2-BaNH Ni metal Hydride H2 nitrogen fixation: 100−500 ℃, 300 min
hydrogenation: 100−500 ℃28800 μmol/(g·h) [94] −: Data not found in the literature. -
[1] 刘化章. 合成氨工业:过去、现在和未来-合成氨工业创立100周年回顾、启迪和挑战[J]. 化工进展,2013,32(9):1995−2005.LIU Huazhang. Ammonia synthesis industry: Past, present and future[J]. Chem Ind Eng Prog,2013,32(9):1995−2005. [2] LIU H Z. Ammonia synthesis catalyst 100 years: Practice, enlightenment and challenge[J]. Chin J Catal,2014,35(10):1619−1640. doi: 10.1016/S1872-2067(14)60118-2 [3] PFROMM P H. Towards sustainable agriculture: Fossil-free ammonia[J]. J Renew Sustainable Energy,2017,9(3):034702. doi: 10.1063/1.4985090 [4] 安广禄, 刘永忠, 康丽霞. 适应季节性氨需求的可再生能源合成氨系统优化设计[J]. 化工学报,2021,72(3):1595−1605.AN Guanglu, LIU Yongzhong, KANG Lixia. Optimal design of synthesis ammonia production system powered by renewable energy for seasonal demands of ammonia[J]. CIESC J,2021,72(3):1595−1605. [5] HAN G F, LI F, CHEN Z W, et al. Mechanochemistry for ammonia synthesis under mild conditions[J]. Nat Nanotechnol,2021,16:325−330. doi: 10.1038/s41565-020-00809-9 [6] KLERKE A, CHRISTENSEN C H, NØRSKOV J K, et al. Ammonia for hydrogen storage: challenges and opportunities[J]. J Mater Chem,2008,18(20):2304−2310. doi: 10.1039/b720020j [7] GUO J P, CHEN P. Catalyst: NH3 as an energy carrier[J]. Chem,2017,3(5):709−712. doi: 10.1016/j.chempr.2017.10.004 [8] ZAC C, MATTHEW I, RICHARD N L, et al. Ammonia to power: Forecasting the levelized cost of electricity from green ammonia in large-scale power plants[J]. Appl Energy,2021,282:116009. doi: 10.1016/j.apenergy.2020.116009 [9] GIDDEY S, BADWAL S P S, MUNNINGS C, et al. Ammonia as a renewable energy transportation media[J]. ACS Sustainable Chem Eng,2017,5(11):10231−10239. doi: 10.1021/acssuschemeng.7b02219 [10] 张谭, 刘光, 李晋平, 等. Ru基氮还原电催化剂性能调控策略[J]. 化工学报,2023,74(6):2264−2280.ZHANG Tan, LIU Guang, LI Jinping, et al. Performance regulation strategies of Ru-based nitrogen reduction electrocatalysts[J]. CIESC J,2023,74(6):2264−2280. [11] VOJVODIC A, MEDFORD A J, STUDT F, et al. Exploring the limits: A low-pressure, low-temperature Haber-Bosch process[J]. Chem Phys Lett,2014,598:108−112. doi: 10.1016/j.cplett.2014.03.003 [12] ERISMAN J W, SUTTON M A, GALLOWAY J, et al. How a century of ammonia synthesis changed the world[J]. Nat Geosci,2008,1:636−639. doi: 10.1038/ngeo325 [13] KITANO M, KANBARA S, INOUE Y, et al. Electride support boosts nitrogen dissociation over ruthenium catalyst and shifts the bottleneck in ammonia synthesis[J]. Nat Commun,2015,6:1−9. [14] LIU Z Y, YU Q B, WANG H, et al. Selecting nitrogen carriers used for chemical looping ammonia generation of biomass and H2O by thermodynamic method[J]. Int J Hydrogen Energy,2023,48(10):4035−4051. doi: 10.1016/j.ijhydene.2022.10.197 [15] AMAR I A, PETIT C T G, MANN G, et al. Electrochemical synthesis of ammonia from N2 and H2O based on (Li, Na, K)2CO3–Ce0.8Gd0.18Ca0.02O2-δ composite electrolyte and CoFe2O4 cathode[J]. Int J Hydrogen Energy,2014,39(9):4322−4330. doi: 10.1016/j.ijhydene.2013.12.177 [16] YE D P, TSANG S C E. Prospects and challenges of green ammonia synthesis[J]. Nat Synth,2023,2:612−623. doi: 10.1038/s44160-023-00321-7 [17] NAKAO T, TADA T, HOSONO H. Transition metal-doped Ru nanoparticles loaded on metal hydrides for efficient ammonia synthesis from first principles[J]. J Phys Chem C,2020,124(2):1529−1534. doi: 10.1021/acs.jpcc.9b10544 [18] 赵斐, 王琪, 刘光, 等. d区过渡金属基催化剂用于电化学合成氨[J]. 化工进展,2021,40(4):1948−1965.ZHAO Fei, WANG Qi, LIU Guang, et al. d-Block transition metal-based catalysts for electrocatalytic ammonia synthesis[J]. Chem Ind Eng Prog,2021,40(4):1948−1965. [19] 任晓玲, 严孝清, 龚湘姣, 等. 光(电)催化氮气还原合成氨研究进展[J]. 化工进展,2020,39(12):4856−4876. doi: 10.16085/j.issn.1000-6613.2020-0942REN Xiaoling, YAN Xiaoqing, GONG Xiangjiao, et al. Overview on photo(electro) catalytic nitrogen fixation for ammonia synthesis[J]. Chem Ind Eng Prog,2020,39(12):4856−4876. doi: 10.16085/j.issn.1000-6613.2020-0942 [20] 张婷, 孙晓红, 于宏兵, 等. 电催化氮气还原合成氨反应中抑制水解析氢竞争的研究进展[J]. 化工进展,2021,40(12):6670−6687. doi: 10.16085/j.issn.1000-6613.2020-2520ZHANG Ting, SUO Xiaohong, YU Hongbing, et al. Research progress of inhibiting hydrogen evolution in electro-catalytic ammonia synthesis[J]. Chem Ind Eng Prog,2021,40(12):6670−6687. doi: 10.16085/j.issn.1000-6613.2020-2520 [21] DUAN G Y, CHEN Y M, TANG Y, et al. Advances in electrocatalytic ammonia synthesis under mild conditions[J]. Prog Energy Combust Sci,2020,81:100860. doi: 10.1016/j.pecs.2020.100860 [22] LONG J, CHEN S M, ZHANG Y L, et al. Direct electrochemical ammonia synthesis from nitric oxide[J]. Angew Chem Int Ed,2020,59:9711−9718. doi: 10.1002/anie.202002337 [23] WU T T, FAN W J, ZHANG Y, et al. Electrochemical synthesis of ammonia: Progress and challenges[J]. Mater Today Phys,2021,16:100310. doi: 10.1016/j.mtphys.2020.100310 [24] 郭建平, 陈萍. 多相化学合成氨研究进展[J]. 科学通报,2019,64(11):1114−1128. doi: 10.1360/N972019-00079GUO Jianping, CHEN Ping. Recent progress in heterogeneous ammonia synthesis[J]. Chin Sci Bull,2019,64(11):1114−1128. doi: 10.1360/N972019-00079 [25] XIONG C H, WU Y, FENG M Q, et al. High thermal stability Si-Al based N-carrier for efficient and stable chemical looping ammonia generation[J]. Appl Energy, 2022, 119519. [26] KOJIMA R, AIKA K-I. Cobalt molybdenum bimetallic nitride catalysts for ammonia synthesis Part 1. Preparation and characterization[J]. Appl Catal A: Gen,2001,215:149−160. doi: 10.1016/S0926-860X(01)00529-4 [27] SCHOLTEN J J F, ZWIETERING P, KONVALINKA J A, et al. Chemisorption of nitrogen on iron catalysts in connection with ammonia synthesis. Part 1.-The kinetics of the adsorption and desorption of nitrogen[J]. Transa Faraday Soc,1959,55:2166−2179. doi: 10.1039/TF9595502166 [28] HOFFMAN B M, LUKOYANOV D, DEAN D R, et al. Nitrogenase: A draft mechanism[J]. Acc Chem Res,2013,46(2):587−595. doi: 10.1021/ar300267m [29] ABILD-PEDERSEN F, GREELEY J, STUDT F, et al. Scaling properties of adsorption energies for hydrogen-containing molecules on transition-metal surfaces[J]. Phy Rev Lett,2007,99(1):016105. doi: 10.1103/PhysRevLett.99.016105 [30] HOSONO H. Spiers memorial lecture: Catalytic activation of molecular nitrogen for green ammonia synthesis: introduction and current status[J]. Faraday Discuss,2023,243:9−26. doi: 10.1039/d3fd00070b [31] HAO Q, LIU C W, JIA G H, et al. Catalytic reduction of nitrogen to produce ammonia by bismuth-based catalysts: State of the art and future prospects[J]. Mater Horiz,2020,7(4):1014−1029. doi: 10.1039/C9MH01668F [32] ZHANG X L, YE Y L, ZHANG L, et al. Designing an alkali-metal-like superatom Ca3B for ambient nitrogen reduction to ammonia[J]. Phys Chem Chem Phys,2021,23:18908−18915. doi: 10.1039/D1CP01533H [33] JIA H P, QUADRELLI E A. Mechanistic aspects of dinitrogen cleavage and hydrogenation to produce ammonia in catalysis and organometallic chemistry: Relevance of metal hydride bonds and dihydrogen[J]. Chem Soc Rev,2014,43(2):547−564. doi: 10.1039/C3CS60206K [34] 刘永卓, 郭庆杰. 化学链基础理论及其在节能减排中的应用[J]. 工程研究-跨学科视野中的工程,2015,7(4):404−412. doi: 10.3724/SP.J.1224.2015.00404LIU Yongzhuo, GUO Qingjie. Fundamental principles and its appliation of chemical looping in the filed of energy-saving and emission reduction[J]. J Eng Stud,2015,7(4):404−412. doi: 10.3724/SP.J.1224.2015.00404 [35] 史晓斐, 杨思宇, 钱宇. 化学链技术在煤炭清洁高效利用中的研究进展[J]. 化工学报,2018,69(12):4931−4946.SHI Xiaofei, YANG Siyu, QIAN Yu. Chemical looping technology for clean and highly effcient coal processes[J]. CIESC J,2018,69(12):4931−4946. [36] MICHALSKY R, PARMAN B J, AMANOR-BOADU V, et al. Solar thermochemical production of ammonia from water, air and sunlight: Thermodynamic and economic analyses[J]. Energy,2012,42(1):251−260. doi: 10.1016/j.energy.2012.03.062 [37] BARTEL C J, RUMPTZ J R, WEIMER A W, et al. High-throughput equilibrium analysis of active materials for solar thermochemical ammonia synthesis[J]. ACS Appl Mater Int,2019,11(28):24850−24858. doi: 10.1021/acsami.9b01242 [38] GAO W B, WANG R Z, FENG S, et al. Thermodynamic and kinetic considerations of nitrogen carriers for chemical looping ammonia synthesis[J]. Disc Chem Eng, 2023, 3(1): 1−16. [39] GREGORY D H. Structural families in nitride chemistry[J]. J Chem Soc, Dalton Trans,1999,(3):259−270. doi: 10.1039/a807732k [40] WU Z G, CHEN X J, STRUZHKIN V V, et al. Trends in elasticity and electronic structure of transition-metal nitrides and carbides from first principles[J]. Phys Rev B,2005,71:214103. doi: 10.1103/PhysRevB.71.214103 [41] MICHALSKY R, PFROMM P H. Thermodynamics of metal reactants for ammonia synthesis from steam, nitrogen and biomass at atmospheric pressure[J]. AIChE J,2012,58(10):3203−3213. doi: 10.1002/aic.13717 [42] JACOBSEN C J, DAHL S, CLAUSEN B S, et al. Catalyst design by interpolation in the periodic table: bimetallic ammonia synthesis catalysts[J]. J Am Chem Soc,2001,123:8404−8405. doi: 10.1021/ja010963d [43] ALEXANDER A M, HARGREAVES J S J, MITCHELL C. The reduction of various nitrides under hydrogen: Ni3N, Cu3N, Zn3N2 and Ta3N5[J]. Top Catal,2012,55:1046−1053. doi: 10.1007/s11244-012-9890-3 [44] ZEINALIPOUR-YAZDI C D, HARGREAVES J S J, CATLOW C R A. Nitrogen activation in a mars-van krevelen mechanism for ammonia synthesis on Co3Mo3N[J]. J Phys Chem C,2015,119(51):28368−28376. doi: 10.1021/acs.jpcc.5b06811 [45] MCKAY D, GREGORY D H, HARGREAVES J S J, et al. Towards nitrogen transfer catalysis: Reactive lattice nitrogen in cobalt molybdenum nitride[J]. Chem Commun,2007,29:3051−3053. [46] HUNTER S M, GREGORY D H, HARGREAVES J S J, et al. A study of 15N/14N isotopic exchange over cobalt molybdenum nitrides[J]. ACS Catal,2013,3(8):1719−1725. doi: 10.1021/cs400336z [47] MICHALSKY R, PFROMM P H. An ionicity rationale to design solid phase metal nitride reactants for solar ammonia production[J]. J Phys Chem C,2012,116(44):23243−23251. doi: 10.1021/jp307382r [48] MICHALSKY R, PFROMM P H, STEINFELD A. Rational design of metal nitride redox materials for solar-driven ammonia synthesis[J]. Interface Focus, 2015, 5(3): 20140084. [49] YE T N, PARK S W, LU Y F, et al. Contribution of nitrogen vacancies to ammonia synthesis over metal nitride catalysts[J]. J Am Chem Soc,2020,142(33):14374−14383. doi: 10.1021/jacs.0c06624 [50] CAO A, BUKAS V J, SHADRAVAN V, et al. A spin promotion effect in catalytic ammonia synthesis[J]. Nat Commun, 2022, 13: 2382. [51] MICHALSKY R, AVRAM A M, PETERSON B A, et al. Chemical looping of metal nitride catalysts: Low-pressure ammonia synthesis for energy storage[J]. Chem Sci,2015,6:3965−3974. doi: 10.1039/C5SC00789E [52] 吴烨, 冯鸣谦, 方婧, 等. 化学链合成氨技术研究进展及展望[J]. 洁净煤技术,2021,27(2):92−106. doi: 10.13226/j.issn.1006-6772.CCUS20091401WU Ye, FENG Mingqian, FANG Jing, et al. Research progress and prospect of chemical looping ammonia synthesis technology[J]. Clean Coal Technol,2021,27(2):92−106. doi: 10.13226/j.issn.1006-6772.CCUS20091401 [53] WANG Q R, GUO J P, CHEN P. Recent progress towards mild-condition ammonia synthesis[J]. J Energy Chem,2019,36:25−36. doi: 10.1016/j.jechem.2019.01.027 [54] 段一菲, 陈存壮, 张军社, 等. 化学链小分子转化研究进展[J]. 中国科学: 化学,2020,50(3):337−365.DUAN Yifei, CHEN Cunzhuang, ZHANG Junshe, et al. Research progress in chemical chain small molecule transformation[J]. Sci Chi Chem,2020,50(3):337−365. [55] 冯圣, 高文波, 曹湖军, 等. 化学链合成氨研究进展[J]. 化学学报,2020,78(9):916−927. doi: 10.6023/A20060207FENG Sheng, GAO Wenbo, CAO Hujun, et al. Advances in the chemical looping ammonia synthesis[J]. Acta Chim Sin,2020,78(9):916−927. doi: 10.6023/A20060207 [56] MCENANEY J M, SINGH A R, SCHWALBE J A, et al. Ammonia synthesis from N2 and H2O using a lithium cycling electrification strategy at atmospheric pressure[J]. Energy Environ Sci,2017,10(7):1621−1630. doi: 10.1039/C7EE01126A [57] ZHU D, ZHANG L H, RUTHER R E, et al. Photo-illuminated diamond as a solid-state source of solvated electrons in water for nitrogen reduction[J]. Nat Mater,2013,12(9):836−841. doi: 10.1038/nmat3696 [58] MONTOYA J H, TSAI C, VOJVODIC A, et al. The challenge of electrochemical ammonia synthesis: A new perspective on the role of nitrogen scaling relations[J]. ChemSusChem,2015,8(13):2180−2186. doi: 10.1002/cssc.201500322 [59] KŐLELI F, KAYAN D B. Low overpotential reduction of dinitrogen to ammonia in aqueous media[J]. J Electroanal Chem,2010,638(1):119−122. doi: 10.1016/j.jelechem.2009.10.010 [60] SWEARER D F, KNOWLES N R, EVERITT H O, et al. Light-driven chemical looping for ammonia synthesis[J]. ACS Energy Lett,2019,4(7):1505−1512. doi: 10.1021/acsenergylett.9b00860 [61] HEIDLAGE M G, KEZAR E A, SNOW K C, et al. Thermochemical synthesis of ammonia and syngas from natural gas at atmospheric pressure[J]. Ind Eng Chem Res,2017,56(47):14014−14024. doi: 10.1021/acs.iecr.7b03173 [62] FU E K, GONG F, WANG S, et al. Ni-Mn-N derived composite nitrogen carriers for enhanced chemical looping ammonia production[J]. Fuel Process Technol, 2023, 252: 107971. [63] WISE R S, MARKEL E J. Synthesis of high surface area molybdenum nit ride in mixtures of nitrogen and hydrogen[J]. J Catal,1994,145:344−355. doi: 10.1006/jcat.1994.1043 [64] MICHALSKY R, PFROMM P H. Chromium as reactant for solar thermochemical synthesis of ammonia from steam, nitrogen, and biomass at atmospheric pressure[J]. Sol Energy,2011,85(11):2642−2654. doi: 10.1016/j.solener.2011.08.005 [65] GÁLVEZ M E, HALMANN M, STEINFELD A. Ammonia production via a two-step Al2O3/AlN thermochemical cycle. 1. Thermodynamic, environmental, and economic analyses[J]. Ind Eng Chem Res,2007,46(7):2042−2046. doi: 10.1021/ie061550u [66] MOLISANI A L, YOSHIMURA H N. Low-temperature synthesis of AlN powder with multicomponent additive systems by carbothermal reduction-nitridation method[J]. Mater Res Bull,2010,45(6):733−738. doi: 10.1016/j.materresbull.2010.02.012 [67] GAO Y, WU Y, ZHANG Q, et al. N-desorption or NH3 generation of TiO2-loaded Al-based nitrogen carrier during chemical looping ammonia generation technology[J]. Int J Hydrogen Energy,2018,43(34):16589−16597. doi: 10.1016/j.ijhydene.2018.07.042 [68] FU R L, ZHOU H P, CHEN L, et al. Morphologies and growth mechanisms of aluminum nitride whiskers synthesized by carbothermal reduction[J]. Mater Sci Eng A,1999,266(1/2):44−51. doi: 10.1016/S0921-5093(99)00047-7 [69] MAO X X, LI J, ZHANG H L, et al. Synthesis of AlN powder by carbothermal reduction-nitridation of alumina/carbon black foam[J]. J Inorg Mater,2017,32(10):1115−1120. doi: 10.15541/jim20170067 [70] IDE T, KOMEYA K, MEGURO T, et al. Synthesis of AlN powder by carbothermal reduction-nitridation of various Al2O3 powders with CaF2[J]. J Am Ceram Soc,1999,82(11):2993−2998. [71] ELAGIN A A, BEKETOV A R, BARANOV M V, et al. Aluminum nitride. Preparation methods[J]. Refract Ind Ceram,2013,53(6):395−403. doi: 10.1007/s11148-013-9534-6 [72] 秦明礼, 曲选辉, 林健凉, 等. 碳热还原法制备氮化铝陶瓷粉末的研究[J]. 材料导报,2001,(7):56−59. doi: 10.3321/j.issn:1005-023X.2001.07.020QIN Mingli, QU Xuanhui, LIN Jianliang, et al. Research on synthesis of aluminum nitride power by carbothermal reduction method[J]. Mater Rep,2001,(7):56−59. doi: 10.3321/j.issn:1005-023X.2001.07.020 [73] WANG B Y, GUO H X, YIN X L, et al. N-sorption capability of Al2O3-supported Mn-/Fe-based nitrogen carriers during chemical looping ammonia synthesis technology[J]. Energy Fuels,2020,34(8):10247−10255. doi: 10.1021/acs.energyfuels.0c01000 [74] ZHANG Q, WU Y, GAO Y, et al. High-performance mesoporous (AlN/Al2O3) for enhanced NH3 yield during chemical looping ammonia generation technology[J]. Int J Hydrogen Energy,2020,45(16):9903−9913. doi: 10.1016/j.ijhydene.2020.01.172 [75] FENG M Q, ZHANG Q, WU Y, et al. Using coal coke for N-sorption with an Al-based nitrogen carrier during chemical looping ammonia generation[J]. Energy Fuels,2020,34(10):12527−12534. doi: 10.1021/acs.energyfuels.0c02733 [76] BARTEL C J, MUHICH C L, WEIMER A W, et al. Aluminum nitride hydrolysis enabled by hydroxyl-mediated surface proton hopping[J]. ACS Appl Mater Inter,2016,8(28):18550−18559. doi: 10.1021/acsami.6b04375 [77] 刘化章, 胡樟能, 李小年, 等. FeO基氨合成催化剂[J]. 化工学报,1994,45(4):385−392.LIU Huazhang, HU Zhangneng, LI Xiaonian, et al. FeO based catalyst for ammonia synthesis[J]. CIESC J,1994,45(4):385−392. [78] WU Y, JIANG G D, ZHANG H B, et al. Fe2O3, a cost effective and environmentally friendly catalyst for the generation of NH3-a future fuel-using a new Al2O3-looping based technology[J]. Chem Commun,2017,53(77):10664−10667. doi: 10.1039/C7CC04742H [79] ARAMEND A M, BORÁU V, JIMÉNEZ C, et al. Synthesis and characterization of ZrO2 as acid-basic catalysts: Reactivity of 2-methyl-3-butyn-2-ol[J]. J Catal,1999,183(2):240−250. doi: 10.1006/jcat.1999.2418 [80] WANG Z Q, MA Y C, LIN J X. Ruthenium catalyst supported on high-surface-area basic ZrO2 for ammonia synthesis[J]. J Mol Catal A: Chem,2013,378:307−313. doi: 10.1016/j.molcata.2013.07.003 [81] ZHANG Y J, ZHAN Y Y, CAO Y N, et al. Low-temperature water-gas shift reaction over Au/ZrO2 catalysts using hydrothermally synthesized zirconia as supports[J]. Chin J Catal,2012,33:230−236. doi: 10.1016/S1872-2067(11)60327-6 [82] WU Y, GAO Y, ZHANG Q, et al. Promising zirconia-mixed Al-based nitrogen carriers for chemical looping of NH3: Reduced NH3 decomposition and improved NH3 yield[J]. Fuel,2020,264:116821. doi: 10.1016/j.fuel.2019.116821 [83] YAMAGUCHI S, ICHIKAWA T, WANG Y M, et al. Nitrogen dissociation via reaction with lithium alloys[J]. ACS Omega,2017,2(3):1081−1088. doi: 10.1021/acsomega.6b00498 [84] GOSHOME K, MIYAOKA H, YAMAMOTO H, et al. Ammonia synthesis via non-equilibrium reaction of lithium nitride in hydrogen flow condition[J]. Mater Trans,2015,56(3):1081−1088. [85] ZHANG T F, ISOBE S, JAIN A, et al. Enhancement of hydrogen desorption kinetics in magnesium hydride by doping with lithium metatitanate[J]. J Alloys Compd,2017,711:400−405. doi: 10.1016/j.jallcom.2017.03.361 [86] BAIK Y J, KWEN M, LEE K, et al. Splitting of hydrogen atoms proton-electron pairs at bao-Ru interfaces for promoting ammonia synthesis under mild conditions[J]. J AM Chem Soc,2023,145:11364−11374. doi: 10.1021/jacs.3c02529 [87] LAASSIRI S, ZEINALIPOUR-YAZDI C D, CATLOW C R A, et al. The potential of manganese nitride based materials as nitrogen transfer reagents for nitrogen chemical looping[J]. Appl Catal B: Environ,2018,223:60−66. doi: 10.1016/j.apcatb.2017.04.073 [88] RAI R K, MAKSOUD W A M, MORLANÉS N, et al. Iron-cobalt-based materials: An efficient bimetallic catalyst for ammonia synthesis at low temperatures[J]. ACS Catal,2022,12:587−599. doi: 10.1021/acscatal.1c05078 [89] WANG S, GONG F, ZHOU Q, et al. Transition metal enhanced chromium nitride as composite nitrogen carrier for sustainable chemical looping ammonia synthesis[J]. Appl Catal B: Environ, 2023, 339: 123134. [90] YANG S, ZHANG T, YANG Y Y, et al. Molybdenum based nitrogen carrier for ammonia production via a chemical looping route[J]. Appl Catal B: Environ, 2022, 312: 121404. [91] 张谭, 余钟亮, 余嘉琪, 等. 基于高性能负载型钼基载氮体的化学链合成氨性能研究[J]. 化学学报,2022,80(6):788−796. doi: 10.6023/A22010057ZHANG Tan, YU Zhongliang, YU Jiaqi, et al. Chemical looping ammonia synthesis with high performance supported molybdenum-based nitrogen carrier[J]. Acta Chim Sin,2022,80(6):788−796. doi: 10.6023/A22010057 [92] BROWN S W, ROBINSON B, WANG Y X, et al. Microwave heated chemical looping ammonia synthesis over Fe and CoMo particles[J]. J Mater Chem A,2022,10:15497−15507. doi: 10.1039/D2TA03241D [93] GOTO Y, DAISLEY A, HARGREAVES J S J. Towards anti-perovskite nitrides as potential nitrogen storage materials for chemical looping ammonia production: Reduction of Co3ZnN, Ni3ZnN, Co3InN and Ni3InN under hydrogen[J]. Catal Today,2021,364:196−201. doi: 10.1016/j.cattod.2020.03.022 [94] GAO W B, GUO J P, WANG P K, et al. Production of ammonia via a chemical looping process based on metal imides as nitrogen carriers[J]. Nat Energy,2018,3(12):1067−1075. doi: 10.1038/s41560-018-0268-z [95] CHEN P, XIONG Z T, LUO J Z, et al. Interaction of hydrogen with metal nitrides and imides[J]. Nature,2002,420(6913):302−304. doi: 10.1038/nature01210 [96] GAO W B, WANG P K, GUO J P, et al. Barium hydride-mediated nitrogen transfer and hydrogenation for ammonia synthesis: A case study of cobalt[J]. ACS Catal,2017,7(5):3654−3661. doi: 10.1021/acscatal.7b00284 [97] TAGAWA K, GI H, SHINZATO K, et al. Improvement of kinetics of ammonia synthesis at ambient pressure by the chemical looping process of lithium hydride[J]. J Phys Chem C,2022,126(5):2403−2409. doi: 10.1021/acs.jpcc.1c09902 [98] HAGEN S, BARFOD R, FEHRMANN R, et al. Ammonia synthesis with barium-promoted iron-cobalt alloys supported on carbon[J]. J Catal,2003,214(2):327−335. doi: 10.1016/S0021-9517(02)00182-3 [99] LIU T, TEMPRANO I, JENKINS S J, et al. Low temperature synthesis of NH3 from atomic N and H at the surfaces of FeS2{100} crystals[J]. J Phys Chem C,2013,117(21):10990−10998. doi: 10.1021/jp308872y [100] MEDFORD A J, WELLENDORFF J, VOJVODIC A, et al. Assessing the reliability of calculated catalytic ammonia synthesis rates[J]. Science,2014,345(6193):197−200. doi: 10.1126/science.1253486 [101] LI L L, ZHANG T H, CAI J H, et al. Operando spectroscopic and isotopic-label-directed observation of LaN-promoted Ru/ZrH2 catalyst for ammonia synthesis via associative and chemical looping route[J]. J Catal,2020,389:218−228. doi: 10.1016/j.jcat.2020.05.039 [102] 张谭. 钼基载氮体涉及的化学链合成氨反应特性研究[D]. 太原: 太原理工大学, 2022.ZHANG Tan. Study on reaction characteristics of ammonia synthesis by chemical looping of molybdenum based nitrogen carriers[D]. Taiyuan: Taiyuan University of Technology, 2022. -



 下载:
下载: