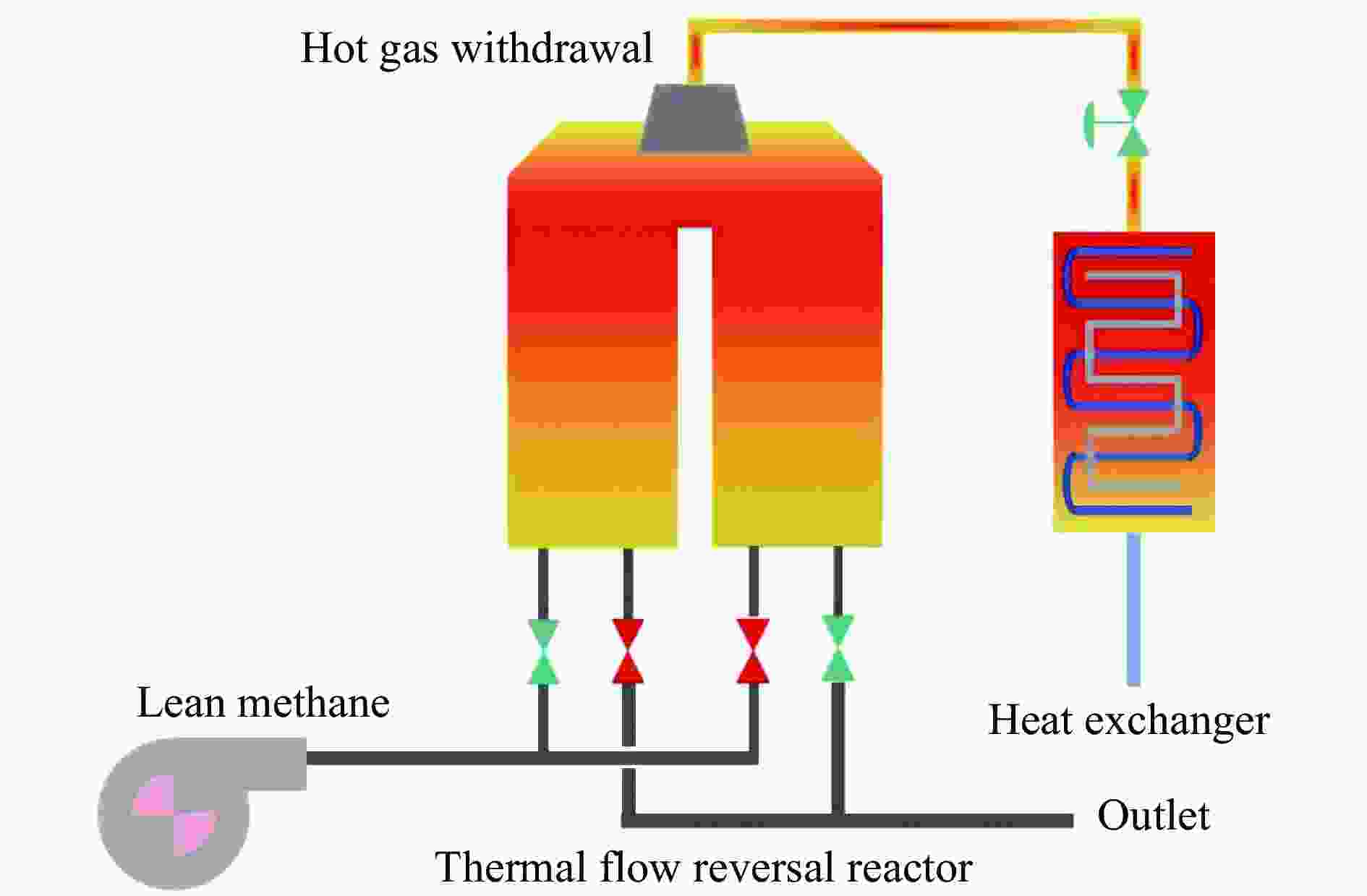
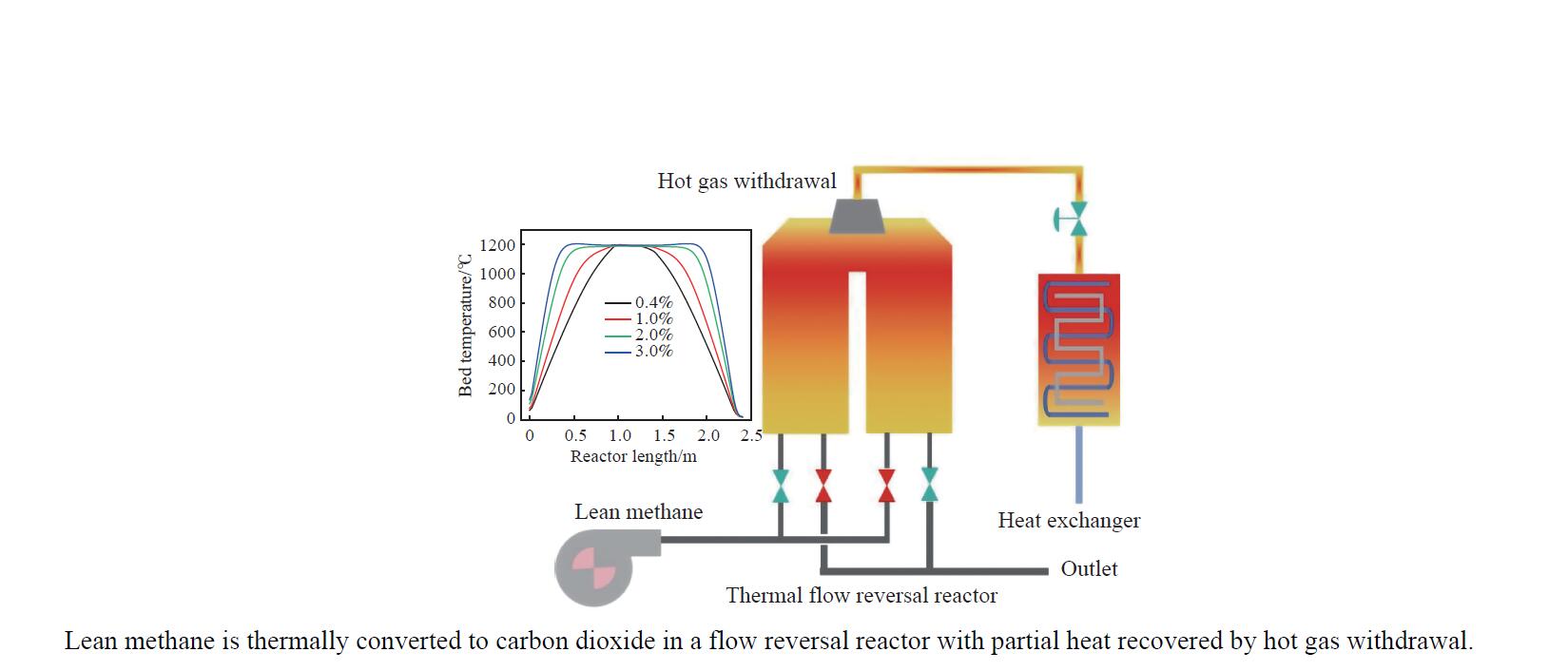
Dynamic behaviors and heat recovery with hot gas withdrawal of flow reversal reactor for thermal oxidation of lean methane
-
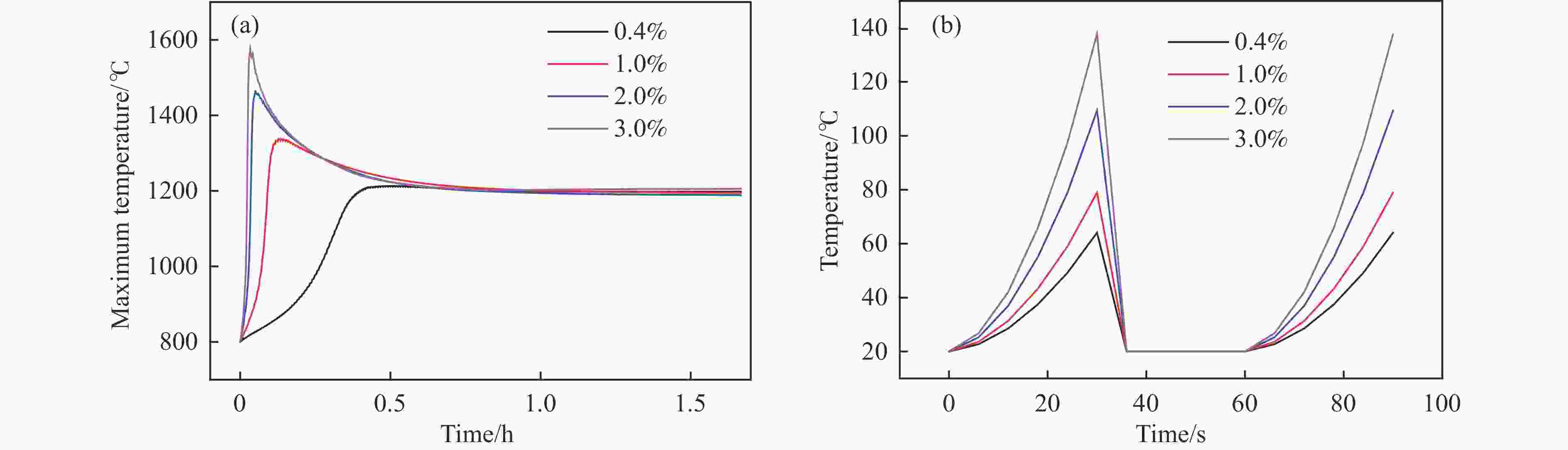
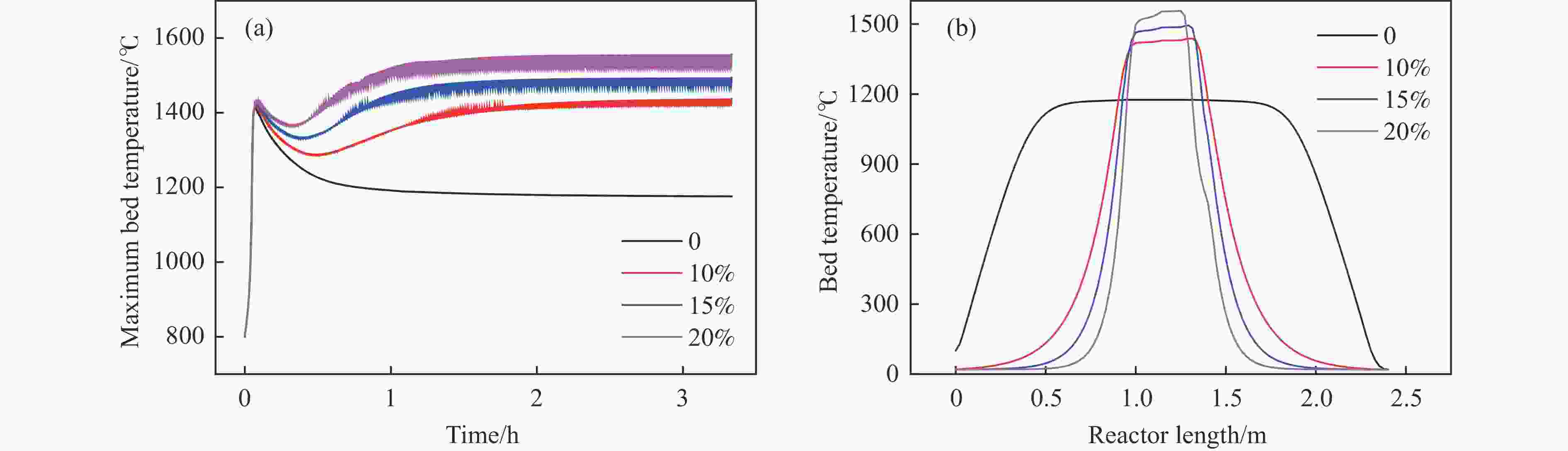
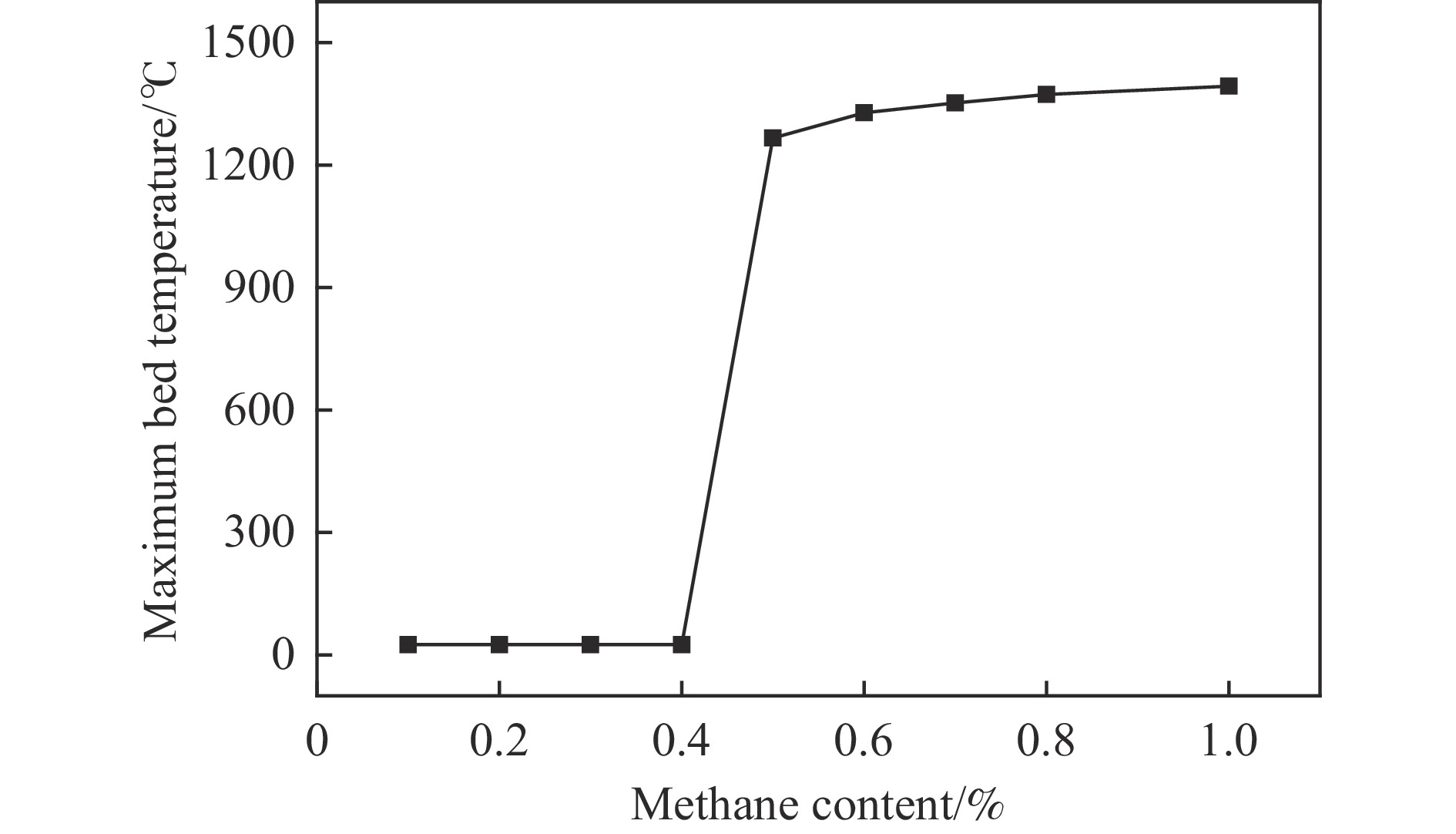
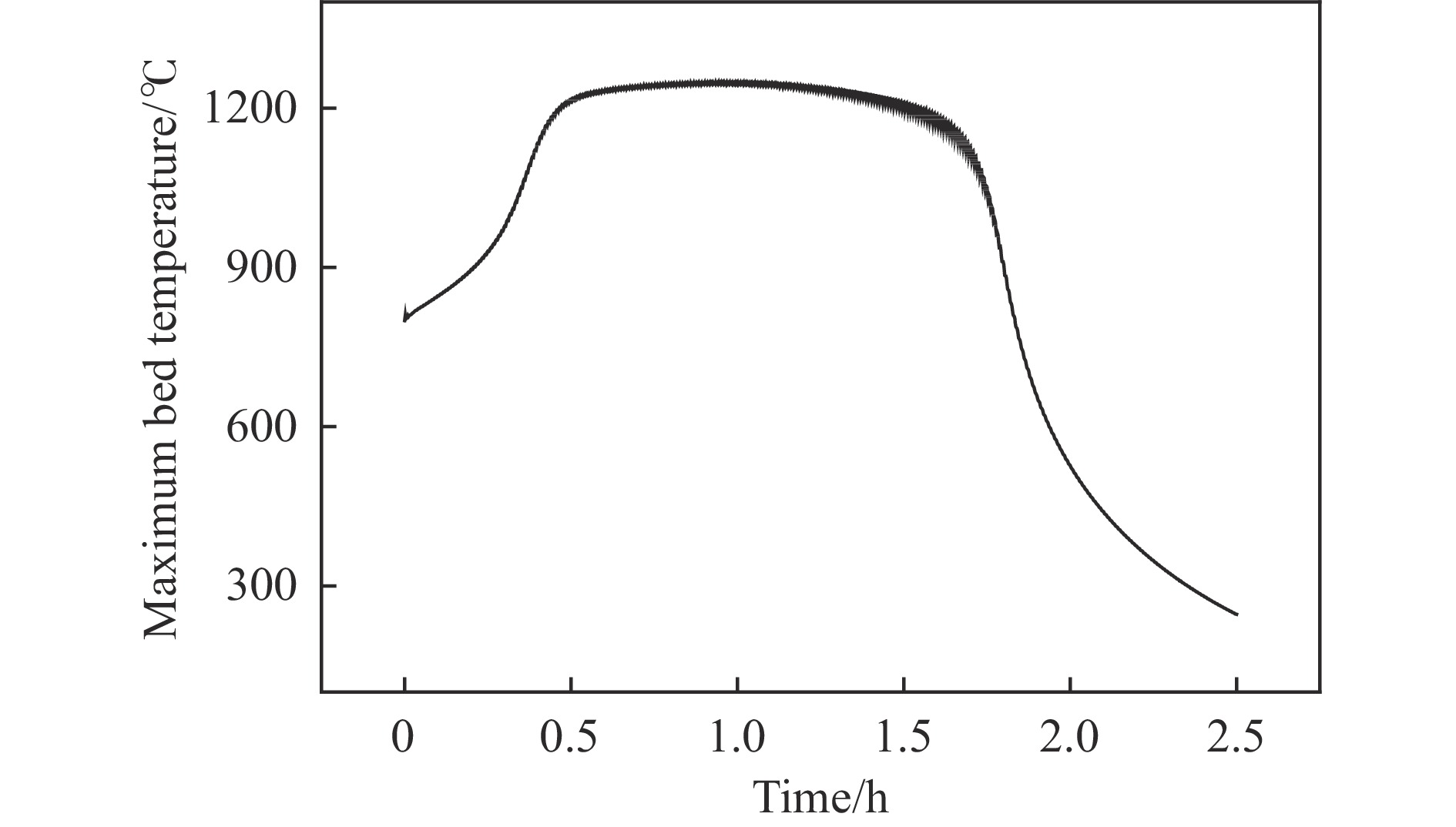
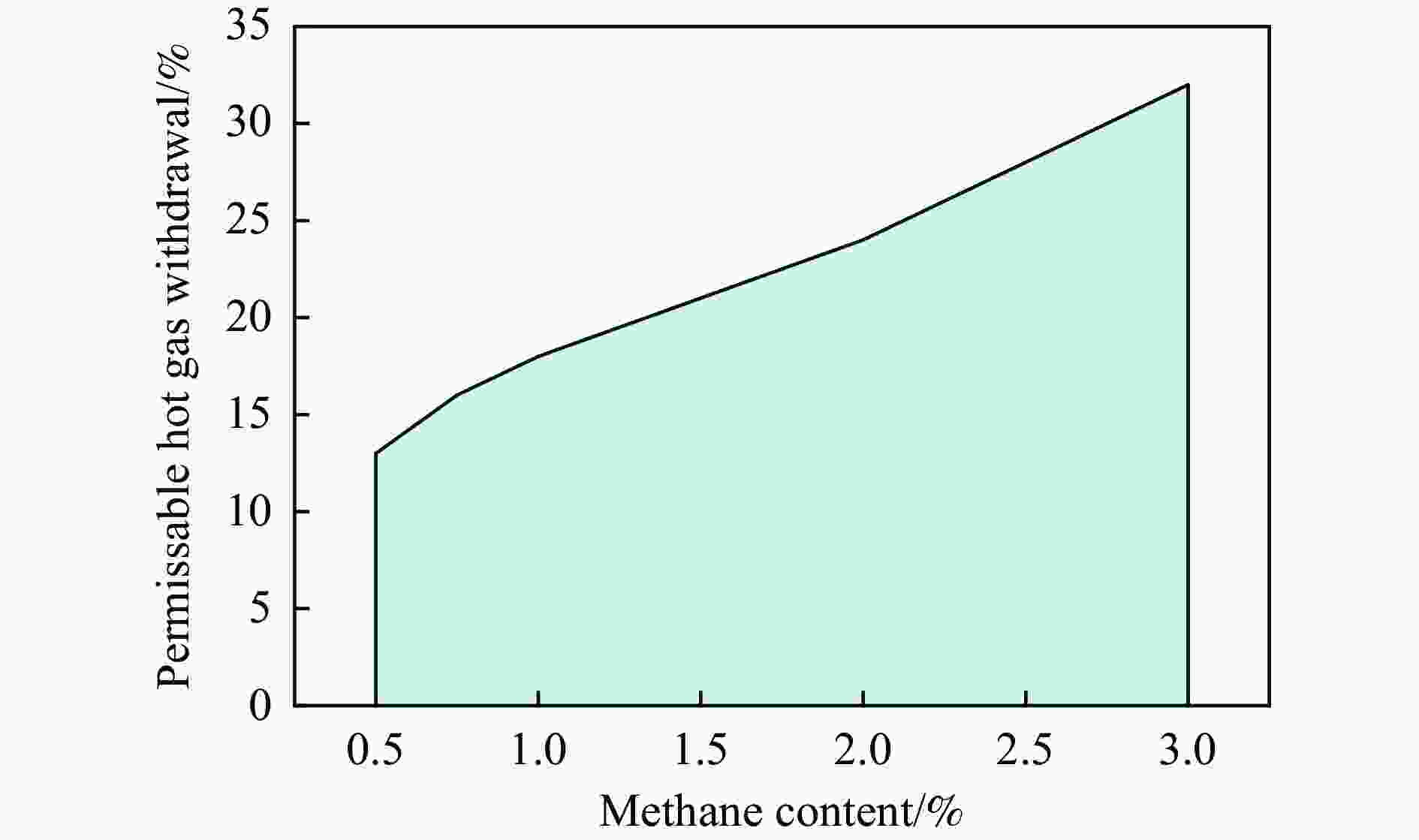
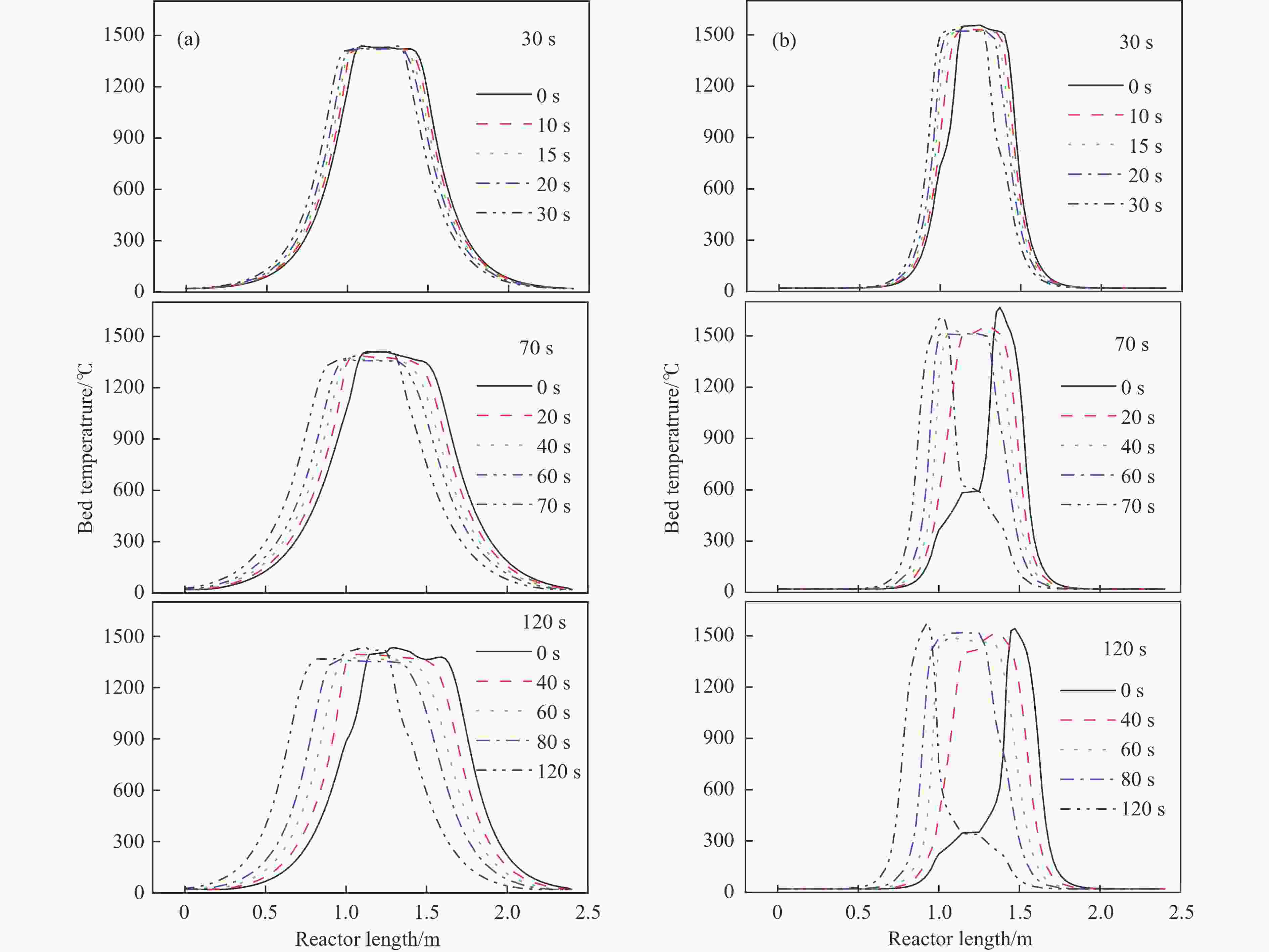
摘要: 废弃煤矿的低体积分数甲烷(1%−3%)通常被直接排放到大气中,但其较高的升温潜势带来了严重的环境问题。在流向变换反应器中直接热氧化甲烷是一种有吸引力的解决方案,但潜在的爆炸和不稳定燃烧等风险限制了其应用。阐明低含量甲烷在流向变换反应器中热氧化的动力学行为是开发工业级反应器的基础。为此,采用数值模拟的方法分析了低含量甲烷热氧化流向变换反应器的自热操作边界,深入研究了热空气导出量对流向变换反应器行为的影响。结果显示,甲烷体积分数超过0.2%即可实现自热操作;甲烷体积分数从0.5%提升至3.0%,最高床温仍维持在1200 °C左右。当甲烷体积分数超过0.5%,可以回收部分热量;相同甲烷含量条件下,最高床温随着热气抽出量的增加而增加;随着甲烷体积分数从0.5%提升到3.0%,允许导出的热空气从12.5%几乎线性地增加到32%。进一步研究发现,以30−50 s的时间间隔反向流动可以确保甲烷的完全转化和床温稳定。上述结果表明,甲烷体积分数在1%−3%时,采用热氧化处理可以实现余热回收;此外,通过调整换向时间和热空气导出量可以实现床层温度控制。Abstract: Lean methane from abandoned coal mines or drainage gas with methane concentration of 1%−3% is in general directly discharged into the atmosphere due to the lack of appropriate technology, which has caused serious environmental concerns due to its high global warming potential. While direct thermal oxidation of ultra-low methane in a flow reversal reactor offers an attractive solution, it poses challenges such as potential explosions and unstable combustion flames. Elucidating the dynamic behavior of thermal oxidation of ultra-low methane in a flow reversal reactor is the basis for practical application. To this end, autothermal operation boundary of a pilot-scale thermal flow reversal reactor was examined and the effects of hot gas withdrawal on the behavior of flow reversal reactor was deeply studied. It was found that autothermal operation can be achieved with a methane content of over 0.2% and heat can be recovered if methane content is over 0.5%. Withdrawal of hot air has a significant impact on the dynamic behavior of the reactor: maximum bed temperature at the pseudo-steady state without hot gas extraction keeps almost constant with methane concentration varying in 0.5%−3.0%; whereas for heat recovery by hot gas withdrawal, the maximum bed temperature increases with the increase of the amount of hot gas extracted, and the allowable hot gas exported from the reactor increases nearly linearly from 12.5% to 32% as the methane content increases from 0.5% to 3.0%. Furthermore, the appropriate switching time decreases with the increase of the amount of hot gas withdrawn; for most cases, reversing flow direction at a time interval of 30−50 s can ensure complete methane conversion and stable bed temperature. Thus, it may be concluded that lean methane (1%−3%) can be mitigated by thermal oxidation without worrying about the bed temperature runaway or explosion.
-
Key words:
- low methane /
- thermal oxidation /
- flow reversal reactor /
- heat recovery /
- hot gas withdrawal
-
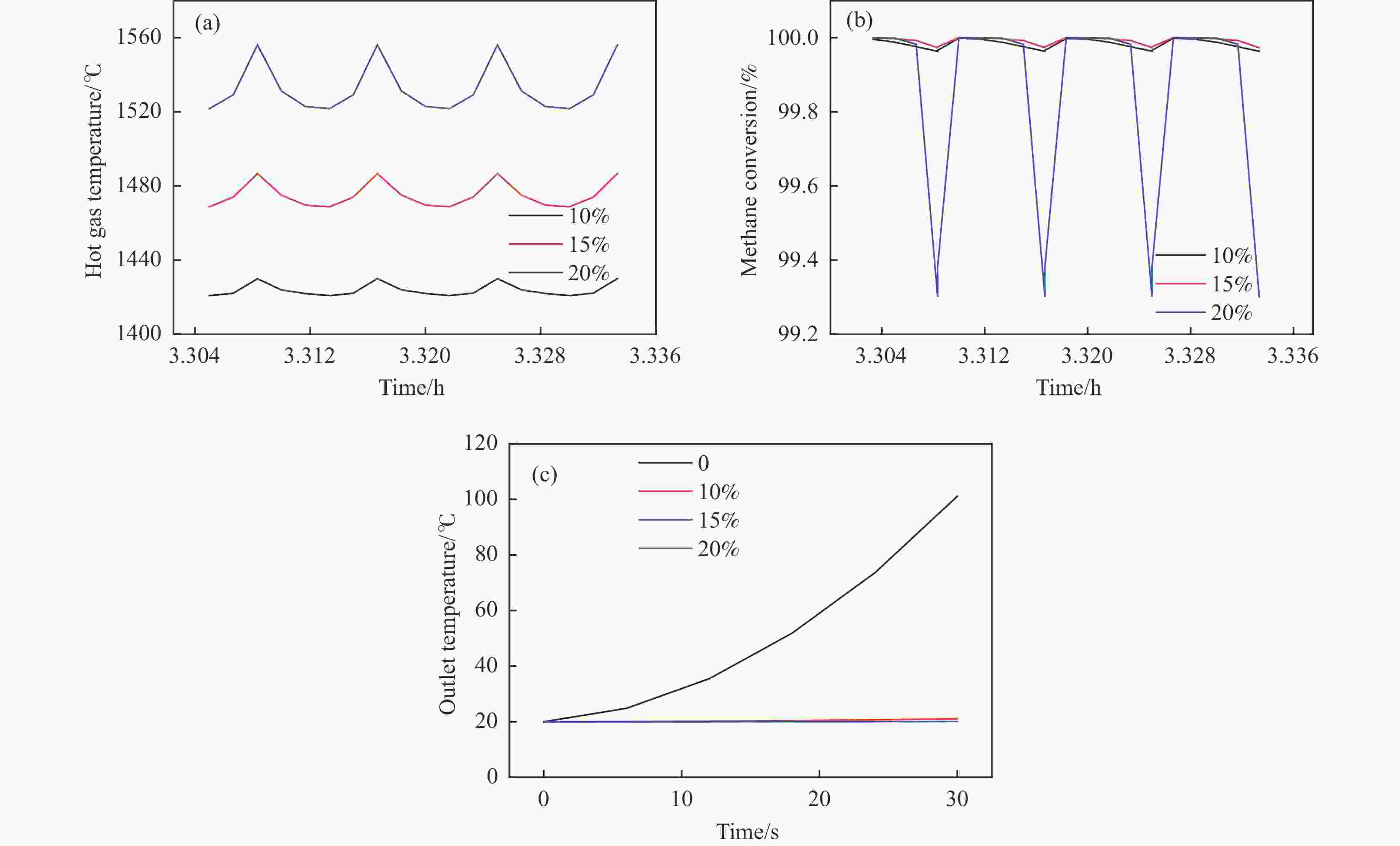
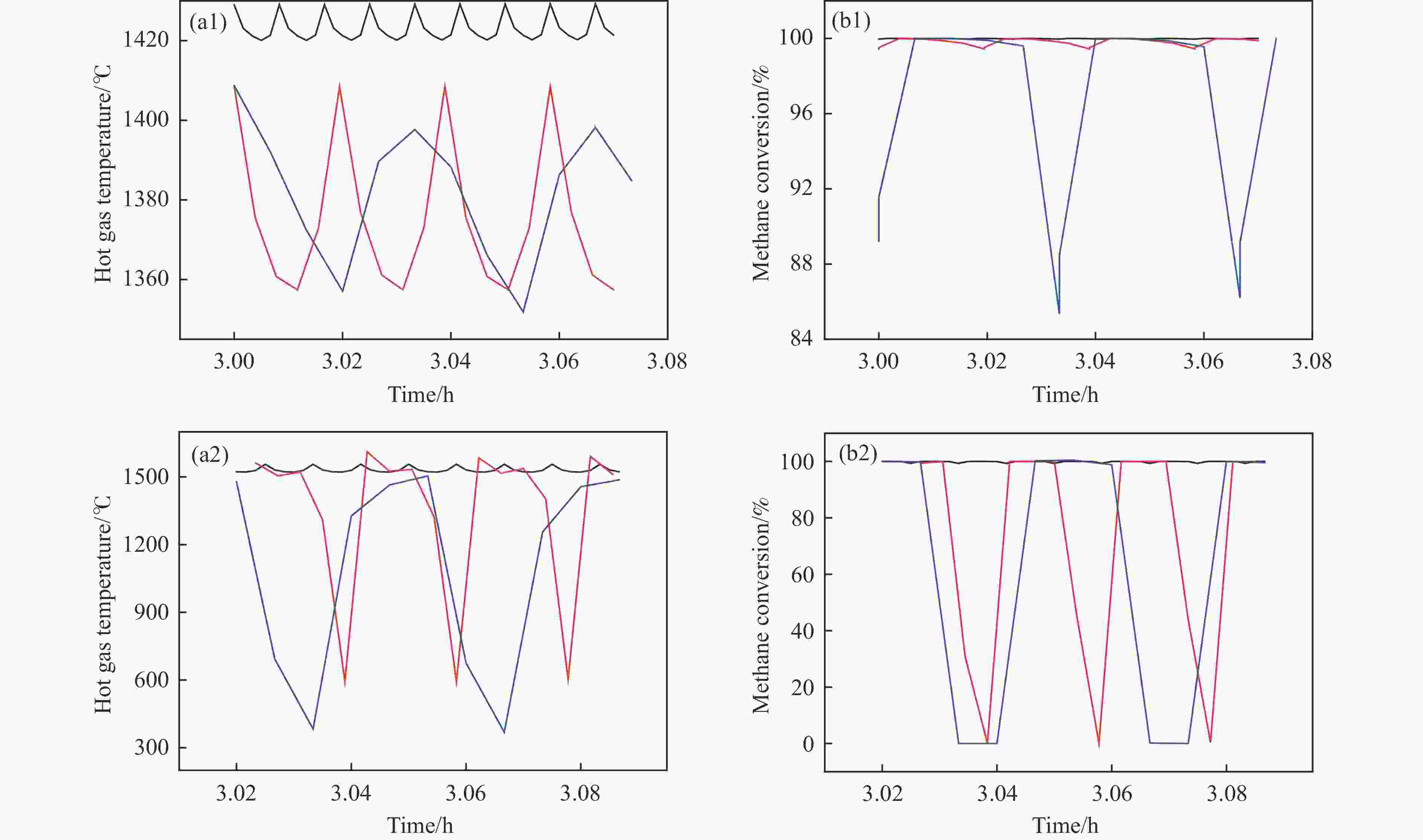
Figure 5 (a) Temperature of hot gas extracted from the reactor at pseudo-steady state; (b) Methane conversion in the extracted hot gas; (c) Outlet bed temperature in a flow reversal cycle (methane content is 1.6%, switching time is 30 s and the fraction of hot gas withdrawal from reactor is 0, 10%, 15% and 20%, respectively)
Table 1 Operation conditions of non-catalytic oxidation of ventilation air methane in flow reversal reactor
Item Unit Value Methane concentration % 0.1−2.8 Feed flow rate (m3·h−1) 10000 Inlet temperature °C 293.15 Inert bed length m 1 Void space m 0.3 Switching time s 30−120 Fraction of hot withdrawal 10%−20% -
[1] PACHAURI R K, ALLEN M R, BARROS V R, et al. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change[M]. 1st ed. Geneva: IPCC, 2014. [2] RAMANATHAN V, HAINES A. Global Mitigation of Non-CO2 Greenhouse Gases[M]. 1st ed. Amsterdam: Springer, 2010. [3] SU S, BEATH A, GUO H, et al. An assessment of mine methane mitigation and utilisation technologies[J]. Prog Energy Combust,2005,31(2):123−170. doi: 10.1016/j.pecs.2004.11.001 [4] YIN J, SU S, YU X, et al. Site trials and demonstration of a novel pilot ventilation air methane mitigator[J]. Energy Fuels,2020,34(8):9885−9893. doi: 10.1021/acs.energyfuels.0c01681 [5] AROMADA S A, KVAMME B. Impacts of CO2 and H2S on the risk of hydrate formation during pipeline transport of natural gas[J]. Front Chem Sci Eng,2019,13(3):616−627. doi: 10.1007/s11705-019-1795-2 [6] KOLIOS G, FRAUHAMMER J, EIGENBERGER G. Autothermal fixed-bed reactor concepts[J]. Chem Eng Sci,2000,55(24):5945−5967. doi: 10.1016/S0009-2509(00)00183-4 [7] GOSIEWSKI K, MATROS Y S, WARMUZINSKI K, et al. Homogeneous vs. catalytic combustion of lean methane—air mixtures in reverse-flow reactors[J]. Chem Eng Sci,2008,63(20):5010−5019. doi: 10.1016/j.ces.2007.09.013 [8] LI Z, QIN Z, ZHANG Y, et al. A control strategy of flow reversal with hot gas withdrawal for heat recovery and its application in mitigation and utilization of ventilation air methane in a reverse flow reactor[J]. Chem Eng J,2013,228(0):243−255. [9] RAMOS H S F, MMBAGA J P, HAYES R E. Catalyst optimization in a catalytic flow reversal reactor for lean methane combustion [J]. Catal Today, 2023, 407182−193. [10] DUFOUR P, COUENNE F, TOURE Y. Model predictive control of a catalytic reverse flow reactor[J]. IEEE Trans Contr Syst Trans,2003,11(5):705−714. doi: 10.1109/TCST.2003.816408 [11] DUFOUR P, TOURé Y. Multivariable model predictive control of a catalytic reverse flow reactor[J]. Comput Chem Eng,2004,28(11):2259−2270. doi: 10.1016/j.compchemeng.2004.04.006 [12] BALAJI S, FUXMAN A, LAKSHMINARAYANAN S, et al. Repetitive model predictive control of a reverse flow reactor[J]. Chem Eng Sci,2007,62(8):2154−2167. doi: 10.1016/j.ces.2006.12.082 [13] EDOUARD D, DUFOUR P, HAMMOURI H. Observer based multivariable control of a catalytic reverse flow reactor: comparison between LQR and MPC approaches[J]. Comput Chem Eng,2005,29(4):851−865. doi: 10.1016/j.compchemeng.2004.09.018 [14] EDOUARD D, HAMMOURI H, ZHOU X G. Control of a reverse flow reactor for VOC combustion[J]. Chem Eng Sci,2005,60(6):1661−1672. doi: 10.1016/j.ces.2004.10.020 [15] BARRESI A A, VANNI M. Control of catalytic combustors with periodical flow reversal[J]. AIChE J,2002,48(3):648−652. doi: 10.1002/aic.690480322 [16] HEVIA M A G, ORDóñEZ S, DíEZ F V, et al. Design and testing of a control system for reverse-flow catalytic afterburners[J]. AIChE J,2005,51(11):3020−3027. doi: 10.1002/aic.10573 [17] LI Z, QIN Z, ZHANG Y, et al. A logic-based controller for the mitigation of ventilation air methane in a catalytic flow reversal reactor[J]. Front Chem Sci Eng,2013,7(3):347−356. doi: 10.1007/s11705-013-1347-0 [18] LI Z, QIN Z, WU Z, et al. Fuzzy logic control of a reverse flow reactor for catalytic oxidation of ventilation air methane[J]. Control Eng Pract,2014,25(0):112−122. [19] GOSIEWSKI K, PAWLACZYK-KUREK A. Aerodynamic CFD simulations of experimental and industrial thermal flow reversal reactors [J]. Chem Eng J, 2019, 3731367−1379. [20] SLEPTEREV A A, SALNIKOV V S, TSYRULNIKOV P G, et al. Homogeneous high-temperature oxidation of methane[J]. React Kinet Catal Lett,2007,91(2):273−282. doi: 10.1007/s11144-007-5158-5 [21] GOSIEWSKI K, PAWLACZYK A, WARMUZINSKI K, et al. A study on thermal combustion of lean methane–air mixtures: Simplified reaction mechanism and kinetic equations[J]. Chem Eng J,2009,154(1):9−16. [22] WANG Y, LIU Y, CAO Q, et al. Homogeneous combustion of fuel Ultra-lean methane–Air mixtures: Experimental study and simplified reaction mechanism[J]. Energy Fuels,2011,25(8):3437−3445. doi: 10.1021/ef200484c [23] PAWLACZYK A, GOSIEWSKI K. Combustion of lean methane–air mixtures in monolith beds: Kinetic studies in low and high temperatures [J]. Chem Eng J, 2015, 28229−36. [24] LAN B, LI Y-R, ZHAO X-S, et al. Industrial-scale experimental study on the thermal oxidation of ventilation air methane and the heat recovery in a multibed thermal flow-reversal reactor[J]. Energies,2018,11(6):1578. doi: 10.3390/en11061578 [25] GOSIEWSKI K, PAWLACZYK A, JASCHIK M. Energy recovery from ventilation air methane via reverse-flow reactors [J]. Energy, 2015, 9213−23. [26] MAO M, SHI J, LIU Y, et al. Experimental investigation on control of temperature asymmetry and nonuniformity in a pilot scale thermal flow reversal reactor [J]. Appl Therm Eng, 2020, 175115375. [27] LI Q, LIN B, YUAN D, et al. Demonstration and its validation for ventilation air methane (VAM) thermal oxidation and energy recovery project [J]. Appl Therm Eng, 2015, 9075−85. [28] GOSIEWSKI K, WARMUZINSKI K. Effect of the mode of heat withdrawal on the asymmetry of temperature profiles in reverse-flow reactors. Catalytic combustion of methane as a test case[J]. Chem Eng Sci,2007,62(10):2679−2689. doi: 10.1016/j.ces.2007.02.013 [29] DOBREGO K V, KOZLOV I M, BUBNOVICH V I, et al. Dynamics of filtration combustion front perturbation in the tubular porous media burner[J]. Int J Heat Mass Tran,2003,46(17):3279−3289. doi: 10.1016/S0017-9310(03)00125-X [30] KIM S G, YOKOMORI T, KIM N I, et al. Flame behavior in heated porous sand bed[J]. Proc Combust Inst,2007,31(2):2117−2124. doi: 10.1016/j.proci.2006.08.070 [31] ZHANG L, ZHENG S, YANG Z. Experimental study of the combustion characteristics of methane at an ultra-low concentration in Inert particles[J]. J Eng Thermal Energy Power,2013,28(1):78−81. [32] MILLER J A, BOWMAN C T. Mechanism and modeling of nitrogen chemistry in combustion[J]. Prog Energy Combust,1989,15(4):287−338. doi: 10.1016/0360-1285(89)90017-8 [33] LI Z, WU Z, QIN Z, et al. Demonstration of mitigation and utilization of ventilation air methane in a pilot scale catalytic reverse flow reactor[J]. Fuel Process Technol,2017,160(Supplement C):102−108. -




 下载:
下载: