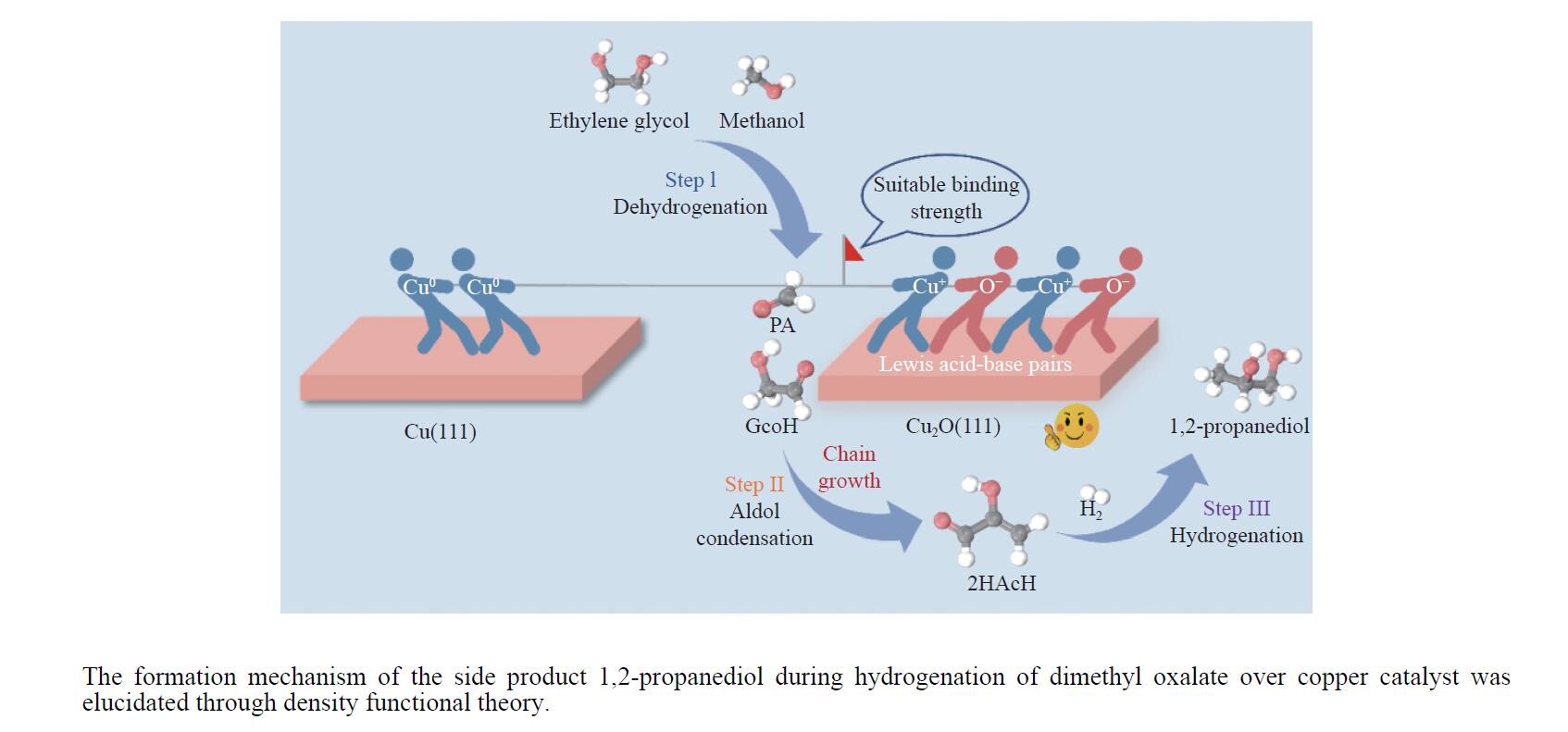
A DFT study on the formation mechanism of side product 1,2-propanediol in the hydrogenation of dimethyl oxalate over copper catalyst
-
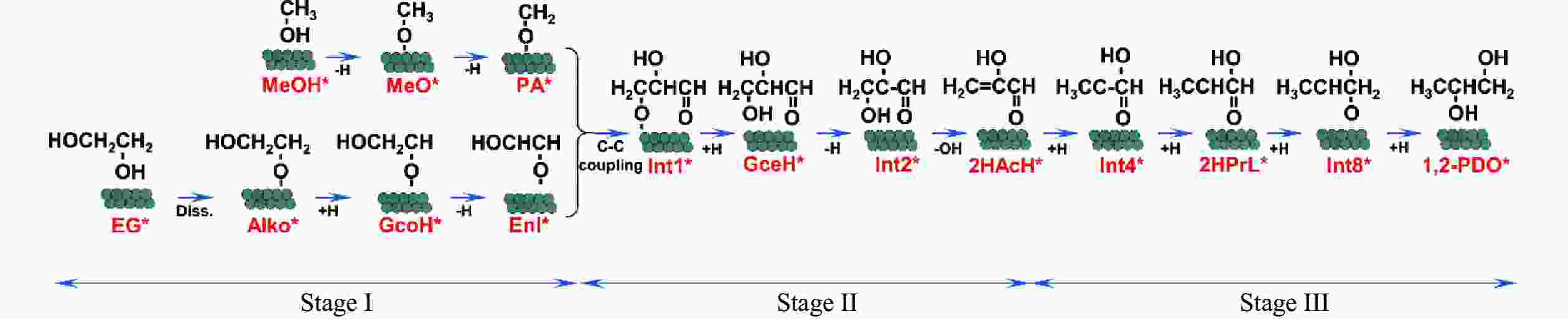
摘要: 利用密度泛函理论对Cu(111)及Cu2O(111)表面上草酸二甲酯加氢副产物1,2-丙二醇(1,2-PDO)的生成机理进行了探究,计算了两种表面上1,2-PDO生成的不同反应路径基元步骤的热力学数据以及所涉及物种的吸附行为,进行了局域态密度以及差分电荷密度分析,阐明了铜催化剂的主要活性位点及1,2-PDO生成的主要路径。结果表明,1,2-PDO主要由乙二醇和甲醇于Cu2O(111)表面通过Guerbet醇缩合反应生成,具体包括醇脱氢、羟醛缩合以及不饱和醛加氢三个过程。Cu2O(111)表面${\rm{Cu}}_{{\rm{us}}}^{+} $及${\rm{O}}_{{\rm{suf}}}^-$位点形成的Lewis酸碱对能够促进反应物、产物及反应中间体的吸附且对于1,2-PDO生成过程的整体催化活性更高。Cu2O(111)表面的${\rm{O}}_{{\rm{suf}}}^- $位点是醇类脱氢生成醛、羟醛缩合过程中生成烯醇物种以及不饱和醛类中间体加氢的主要活性中心,而C−C偶联反应则发生在${\rm{Cu}}_{{\rm{us}}}^{+} $金属位点上。论文研究结果可为铜催化剂设计和改性以及草酸酯加氢工艺的优化提供理论指导。Abstract: The costly separation of 1,2-propanediol (1,2-PDO), an unavoidable byproduct in the hydrogenation of dimethyl oxalate (DMO), significantly hampers the economic viability of coal-to-ethylene glycol (EG) technology. To address this challenge, the formation mechanism of the side product 1,2-PDO on the Cu(111) and Cu2O(111) surfaces during DMO hydrogenation was investigated, which focused on the active sites of copper catalyst and the dominant pathway through density functional theory calculation. The thermodynamics of each elementary step and the adsorption behavior of various species involved in the reaction network along with the local density of states and charge density difference were systematically analyzed. The results indicate that 1,2-PDO is generated more favorably on the Cu2O(111) surface than that on the Cu(111) surface, owing to the Lewis acid-base pairs, i.e. ${\rm{Cu}}_{{\rm{us}}}^{+} $ and ${\rm{O}}_{{\rm{suf}}}^- $ sites, present on the Cu2O(111) surface, which strengthens the binding of reactants, products, and reaction intermediates to the substrate. EG reacts primarily with methanol (MeOH) to form 1,2-PDO through Guerbet alcohol condensation reaction through three consecutive steps: alcohol dehydrogenation, aldol condensation, and unsaturated aldehyde hydrogenation. The ${\rm{O}}_{{\rm{suf}}}^- $ sites promote the dehydrogenation of alcohols into aldehydes, the generation of enolates during aldol condensation and the hydrogenation of unsaturated aldehydes, while the ${\rm{Cu}}_{{\rm{us}}}^{+} $ sites are responsible for the C–C coupling reaction. These findings may shed light on the mechanism of 1,2-PDO formation over Cu catalyst and provide fundamental knowledge for the development of more efficient catalysts and process optimization.
-
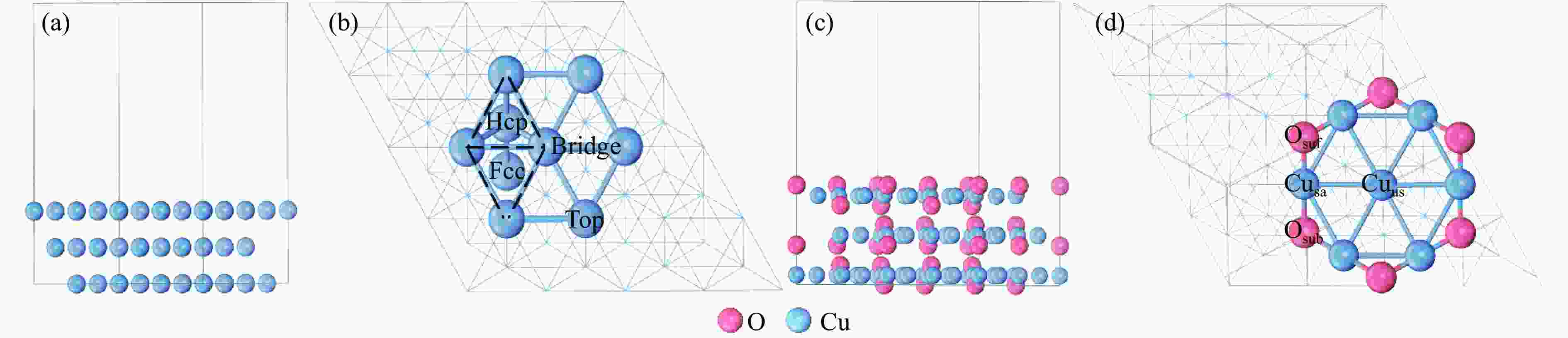
图 1 Cu(111)及Cu2O(111)表面模型侧视图及活性位点
(a): Cu(111)表面模型;(b): Cu(111)表面活性位点;(c): Cu2O(111)表面模型;(d): Cu2O(111)表面活性位点
Figure 1 Side views and active sites of Cu(111) and Cu2O(111) surfaces
(a): side view of Cu(111) surface; (b): possible active sites of Cu(111) surface; (c): side view of Cu2O(111) surface; (d): possible active sites of Cu2O(111) surface.
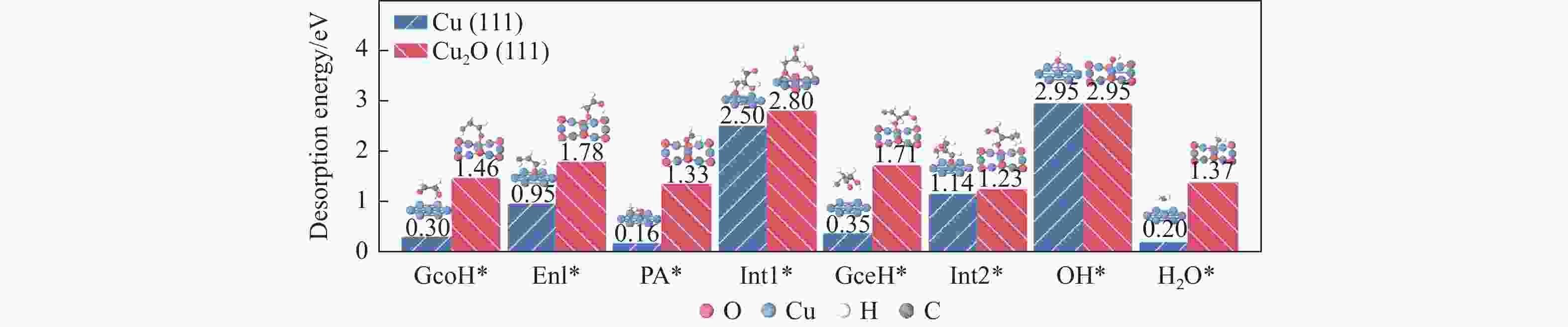
图 4 Cu(111)及Cu2O(111)表面Stage II中GcoH*、Enl*、PA*、Int1*、GceH*、Int2*、OH*、H2O*物种最稳定吸附构型及对应脱附能
Figure 4 Most stable adsorption configurations and the corresponding desorption energies of the GcoH*, Enl*, PA*, Int1*, GceH*, Int2*, OH*, H2O* species on the Cu(111) and Cu2O(111) surfaces during Stage II for the generation of 1,2-PDO
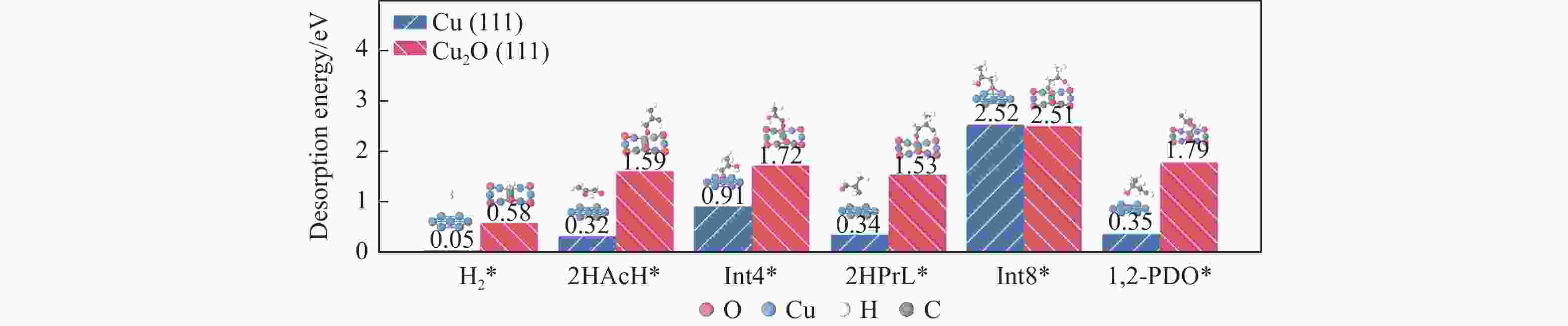
图 5 Cu(111)及Cu2O(111)表面Stage III中H2*、2HAcH*、Int4*、2HPrL*、Int8*、1,2-PDO*物种最稳定吸附构型及对应脱附能
Figure 5 Most stable adsorption configurations and corresponding desorption energies of the H2*, 2HAcH*, Int4*, 2HPrL*, Int8*, 1,2-PDO* species on the Cu(111) and Cu2O(111) surfaces during Stage III for the generation of 1,2-PDO
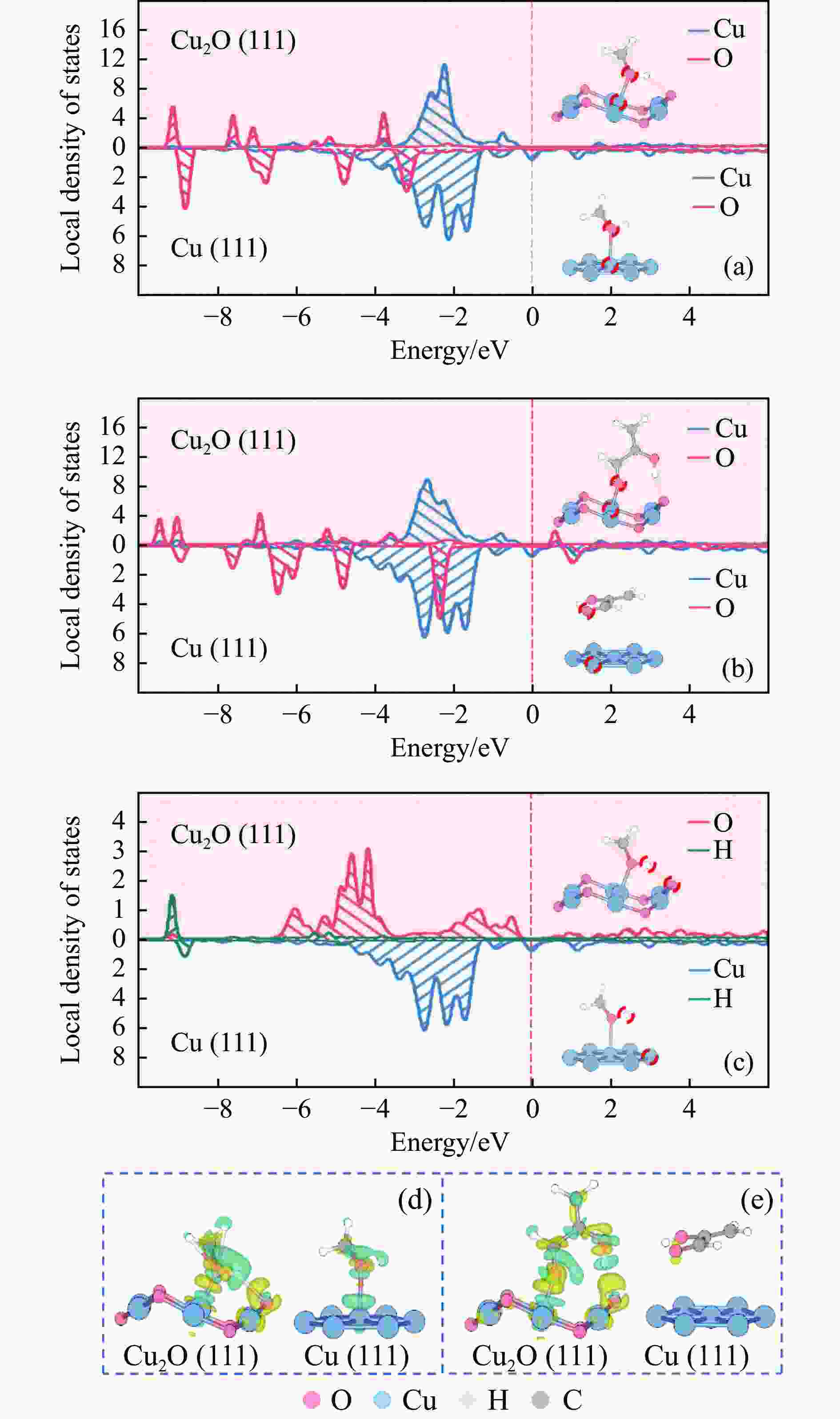
图 6 MeOH*及2HAcH*物种在Cu2O(111)及Cu(111)表面最稳定吸附构型的局域态密度及片段差分电荷密度
(a): MeOH*物种中羟基O原子与Cu原子轨道的局域态密度;(b): 2HAcH*物种中酰基O原子与Cu原子轨道的局域态密;(c): MeOH*物种中羟基H原子与O原子及Cu原子轨道的局域态密度;(d): MeOH*物种最稳定吸附构型的片段差分电荷密度;(e): 2HAcH*物种最稳定吸附构型的片段差分电荷密度
Figure 6 LDOSs and the charge density differences of the most stable adsorption configurations of MeOH* and 2HAcH* on the Cu2O(111) and Cu(111) surfaces
(a): LDOSs for the orbitals of O atom in the hydroxy group of MeOH* with Cu atom; (b): LDOSs for the orbitals of O atom in the acyl group of 2HAcH* with Cu atom; (c): LDOSs for the orbitals of H atom in the hydroxy group of MeOH* with O atom and Cu atom; (d): charge density differences of the most stable adsorption configurations of MeOH*; (e): charge density differences of the most stable adsorption configurations of 2HAcH*
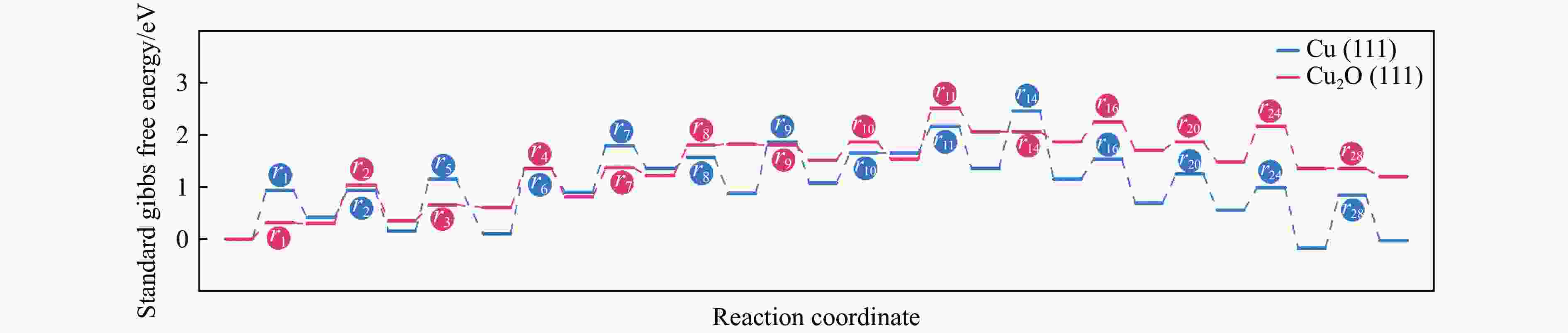
表 1 473.15 K条件下Cu(111)及Cu2O(111)表面Stage I各基元步的标准吉布斯自由能垒(Ea)及反应热(ΔE)
Table 1 Standard Gibbs free energy barriers ( Ea ) and reaction energies ( ΔE ) for each elementary step of Stage I on the Cu(111) and Cu2O(111) surfaces at 473.15 K
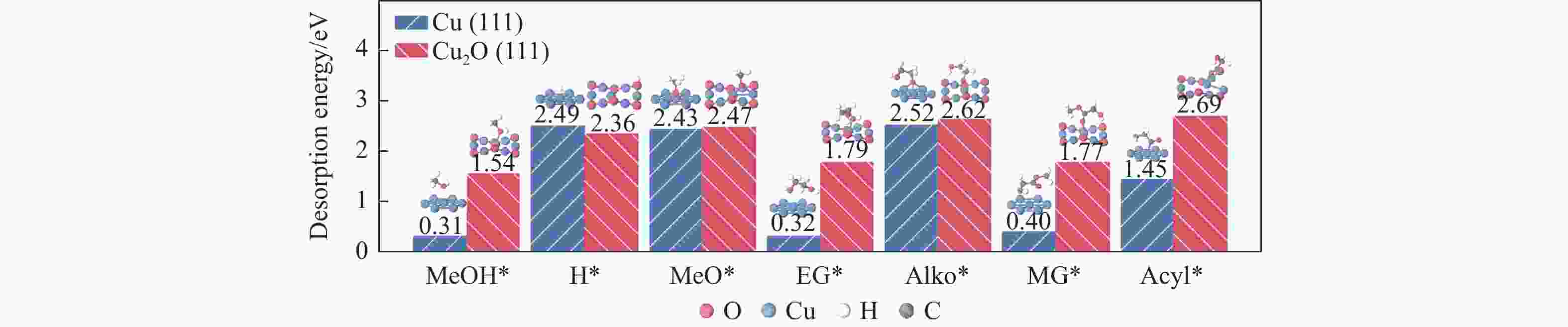
No. Elementary step Cu(111) Cu2O(111) Ea/eV ΔE/eV Ea/eV ΔE/eV r1 MeOH* → MeO*+H* 1.00 −0.05 0.32 0.27 r2 MeO* → PA*+H* 1.24 0.79 0.75 0.20 r3 EG* → Alko*+H* 0.88 −0.25 0.30 0.28 r4 Alko* → GcoH*+H* 1.06 0.67 0.76 0.05 r5 MG* → Acyl*+MeO* 0.94 0.40 2.47 2.00 r6 Acyl*+H* → GcoH* 0.54 −0.25 0.77 −0.58 表 2 473.15 K条件下Cu(111)及Cu2O(111)表面Stage II各基元步的标准吉布斯自由能垒(Ea)及反应热(ΔE)
Table 2 Standard Gibbs free energy barriers (Ea) and reaction energies (ΔE) for each elementary step of Stage II on the Cu(111) and Cu2O(111) surfaces at 473.15 K
No. Elementary step Cu(111) Cu2O(111) Ea/eV ΔE/eV Ea/eV ΔE/eV r7 GcoH* → Enl*+H* 0.90 0.46 0.57 0.42 r8 Enl*+PA* → Int1* 0.21 −0.48 0.59 0.06 r9 Int1*+H* → GceH* 1.01 0.21 −0.02 −0.30 r10 GceH* → Int2*+H* 0.57 0 0.36 0.02 r11 GceH* → Int3*+OH* 1.49 0.30 2.18 −0.03 r12 Int2* → 2HAcH*+OH* 0.51 −0.31 0.98 0.52 r14 H*+OH* → H2O* 1.13 −0.19 −0.01 −0.19 表 3 473.15 K条件下Cu(111)及Cu2O(111)表面Stage III各基元步的标准吉布斯自由能垒(Ea)及反应热(ΔE)
Table 3 Standard Gibbs free energy barriers ( Ea ) and reaction energies ( ΔE ) for each elementary step of Stage III on the Cu(111) and Cu2O(111) surfaces at 473.15 K
No. Elementary step Cu(111) Cu2O(111) Ea/eV ΔE/eV Ea/eV ΔE/eV r15 H2* → H*+H* 1.01 0.08 0.57 0.19 r16 2HAcH*+H* → Int4* 0.39 −0.46 0.38 −0.17 r17 2HAcH*+H* → Int5* 0.90 0.17 1.42 0.47 r18 2HAcH*+H* → Int6* 0.79 −0.03 0.64 0.70 r19 2HAcH*+H* → Int7* 0.89 −0.43 0.49 −0.15 r20 Int4*+H* → 2HPrL* 0.56 −0.14 0.17 −0.21 r24 2HPrL*+H* → Int8* 0.45 −0.74 0.67 −0.15 r25 2HPrL*+H* → Int9* 1.05 0.63 1.01 1.06 r28 Int8*+H* → 1,2-PDO* 1.03 0.15 0 −0.14 -
[1] 范晨亮, 张育红, 王川, 等. 气相色谱法检测工业用乙二醇纯度及杂质[J]. 色谱,2019,37(1):116−120. doi: 10.3724/SP.J.1123.2018.08030FAN Chenliang, ZHANG Yuhong, WANG Chuan, et al. Determination of purity and impurities of ethylene glycol for industrial use by gas chromatography[J]. Se P'u Chin J Chromatogr,2019,37(1):116−120. doi: 10.3724/SP.J.1123.2018.08030 [2] ZHU Y, ZHU Y, DING G, et al. Highly selective synthesis of ethylene glycol and ethanol via hydrogenation of dimethyl oxalate on Cu catalysts: Influence of support[J]. Appl Catal A: Gen,2013,468:296−304. doi: 10.1016/j.apcata.2013.09.019 [3] XU C, CHEN G, ZHAO Y, et al. Interfacing with silica boosts the catalysis of copper[J]. Nat Commun,2018,9(1):3367. doi: 10.1038/s41467-018-05757-6 [4] GIORGIANNI G, MEBRAHTU C, PERATHONER S, et al. Hydrogenation of dimethyl oxalate to ethylene glycol on Cu/SiO2 catalysts prepared by a deposition-decomposition method: Optimization of the operating conditions and pre-reduction procedure[J]. Catal Today,2021,390-391:343−353. [5] YUE WANG, YANG W, YAO D, et al. Effect of surface hydroxyl group of ultra-small silica on the chemical states of copper catalyst for dimethyl oxalate hydrogenation[J]. Catal Today,2020,(350):127−135. [6] SONG Y, ZHANG J, LV J, et al. Hydrogenation of dimethyl oxalate over copper-based catalysts: Acid-base properties and reaction paths[J]. Ind Eng Chem Res,2015,54(40):9699−9707. doi: 10.1021/acs.iecr.5b01928 [7] YUE H, MA X, GONG J. An alternative synthetic approach for efficient catalytic conversion of syngas to ethanol[J]. Acc Chem Res,2014,47(5):1483−1492. doi: 10.1021/ar4002697 [8] ZHU Y, KONG X, ZHU S, et al. Construction of Cu/ZrO2/Al2O3 composites for ethanol synthesis: Synergies of ternary sites for cascade reaction[J]. Appl Catal B: Environ,2015,166-167:551−559. doi: 10.1016/j.apcatb.2014.12.015 [9] YUE H, ZHAO Y, ZHAO S, et al. A copper-phyllosilicate core-sheath nanoreactor for carbon-oxygen hydrogenolysis reactions[J]. Nat Commun,2013,4:2339. doi: 10.1038/ncomms3339 [10] YAN W-Q, ZHANG J-B, ZHOU R-J, et al. Identification of synergistic actions between Cu0 and Cu+ sites in hydrogenation of dimethyl oxalate from microkinetic analysis[J]. Ind Eng Chem Res,2020,59(52):22451−22459. doi: 10.1021/acs.iecr.0c04525 [11] SMIDSTRUP S, PEDERSEN A, STOKBRO K, et al. Improved initial guess for minimum energy path calculations[J]. J Chem Phys,2014,140(21):214106. doi: 10.1063/1.4878664 [12] XU H, MIAO B, ZHANG M, et al. Mechanism of C−C and C−H bond cleavage in ethanol oxidation reaction on Cu2O(111): A DFT-D and DFT+U study[J]. Phys Chem Chem Phys,2017,19(38):26210−26220. doi: 10.1039/C7CP04630H [13] HENKELMAN G, JóNSSON H. A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives[J]. J Chem Phys,1999,111(15):7010−7022. doi: 10.1063/1.480097 [14] OLSEN R A, KROES G J, HENKELMAN G, et al. Comparison of methods for finding saddle points without knowledge of the final states[J]. J Chem Phys,2004,121(20):9776−9792. doi: 10.1063/1.1809574 [15] HENKELMAN G, UBERUAGA B P, JÓNSSON H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths[J]. J Chem Phys,2000,113(22):9901−9904. doi: 10.1063/1.1329672 [16] GARCÍA-MUELAS R, LI Q, LÓPEZ N. Density functional theory comparison of methanol decomposition and reverse reactions on metal surfaces[J]. ACS Catal,2015,5(2):1027−1036. doi: 10.1021/cs501698w [17] HOYT R A, MONTEMORE M M, SYKES E C H, et al. Anhydrous methanol and ethanol dehydrogenation at Cu(111) step edges[J]. J Phys Chem C,2018,122(38):21952−21962. doi: 10.1021/acs.jpcc.8b06730 [18] WANG V, XU N, LIU J-C, et al. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code[J]. Comput Phys Commun,2021,267:108033. doi: 10.1016/j.cpc.2021.108033 [19] WANG W-W, DU P-P, ZOU S-H, et al. Highly dispersed copper oxide clusters as active species in copper-ceria catalyst for preferential oxidation of carbon monoxide[J]. ACS Catal,2015,5(4):2088−2099. doi: 10.1021/cs5014909 [20] ANTON J, NEBEL J, SONG H, et al. The effect of sodium on the structure-activity relationships of cobalt-modified Cu/ZnO/Al2O3 catalysts applied in the hydrogenation of carbon monoxide to higher alcohols[J]. J Catal,2016,335:175−186. doi: 10.1016/j.jcat.2015.12.016 [21] METHFESSEL M, PAXTON A T. High-precision sampling for Brillouin-zone integration in metals[J]. Phys Rev B,1989,40(6):3616−3621. doi: 10.1103/PhysRevB.40.3616 [22] PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple[J]. Phys Rev Lett,1996,77(18):3865−3868. doi: 10.1103/PhysRevLett.77.3865 [23] GUERBET M. Condensation de l’alcool isopropylique avec son dérivé sodé; formation du méthylisobutylcarbinol et du diméthyl-2.4-heptanol-6[J]. Compt rend, 1909, 149: 129-132. [24] MATSU-URA T, SAKAGUCHI S, OBORA Y, et al. Guerbet reaction of primary alcohols leading to β-alkylated dimer alcohols catalyzed by iridium complexes[J]. J Org Chem,2006,71(21):8306−8308. doi: 10.1021/jo061400t [25] ZHAO Y, KONG L, XU Y, et al. Deactivation mechanism of Cu/SiO2 catalysts in the synthesis of ethylene glycol via methyl glycolate hydrogenation[J]. Ind Eng Chem Res,2020,59(27):12381−12388. doi: 10.1021/acs.iecr.0c01619 [26] AN J, WANG X, ZHAO J, et al. Density-functional theory study on hydrogenation of dimethyl oxalate to methyl glycolate over copper catalyst: Effect of copper valence state[J]. Mol Catal, 2020, 482: 110667. [27] CHRISTENSEN J M, JENSEN P A, SCHIøDT N C, et al. Coupling of alcohols over alkali-promoted cobalt-molybdenum sulfide[J]. ChemCatChem,2010,2(5):523−526. doi: 10.1002/cctc.200900239 [28] OGO S, ONDA A, YANAGISAWA K. Selective synthesis of 1-butanol from ethanol over strontium phosphate hydroxyapatite catalysts[J]. Appl Catal, A,2011,402(1):188−195. [29] DI COSIMO J I, APESTEGUÍ A C R, et al. Structural requirements and reaction pathways in condensation reactions of alcohols on MgyAlOx catalysts[J]. J Catal,2000,190(2):261−275. doi: 10.1006/jcat.1999.2734 [30] LUGGREN P J, APESTEGUÍA C R, DI COSIMO J I. Conversion of biomass-derived 2-hexanol to liquid transportation fuels: Study of the reaction mechanism on Cu-Mg-Al mixed oxides[J]. Top Catal,2015,59(2/4):196−206. [31] SUN Z, COUTO VASCONCELOS A, BOTTARI G, et al. Efficient catalytic conversion of ethanol to 1-butanol via the guerbet reaction over copper- and nickel-doped porous[J]. ACS Sustainable Chem Eng,2016,5(2):1738−1746. [32] ZACCHERIA F, SCOTTI N, RAVASIO N. The role of copper in the upgrading of bioalcohols[J]. ChemCatChem,2018,10(7):1526−1535. doi: 10.1002/cctc.201701844 [33] CARVALHO D L, BORGES L E P, APPEL L G, et al. In situ infrared spectroscopic study of the reaction pathway of the direct synthesis of n-butanol from ethanol over MgAl mixed-oxide catalysts[J]. Catal Today,2013,213:115−121. doi: 10.1016/j.cattod.2013.03.034 [34] CARLINI C, DI GIROLAMO M, MACINAI A, et al. Selective synthesis of isobutanol by means of the Guerbet reaction Part 2. Reaction of methanol/ethanol and methanol/ethanol/n-propanol mixtures over copper based/MeONa catalytic systems[J]. J Mol Catal A: Chem,2002,200(1-2):137−146. [35] LI Q, GARCÍA-MUELAS R, LÓPEZ N. Microkinetics of alcohol reforming for H2 production from a FAIR density functional theory database[J]. Nat Commun,2018,9(1):526. doi: 10.1038/s41467-018-02884-y [36] BHASKER-RANGANATH S, RAHMAN M S, ZHAO C, et al. Elucidating the mechanism of ambient-temperature aldol condensation of acetaldehyde on ceria[J]. ACS Catal,2021,11(14):8621−8634. doi: 10.1021/acscatal.1c01216 [37] FAN D, DONG X, YU Y, et al. A DFT study on the aldol condensation reaction on MgO in the process of ethanol to 1, 3-butadiene: understanding the structure-activity relationship[J]. Phys Chem Chem Phys,2017,19(37):25671−25682. doi: 10.1039/C7CP04502F [38] LAN X, WANG T. Highly selective catalysts for the hydrogenation of unsaturated aldehydes: A review[J]. ACS Catal,2020,10(4):2764−2790. doi: 10.1021/acscatal.9b04331 [39] DELBECQ F, SAUTET P. Competitive C=C and C=O adsorption of α-β-unsaturated aldehydes on Pt and Pd surfaces in relation with the selectivity of hydrogenation reactions: A theoretical approach[J]. J Catal,1995,152(2):217−236. doi: 10.1006/jcat.1995.1077 [40] MEDFORD A J, VOJVODIC A, HUMMELSHØJ J S, et al. From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis[J]. J Catal,2015,328:36−42. doi: 10.1016/j.jcat.2014.12.033 [41] ZHAO ZJ, GONG J. Catalyst design via descriptors[J]. Nat Nanotechnol,2022,17(6):563−564. doi: 10.1038/s41565-022-01120-5 [42] KOZUCH S, SHAIK S. How to conceptualize catalytic cycles? The energetic span model[J]. Acc Chem Res,2011,44(2):101−110. doi: 10.1021/ar1000956 [43] KOZUCH S, MARTIN J M L. What makes for a bad catalytic cycle? A theoretical study on the Suzuki-Miyaura reaction within the energetic span model[J]. ACS Catal,2011,1(4):246−253. doi: 10.1021/cs100129u [44] YUE H, ZHAO Y, MA X, et al. Ethylene glycol: Properties, synthesis, and applications[J]. Chem Soc Rev,2012,41(11):4218−4244. doi: 10.1039/c2cs15359a [45] WEN C, YIN A, CUI Y, et al. Enhanced catalytic performance for SiO2-TiO2 binary oxide supported Cu-based catalyst in the hydrogenation of dimethyloxalate[J]. Appl Catal A: Gen,2013,458:82−89. doi: 10.1016/j.apcata.2013.03.021 [46] WANG M, YAO D, LI A, et al. Enhanced selectivity and stability of Cu/SiO2 catalysts for dimethyl oxalate hydrogenation to ethylene glycol by using silane coupling agents for surface modification[J]. Ind Eng Chem Res,2020,59(20):9414−9422. doi: 10.1021/acs.iecr.0c00789 [47] WANG M, HOU S, YANG Y, et al. Surface amine speciespromoted Cu/SiO2 catalysts for the hydrogenation of dimethyl oxalate to ethylene glycol[J]. Ind Eng Chem Res,2023,62(27):10399−10408. doi: 10.1021/acs.iecr.3c01162 -




 下载:
下载: