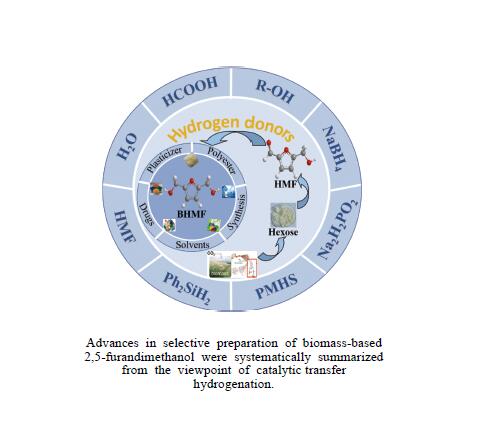
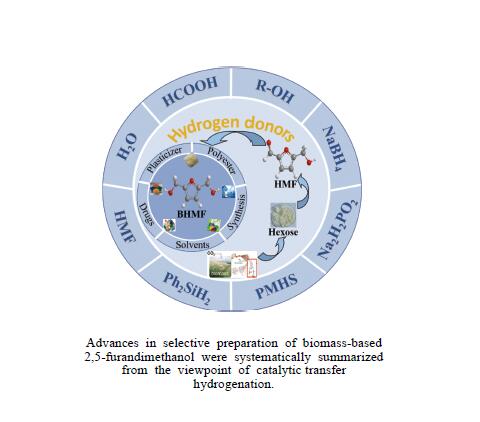
Recent advances in preparing biomass-based 2,5-bis(hydroxymethyl)furan by catalytic transfer hydrogenation
-
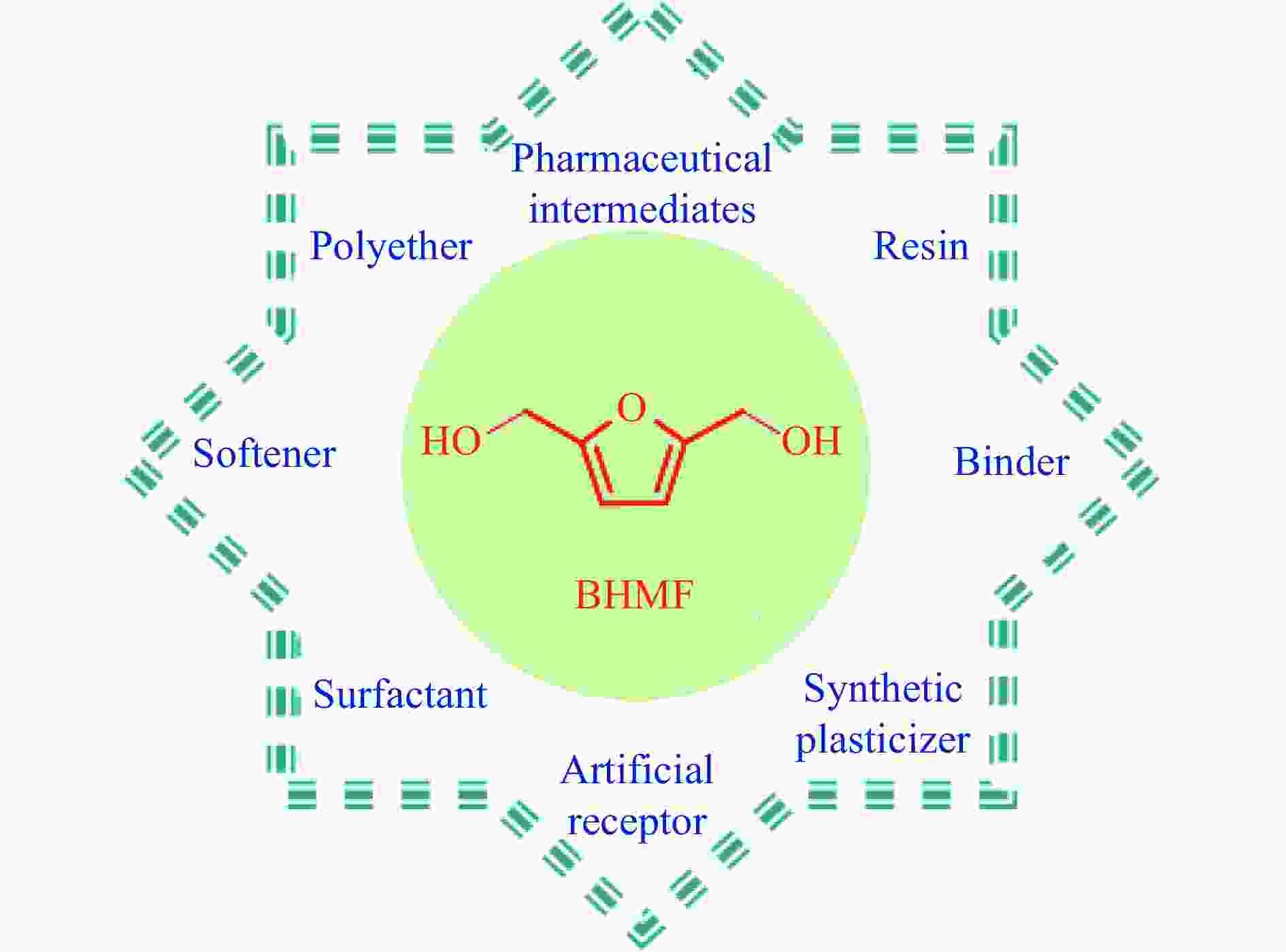
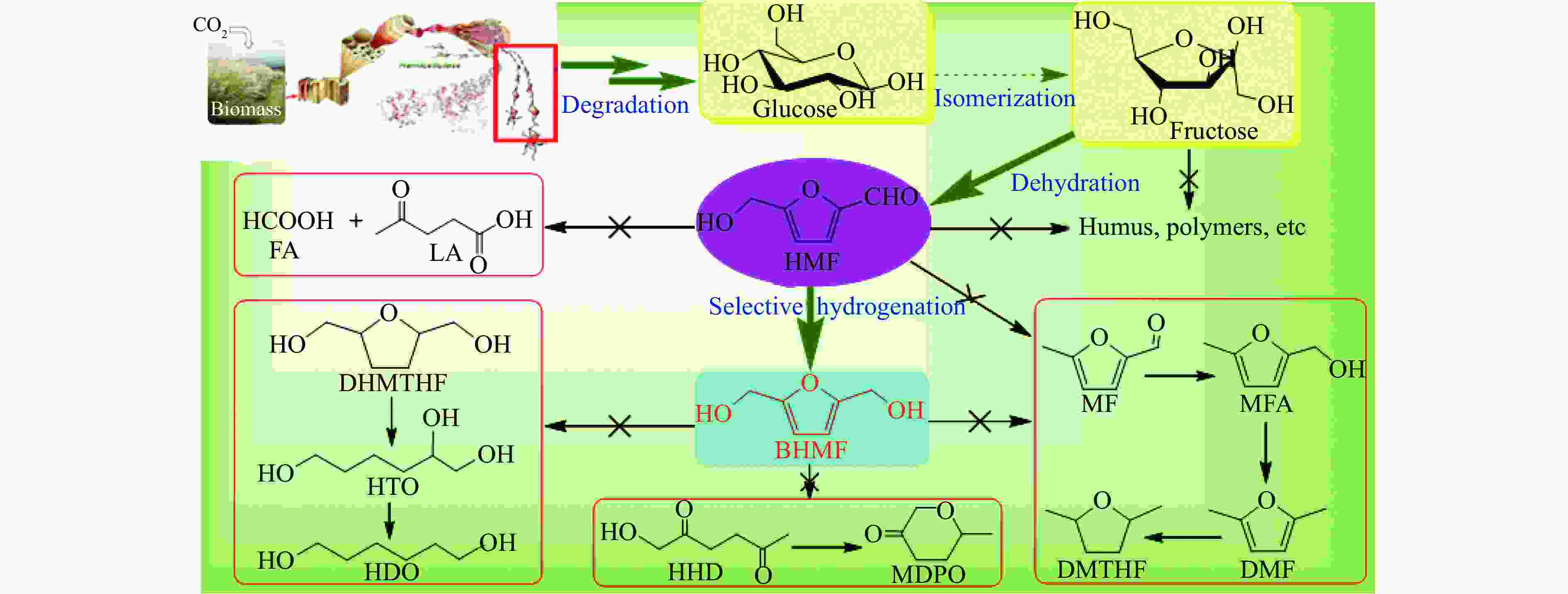
摘要: 生物质基2,5-呋喃二甲醇(BHMF)可从廉价易得的糖类出发,经催化转化-选择性氢化制取,并作为一种用途广泛的化工中间体及燃料前体,尤其在改善传统聚酯性能以及合成绿色可降解的生物基聚酯新材料方面具有独特优势。BHMF制取过程中,传统的氢化方式消耗了大量高品位能源氢气,且高压氢气存在安全隐患并导致基础设施投入多。本工作立足于催化转移氢化的优势,综述了甲酸、醇类及其他类型氢供体通过催化转移氢化的方式选择性加氢制取BHMF的研究进展;并针对催化转移氢化过程中不同类型氢供体、催化剂和反应工艺的特点及存在的问题,分析了反应条件、强化手段等对BHMF选择性和收率的影响以及反应体系的优劣。在此基础上,提出了转移氢化制取BHMF新型催化体系的研究方向,并对清洁高效、本质安全BHMF制取工艺的发展进行了展望,为生物质转化中特定催化体系的研发提供科学参考。Abstract: Biomass-based 2,5-bis(hydroxymethyl)furan (BHMF) is one of the important high value-added chemicals, which can be prepared from inexpensive and renewable carbohydrates through the way of catalytic conversion and selective hydrogenation, and as a widely used chemical intermediate and fuel precursor, it has unique advantages in improving the performance of traditional polyesters and synthesizing new biodegradable bio-based polyesters. In recent years, the research on the production of high value-added chemicals such as BHMF from carbohydrate has been attracting much attention from both academia and industry. However, cleanliness, high efficiency, high selectivity and low-cost remain key challenges in this area, especially for practical applications. In the process of BHMF production, the traditional hydrogenation method consumed a large amount of high-grade energy of hydrogen, and an excessive investment in infrastructure would be generated due to the security risks of higher pressure of hydrogen. On account of the advantages of catalytic transfer hydrogenation, the advances in selective hydrogenation to prepare BHMF using formic acid, alcohols and other types of hydrogen donors by the approach of catalytic transfer hydrogenation is systematically discussed in this review. In view of the features and problems of different types of hydrogen donors, catalysts and reaction processes during the catalytic transfer hydrogenation process, the effects of reaction conditions and process intensifications on the selectivity and yield of BHMF, and the merits and demerits of the reaction system were all investigated. On this basis, the future directions of new catalytic systems for preparation of BHMF by transfer hydrogenation is proposed, and the cleaner, more efficient and essential safety technologies for the production of BHMF is predicted, which will provide some scientific reference for the research and development of related catalytic systems in biomass conversion.
-
表 1 不同醇类化合物的还原电势
Table 1 Reduction potential of different alcohol compounds
No. Alcohol Reduction potential/(kJ·mol−1) 1 Methanol 130.1 2 Propanol 87.3 3 Ethanol 85.4 4 1-Butanol 79.7 5 Isopropanol 70.0 6 2-Butanol 69.3 表 2 以乙醇为溶剂和氢供体催化HMF选择性氢化效果
Table 2 Results of selective catalytic hydrogenation of HMF to BHMF using ethanol as the solvent and hydrogen donor
表 3 以异丙醇为溶剂和氢供体催化HMF选择性氢化效果
Table 3 Results of selective catalytic hydrogenation of HMF to BHMF using isopropanol as the solvent and hydrogen donor
No. Catalyst Temperature/℃ Time/h HMF conversion rate/% BHMF yield/% Ref. 1 Pd/Fe2O3 150 − 50 35 [45] 2 Ru/Co3O4 190 6 100 82.8 [46] 3 RuCu@NFC 210 12 97 91.5 [47] 4 ZrBa-SBA 150 2.5 98.3 90.6 [48] 5 ZrCa@CNS 190 10 91.2 84.2 [49] 6 Zr/NC 130 2.5 99.9 99.9 [42] 7 Zr-DTPA 140 4 98.7 95.2 [50] 8 Zr-HTC 120 4 100 99.2 [44] 9 Zr-PN 140 2 99 98 [51] 10 m-PhP-Zr 120 2 99 93 [52] 11 Zr-FDCA 140 8 100 87 [53] 12 Zr-MOF-808 82 2 98.2 96.2 [54] 13 DUT-69(Zr) 130 6 97.2 86.2 [55] 14 Zr-tannin 100 5 95 89.3 [56] 15 Zr-LS 100 2 98 89 [57] 16 Hf-LigS 100 2 97.3 89.8 [58] 17 Hf-H3IDC-T 100 4 94 92 [59] 18 FDCA-Hf 100 5 98 95 [60] 19 Hf-MOF-808 100 1.5 − 98 [61] 20 Co/UiO-66-NH2 100 4 92.6 88.8 [62] 21 UiO-66 180 4 87 82 [63] 22 Co3O4/MC 140 12 100 97 [64] 23 MnO@C-N 170 21 98 93 [65] 表 4 以丁醇为氢供体和溶剂HMF选择性加氢反应效果
Table 4 Results of selective catalytic hydrogenation of HMF to BHMF using different butanols as the solvent and hydrogen donor
表 5 催化转移氢化制BHMF不同氢供体的优劣
Table 5 Advantages and disadvantages of different hydrogen donors in catalytic transfer hydrogenation for the synthesis of BHMF
Hydrogen donor Advantage Disadvantage Formic acid or formate salts High atomic utilization rate, high safety and environmental friendliness, ideal liquid hydrogen storage material Stronger acidity and corrosiveness, special requirements of catalyst structure Methanol Renewable, lower price, high hydrogen density Higher reduction potential, harsher reaction conditions Ethanol Renewable, economical efficiency Higher reduction potential Isopropanol Lower reduction potential, small steric-hinerance, and strong hydrogen supply ability Higher cost and equipment corrosion Butanol More sources of hydrogen, high value-added by-product γ- butyrolactone Toxic and cost-effective compared to methanol, ethanol, etc Benzyl alcohol Low price and low volatility Flammable, toxic, irritating Cyclohexanol Higher boiling point, continuous hydrogen-supply, by-product cyclohexanone Higher cost, less research NaBH4 High hydrogen storage capacity, high energy density, safety and reliability Expensive and not easy to be separated NaH2PO2 No obvious toxicity, stable to air and water, easy to handle, and large-scale application Releasing heat, leading to flammability and corrosiveness PMHS It is a byproduct of the silicon industry, which is stable, inexpensive, and low toxic to water and air The overall price is relatively expensive, which in turn leads to higher production costs Ph2SiH2 Non toxic, biodegradable, and stable for air and water Hydrogen production requires activation by metal containing catalysts HMF No additional hydrogen donors need to be added Strong alkaline condition, complicated separation pathways Water or protons Electrocatalysis can be carried out at lower temperatures and pressures, HMF can simultaneously undergo hydrogenation and oxidation to obtain different types of high value-added chemicals Lower efficiency -
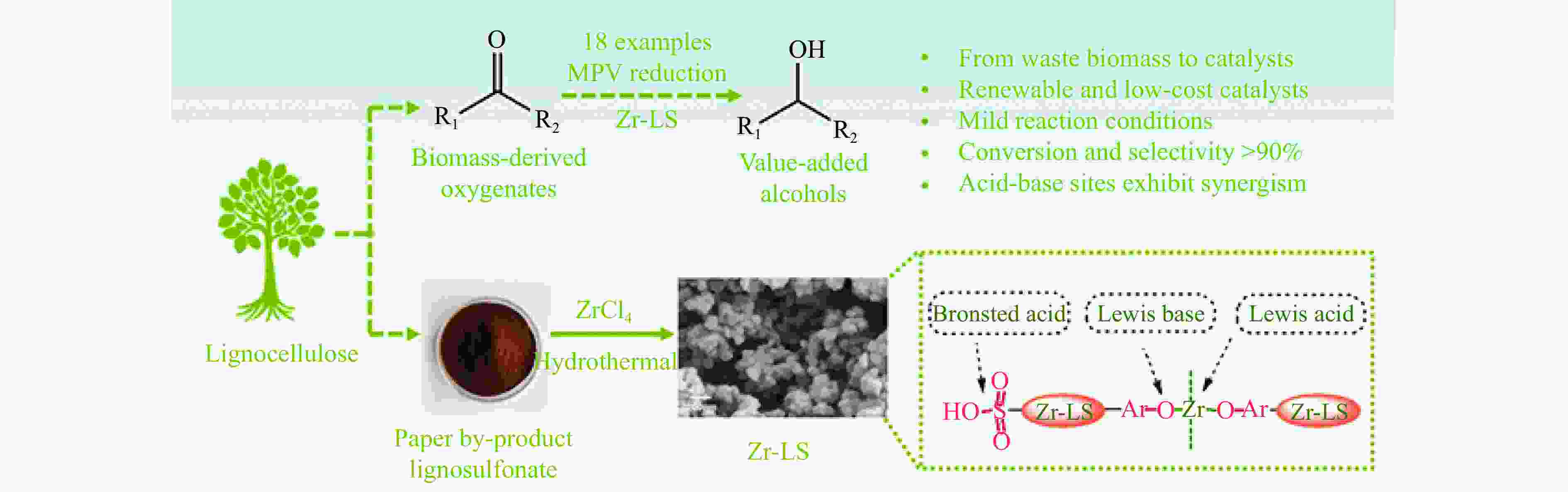
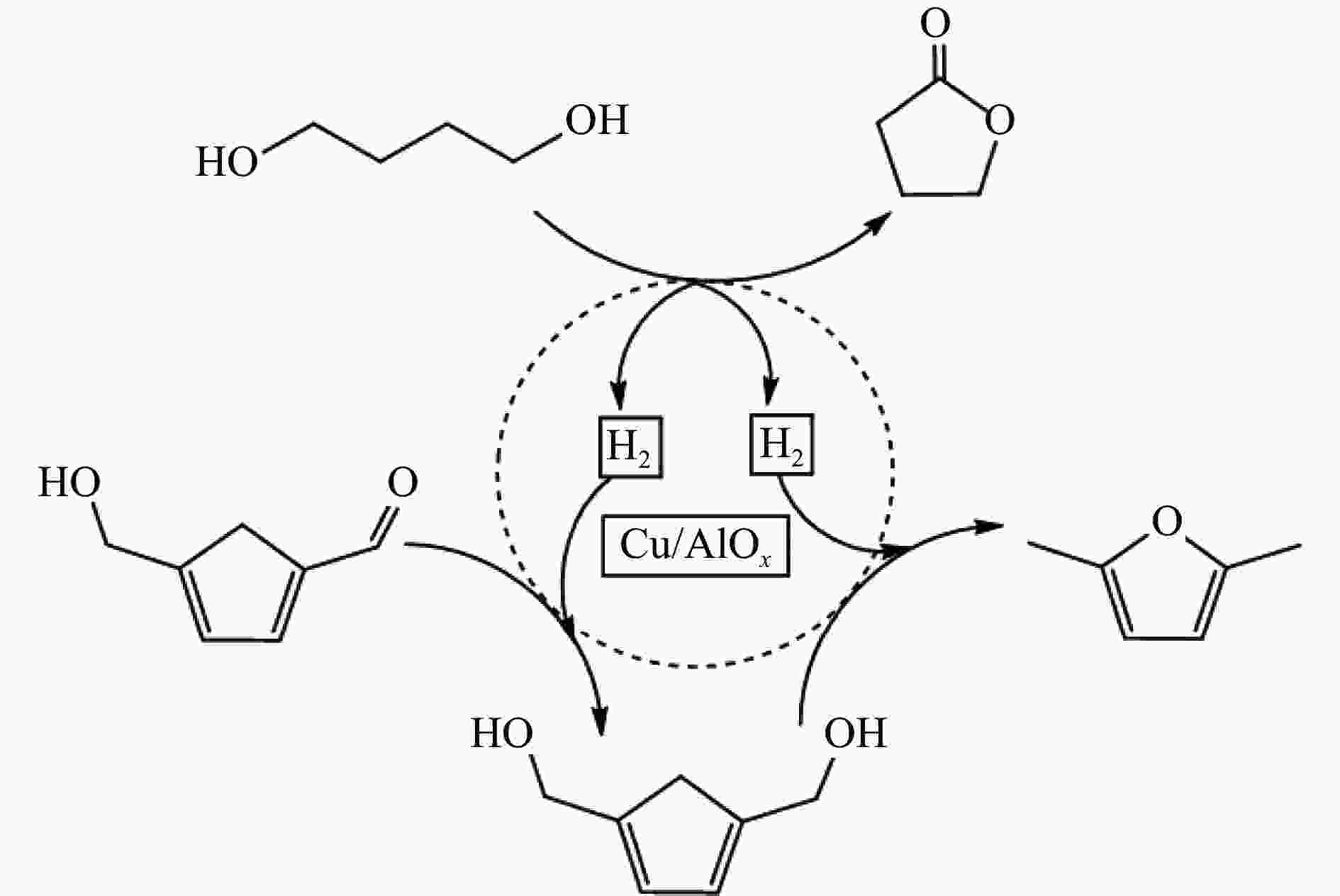
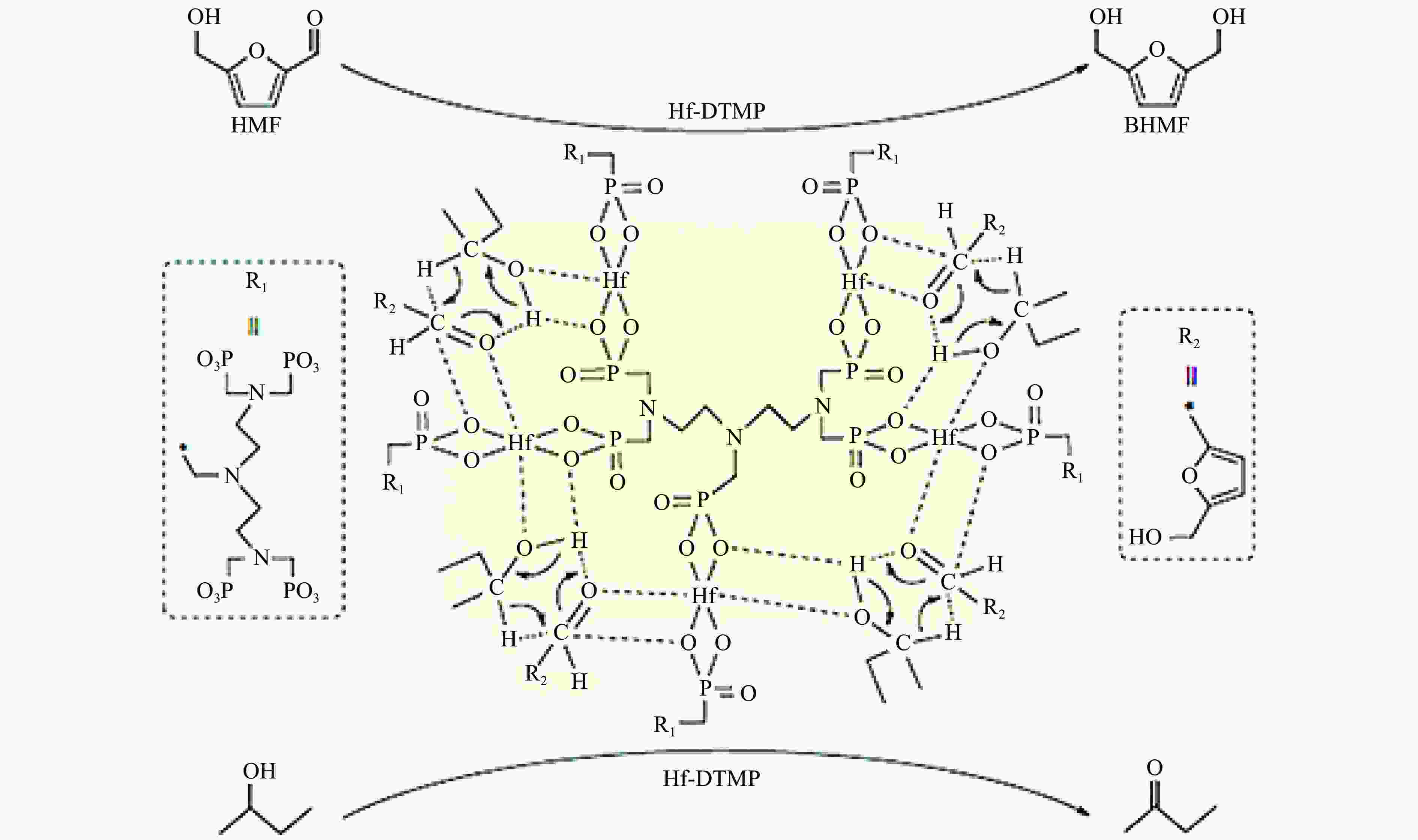
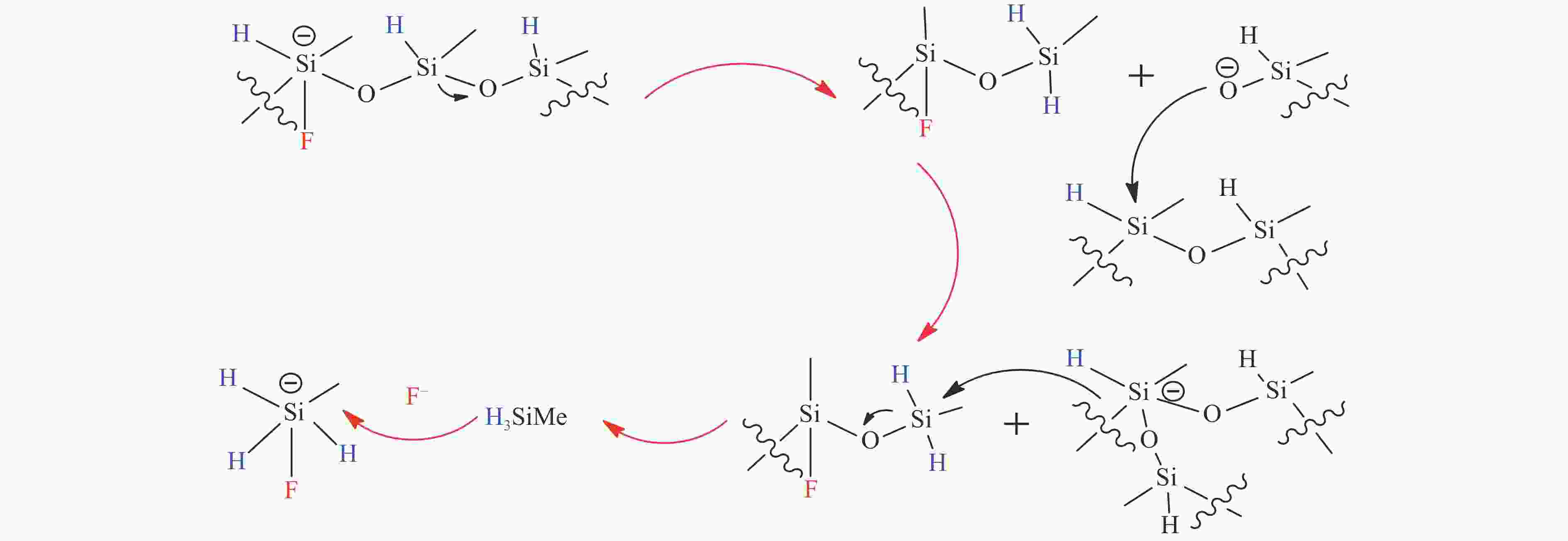
[1] LIAO Y H, KOELEWIJN S F, VAN DEN B G, et al. A sustainable wood biorefinery for low-carbon footprint chemicals production[J]. Science,2020,367(6484):1385−1390. doi: 10.1126/science.aau1567 [2] 加快非粮生物基材料创新发展三年行动方案. 工业和信息化部等六部门[Z]. 2023.A three-year action plan to accelerate the innovative development of non-food biobased materials, MIIT[Z]. 2023. [3] MIKA L T, CSÉFALVAY E, NÉMETH Á. Catalytic conversion of carbohydrates to initial platform chemicals: chemistry and sustainability[J]. Chem Rev,2018,118(2):505−613. doi: 10.1021/acs.chemrev.7b00395 [4] GÉRARDY R, DEBECKER D P, ESTAGER J, et al. Continuous flow upgrading of selected C2-C6 platform chemicals derived from biomass[J]. Chem Rev,2020,120(15):7219−7347. doi: 10.1021/acs.chemrev.9b00846 [5] SU L, FENG Y L, WEI K C, et al. Carbohydrate-based macromolecular biomaterials[J]. Chem Rev,2021,121(18):10950−11029. doi: 10.1021/acs.chemrev.0c01338 [6] CYWAR R M, BECKHAM G T. Producing performance-advantaged bioplastics[J]. Nat Chem,2022,14(9):967−969. doi: 10.1038/s41557-022-01030-y [7] LANE M K M, RUDEL H E, WILSON J A, et al. Green chemistry as just chemistry[J]. Nat Sustain,2023,6(5):502−512. [8] LIN L F, HAN X, HAN B X, et al. Emerging heterogeneous catalysts for biomass conversion: studies of the reaction mechanism[J]. Chem Soc Rev,2021,50(20):11270−11292. doi: 10.1039/D1CS00039J [9] 张军, 李丹妮, 袁浩然, 等. 生物质基糠醛和5-羟甲基糠醛加氢转化研究进展[J]. 燃料化学学报,2021,49(12):1752−1766. doi: 10.1016/S1872-5813(21)60135-4ZHANG Jun, LI Danni, YUAN Haoran, et al. Advances on the catalytic hydrogenation of biomass-derived furfural and 5-hydroxymethylfurfural[J]. J Fuel Chem Technol,2021,49(12):1752−1766. doi: 10.1016/S1872-5813(21)60135-4 [10] XU H C, LI X Y, HU W X, et al. Recent advances on solid acid catalyic systems for production of 5-hydroxymethylfurfural from biomass derivatives[J]. Fuel Process Technol,2022,234:107338. doi: 10.1016/j.fuproc.2022.107338 [11] HU D, ZHANG M, XU H, et al. Recent advance on the catalytic system for efficient production of biomass-derived 5-hydroxymethylfurfural[J]. Renewable Sustainable Energy Rev,2021,147:111253. doi: 10.1016/j.rser.2021.111253 [12] HU Y X, LI H, HU P, et al. Probing the effects of fructose concentration on the evolution of humins during fructose dehydration[J]. React Chem Eng,2023,8(1):175−183. doi: 10.1039/D2RE00324D [13] WENG R G, LU X B, JI N, et al. Taming the butterfly effect: Modulating catalyst nanostructures for better selectivity control of the catalytic hydrogenation of biomass-derived furan platform chemicals[J]. Catal Sci Technol,2021,11(24):7785−7806. doi: 10.1039/D1CY01708J [14] CAI H L, LI C Z, WANG A Q, et al. Biomass into chemicals: one-pot production of furan-based diols from carbohydrates via tandem reactions[J]. Catal Today,2014,234:59−65. doi: 10.1016/j.cattod.2014.02.029 [15] RODRĹGUEZ-PADRÓN D, PEROSA A, LONGO L, et al. Tuning the selectivity of the hydrogenation/hydrogenolysis of 5-hydroxymethylfurfural under batch multiphase and continuous‐flow conditions[J]. ChemSusChem,2022,15(13):e202200503. doi: 10.1002/cssc.202200503 [16] ZHANG J, CHEN J Z. Selective transfer hydrogenation of biomass-based furfural and 5-hydroxymethylfurfural over hydrotalcite-derived copper catalysts using methanol as a hydrogen donor[J]. ACS Sustainable Chem,2017,5(7):5982−5993. doi: 10.1021/acssuschemeng.7b00778 [17] GYNGAZOVA M S, NEGAHDAR L, BLUMENTHAL L C, et al. Experimental and kinetic analysis of the liquid phase hydrodeoxygenation of 5-hydroxymethylfurfural to 2, 5-dimethylfuran over carbon-supported nickel catalysts[J]. Chem Eng Sci,2017,173:455−464. doi: 10.1016/j.ces.2017.07.045 [18] LI D, LIU Q Y, ZHU C H, et al. Selective hydrogenolysis of 5-hydroxymethylfurfural to 2, 5-dimethylfuran over Co3O4 catalyst by controlled reduction[J]. J Energy Chem,2019,30:34−41. doi: 10.1016/j.jechem.2018.03.008 [19] UNTARA T, NOEL S, PHUA P H, et al. From 5-hydroxymethylfurfural (HMF) to polymer precursors: Catalyst screening studies on the conversion of 1, 2, 6-hexanetriol to 1, 6-hexanediol[J]. Top Catal,2012,55:612−619. doi: 10.1007/s11244-012-9839-6 [20] GAO Z, LI C Y, FAN G L, et al. Nitrogen-doped carbon-decorated copper catalyst for highly efficient transfer hydrogenolysis of 5-hydroxymethylfurfural to convertibly produce 2, 5-dimethylfuran or 2, 5-dimethyltetrahydrofuran[J]. Appl Catal B: Environ,2018,226:523−533. doi: 10.1016/j.apcatb.2018.01.006 [21] HU L, XU J X, ZHOU S Y, et al. Catalytic advances in the production and application of biomass-derived 2, 5-dihydroxymethylfuran[J]. ACS Catal,2018,8(4):2959−2980. doi: 10.1021/acscatal.7b03530 [22] NAKAGAWA Y, TAMURA M, TOMISHIGE K. Catalytic reduction of biomass-derived furanic compounds with hydrogen[J]. ACS Catal,2013,3(12):2655−2668. doi: 10.1021/cs400616p [23] ZHANG Y S, REZAYAN A, WANG K, et al. On-demand, highly tunable, and selective 5-hydroxymethylfurfural hydrogenation to furan diols enabled by Ni and Ni3Ga alloy catalysts[J]. ACS Catal,2022,13(1):803−814. [24] 张凯莉, 刘颖, 武书彬. 5-羟甲基糠醛催化氢化制备 2, 5-呋喃二甲醇的研究进展[J]. 化工进展,2019,38(6):2707−2713.ZHANG Kaili, LIU Ying, WU Shubin. Research progress in catalytic hydrogenation of 5-hydroxymethylfurfural to prepare 2, 5-furan dimethanol[J]. Chem Ind Eng Prog,2019,38(6):2707−2713. [25] ZHAO W G, WANG F, ZHAO K Y, et al. Recent advances in the catalytic production of bio-based diol 2, 5-bis (hydroxymethyl) furan[J]. Carbon Resour Convers,2023,6(2):116−131. doi: 10.1016/j.crcon.2023.01.001 [26] CHU G B, YU Z F, GAO F, et al. One-pot formylation and dimerization of p-Alkyl phenols using DCMT-activated DMSO[J]. Synth Commun,2013,43(1):44−51. doi: 10.1080/00397911.2011.590916 [27] FANG W T, RIISAGER A. Recent advances in heterogeneous catalytic transfer hydrogenation/hydrogenolysis for valorization of biomass-derived furanic compounds[J]. Green Chem,2021,23(2):670−688. doi: 10.1039/D0GC03931D [28] THANANATTHANACHON T, RAUCHFUSS T B. Efficient production of the liquid fuel 2, 5-dimethylfuran from fructose using formic acid as a reagent[J]. Angew Chem,2010,37(122):6766−6768. [29] THANANATTHANACHON T, RAUCHFUSS T B. Efficient route to hydroxymethylfurans from sugars via transfer hydrogenation[J]. ChemSusChem,2010,3(10):1139−1141. doi: 10.1002/cssc.201000209 [30] XU L, NIE R F, CHEN X J, et al. Formic acid enabled selectivity boosting in transfer hydrogenation of 5-hydroxymethylfurfural to 2, 5-furandimethanol on highly dispersed Co-Nx sites[J]. Catal Sci Technol,2021,11(4):1451−1457. doi: 10.1039/D0CY01969K [31] XU L, NIE R F, LYU X L, et al. One-pot production of 2, 5-furandimethanol from fructose Co-catalyzed with formic acid and heterogeneous Co catalysts[J]. Energy Fuels,2021,36(1):480−487. [32] XU L, LYU X L, JIANG Y X, et al. Highly selective one-pot production of 2, 5-furandimethanol from saccharides[J]. Green Chem,2022,24(12):4935−4940. doi: 10.1039/D2GC00500J [33] VAN DER WAAL J C, KUNKELER P J, TAN K, et al. Zeolite titanium beta: A selective catalyst for the gas-phase Meerwein-Ponndorf-Verley, and oppenauer reactions[J]. J Catal,1998,173(1):74−83. [34] CHATTERJEE M, ISHIZAKA T, KAWANAMI H. Selective hydrogenation of 5-hydroxymethylfurfural to 2, 5-bis-(hydroxymethyl) furan using Pt/MCM-41 in an aqueous medium: A simple approach[J]. Green Chem,2014,16(11):4734−4739. doi: 10.1039/C4GC01127A [35] TANG X, WEI J N, DING N, et al. Chemoselective hydrogenation of biomass derived 5-hydroxymethylfurfural to diols: Key intermediates for sustainable chemicals, materials and fuels[J]. Renewable Sustainable Energy Rev,2017,77:287−296. doi: 10.1016/j.rser.2017.04.013 [36] HE Y M, DENG L M, LEE Y, et al. A review on the critical role of H2 donor in the selective hydrogenation of 5-hydroxymethylfurfural[J]. ChemSusChem,2022,15(13):e202200232. doi: 10.1002/cssc.202200232 [37] PASINI T, LOLLI A, ALBONETTI S, et al. Methanol as a clean and efficient h-transfer reactant for carbonyl reduction: Scope, limitations, and reaction mechanism[J]. J Catal,2014,317:206−219. doi: 10.1016/j.jcat.2014.06.023 [38] HAO W W, LI W F, TANG X, et al. Catalytic transfer hydrogenation of biomass-derived 5-hydroxymethyl furfural to the building block 2, 5-bishydroxymethyl furan[J]. Green Chem,2016,18(4):1080−1088. doi: 10.1039/C5GC01221J [39] ELSAYED I, JACKSON M A, HASSAN E B. Hydrogen-free catalytic reduction of biomass-derived 5-hydroxymethylfurfural into 2, 5-bis (hydroxymethyl) furan using copper-iron oxides bimetallic nanocatalyst[J]. ACS Sustainable Chem Eng,2020,8(4):1774−1785. doi: 10.1021/acssuschemeng.9b05575 [40] LIU Z X, HUANG Z X, ZHAO W G, et al. Highly efficient Ni-NiO/carbon nanotubes catalysts for the selective transfer hydrogenation of 5-hydroxymethylfurfural to 2, 5-bis (hydroxymethyl) furan[J]. React Chem Eng,2022,7(8):1873−1878. doi: 10.1039/D2RE00134A [41] HUANG Z X, ZENG Z J, ZHU X T, et al. Boehmite-supported CuO as a catalyst for catalytic transfer hydrogenation of 5-hydroxymethylfurfural to 2,5-bis (hydroxymethyl) furan[J]. Front Chem Sci Eng,2023,17(4):415−424. [42] HU L, HE A Y, SHEN X M, et al. A high-efficiency zirconium-based single-atom catalyst for the transformation of biomass-derived 5 hydroxymethylfurfural to 2, 5-bis (hydroxymethyl) furan with nearly 100% selectivity[J]. Green Chem,2022,24(18):6931−6944. doi: 10.1039/D2GC02770D [43] TANG X, ZENG X H, LI Z, et al. In situ generated catalyst system to convert biomass-derived levulinic acid to γ-valerolactone[J]. ChemCatChem,2015,7(8):1372−1379. doi: 10.1002/cctc.201500115 [44] HE A Y, HU L, ZHANG Y M, et al. High-efficiency catalytic transfer hydrogenation of biomass-based 5-hydroxymethylfurfural to 2, 5-bis (hydroxymethyl) furan over a zirconium-carbon coordination catalyst[J]. ACS Sustainable Chem Eng,2021,9(46):15557−15570. doi: 10.1021/acssuschemeng.1c05618 [45] SCHOLZ D, AELLIG C, HERMANS I. Catalytic transfer hydrogenation/hydrogenolysis for reductive upgrading of furfural and 5-(hydroxymethyl) furfural[J]. ChemSusChem,2014,7(1):268−275. doi: 10.1002/cssc.201300774 [46] WANG T, ZHANG J H, XIE W X, et al. Catalytic transfer hydrogenation of biobased HMF to 2, 5-bis-(hydroxymethyl) furan over Ru/Co3O4[J]. Catalysts,2017,7(3):92. [47] ZHANG J H, XIE W X, LIANG Q D, et al. Nano-fibrillated cellulose as a versatile carrier of Ru/Cu nanoparticles for the catalytic transfer hydrogenation of 5-hydroxymethyfural to 2, 5-bishydroxymethylfuran[J]. ChemistrySelect,2019,4(9):2846−2850. doi: 10.1002/slct.201900285 [48] WEI J N, CAO X J, WANG T, et al. Catalytic transfer hydrogenation of biomass-derived 5-hydroxymethylfurfural into 2, 5-bis (hydroxymethyl) furan over tunable Zr-based bimetallic catalysts[J]. Catal Sci Technol,2018,8(17):4474−4484. doi: 10.1039/C8CY00500A [49] ZHANG J H, QI Z H, LIU Y, et al. Selective hydrogenation of 5-hydroxymethylfurfural into 2, 5-bis (hydroxymethyl) furan over a cheap carbon-nanosheets-supported Zr/Ca bimetallic catalyst[J]. Energy Fuels,2020,34(7):8432−8439. doi: 10.1021/acs.energyfuels.0c01128 [50] HU L, LIU S, SONG J, et al. Zirconium-containing organic-inorganic nanohybrid as a highly efficient catalyst for the selective synthesis of biomass-derived 2, 5-dihydroxymethylfuran in isopropanol[J]. Waste Biomass Valorization,2020,11:3485−3499. doi: 10.1007/s12649-019-00703-z [51] LI H, HE J, RIISAGER A, et al. Acid-base bifunctional zirconium n-alkyltriphosphate nanohybrid for hydrogen transfer of biomass-derived carboxides[J]. ACS Catal,2016,6(11):7722−7727. doi: 10.1021/acscatal.6b02431 [52] LI H, FANG Z, HE J, et al. Orderly layered Zr-benzylphosphonate nanohybrids for efficient acid-base-mediated bifunctional/cascade catalysis[J]. ChemSusChem,2017,10(4):681−686. doi: 10.1002/cssc.201601570 [53] LI H, LIU X F, YANG T T, et al. Porous zirconium-furandicarboxylate microspheres for efficient redox conversion of biofuranics[J]. ChemSusChem,2017,10(8):1761−1770. doi: 10.1002/cssc.201601898 [54] VALEKAR A H, LEE M, YOON J W, et al. Catalytic transfer hydrogenation of furfural to furfuryl alcohol under mild conditions over Zr-MOFs: Exploring the role of metal node coordination and modification[J]. ACS Catal,2020,10(6):3720−3732. doi: 10.1021/acscatal.9b05085 [55] WANG T, HU A Y, XU G Z, et al. Porous Zr-thiophenedicarboxylate hybrid for catalytic transfer hydrogenation of bio-based furfural to furfuryl alcohol[J]. Catal Lett,2019,149:1845−1855. doi: 10.1007/s10562-019-02748-0 [56] LENG Y, SHI L C, DU S Y, et al. A tannin-derived zirconium-containing porous hybrid for efficient Meerwein-Ponndorf-Verley reduction under mild conditions[J]. Green Chem,2020,22(1):180−186. doi: 10.1039/C9GC03393A [57] ZHOU S H, DAI F L, XIANG Z Y, et al. Zirconium-lignosulfonate polyphenolic polymer for highly efficient hydrogen transfer of biomass-derived oxygenates under mild conditions[J]. Appl Catal B: Environ,2019,248:31−43. doi: 10.1016/j.apcatb.2019.02.011 [58] ZHOU S H, DAI F L, CHEN Y A, et al. Sustainable hydrothermal self-assembly of hafnium-lignosulfonate nanohybrids for highly efficient reductive upgrading of 5-hydroxymethylfurfural[J]. Green Chem,2019,21(6):1421−1431. doi: 10.1039/C8GC03710H [59] DAI F L, ZHOU S H, QIN X Z, et al. Surfactant-assisted synthesis of mesoporous hafnium-imidazoledicarboxylic acid hybrids for highly efficient hydrogen transfer of biomass-derived carboxides[J]. Mol Catal,2019,479:110611. doi: 10.1016/j.mcat.2019.110611 [60] LI H, YANG T T, FANG Z. Biomass-derived mesoporous Hf-containing hybrid for efficient Meerwein-Ponndorf-Verley reduction at low temperatures[J]. Appl Catal B: Environ,2018,227:79−89. doi: 10.1016/j.apcatb.2018.01.017 [61] ROJAS-BUZO S, GARCĹA-GARCĹA P, CORMA A. Catalytic transfer hydrogenation of biomass-derived carbonyls over hafnium-based metal-organic frameworks[J]. ChemSusChem,2018,11(2):432−438. doi: 10.1002/cssc.201701708 [62] WANG L, ZUO J L, ZHANG Q T, et al. Catalytic transfer hydrogenation of biomass-derived 5-hydroxymethylfurfural into 2, 5-dihydroxymethylfuran over Co/UiO-66-NH2[J]. Catal Lett,2022,15(2):361−371. [63] QIU M, GUO T M, XI R, et al. Highly efficient catalytic transfer hydrogenation of biomass-derived furfural to furfuryl alcohol using UiO-66 without metal catalysts[J]. Appl Catal A: Gen,2020,602:117719. doi: 10.1016/j.apcata.2020.117719 [64] WANG G H, DENG X H, GU D, et al. Co3O4 nanoparticles supported on mesoporous carbon for selective transfer hydrogenation of α, β-unsaturated aldehydes[J]. Angew Chem Int Ed,2016,55(37):11101−11105. doi: 10.1002/anie.201604673 [65] FENG Y C, LONG S S, YAN G H, et al. Manganese catalyzed transfer hydrogenation of biomass-derived aldehydes: Insights to the catalytic performance and mechanism[J]. J Catal,2020,389:157−165. doi: 10.1016/j.jcat.2020.05.037 [66] HUANG J, DAI W L, LI H X, et al. Au/TiO2 as high efficient catalyst for the selective oxidative cyclization of 1, 4-butanediol to γ-butyrolactone[J]. J Catal,2007,252(1):69−76. doi: 10.1016/j.jcat.2007.09.011 [67] AELLIG C, JENNY F, SCHOLZ D, et al. Combined 1, 4-butanediol lactonization and transfer hydrogenation/hydrogenolysis of furfural-derivatives under continuous flow conditions[J]. Catal Sci Technol,2014,4(8):2326−2331. doi: 10.1039/C4CY00213J [68] HU L, LI T, XU J X, et al. Catalytic transfer hydrogenation of biomass-derived 5-hydroxymethylfurfural into 2, 5-dihydroxymethylfuran over magnetic zirconium-based coordination polymer[J]. Chem Eng J,2018,352:110−119. doi: 10.1016/j.cej.2018.07.007 [69] HU L, YANG M, XU N, et al. Selective transformation of biomass-derived 5-hydroxymethylfurfural into 2, 5-dihydroxymethylfuran via catalytic transfer hydrogenation over magnetic zirconium hydroxides[J]. Korean J Chem Eng,2018,35:99−109. doi: 10.1007/s11814-017-0238-3 [70] HU L, LI N, DAI X L, et al. Highly efficient production of 2, 5-dihydroxymethylfuran from biomass-derived 5-hydroxymethylfurfural over an amorphous and mesoporous zirconium phosphonate catalyst[J]. J Energy Chem,2019,37:82−92. doi: 10.1016/j.jechem.2018.12.001 [71] HU L, DAI X L, LI N, et al. Highly selective hydrogenation of biomass-derived 5-hydroxymethylfurfural into 2, 5-bis (hydroxymethyl) furan over an acid-base bifunctional hafnium-based coordination polymer catalyst[J]. Sustainable Energy Fuels,2019,3(4):1033−1041. doi: 10.1039/C8SE00545A [72] GAO Z, YANG L, FAN G L, et al. Promotional role of surface defects on carbon-Supported ruthenium-based catalysts in the transfer hydrogenation of furfural[J]. ChemCatChem,2016,8(24):3769−3779. doi: 10.1002/cctc.201601070 [73] SIDDIQUI N, KHATUN R, MISHRA V K, et al. Selective transfer hydrogenation of biomass derived furanic molecules using cyclohexanol as a hydrogen donor over nanostructured Cu/MgO catalyst[J]. Mol Catal,2021,513:111812. doi: 10.1016/j.mcat.2021.111812 [74] ZENG C, SEINO H, REN J, et al. Bio-based furan polymers with self-healing ability[J]. Macromol,2013,46(5):1794−1802. doi: 10.1021/ma3023603 [75] GOSWAMI S, DEY S, JANA S. Design and synthesis of a unique ditopic macrocyclic fluorescent receptor containing furan ring as a spacer for the recognition of dicarboxylic acids[J]. Tetrahedron,2008,64(27):6358−6363. doi: 10.1016/j.tet.2008.04.086 [76] WRIGSTEDT P, KESKIVÄLI J, PEREA-BUCETA J E, et al. One-pot transformation of carbohydrates into valuable furan derivatives via 5-hydroxymethylfurfural[J]. ChemCatChem,2017,9(22):4244−4255. doi: 10.1002/cctc.201701106 [77] WANG T, WEI J N, LIU H, et al. Synthesis of renewable monomer 2, 5-bishydroxymethylfuran from highly concentrated 5-hydroxymethylfurfural in deep eutectic solvents[J]. J Ind Eng Chem,2020,81:93−98. doi: 10.1016/j.jiec.2019.08.057 [78] KANG E S, HONG Y W, CHAE D W, et al. From lignocellulosic biomass to furans via 5-acetoxymethylfurfural as an alternative to 5-hydroxymethylfurfural[J]. ChemSusChem,2015,8(7):1179−1188. doi: 10.1002/cssc.201403252 [79] NISHIMURA S, SHIMURA T, EBITANI K. Transfer hydrogenation of furaldehydes with sodium phosphinate as a hydrogen source using Pd-supported alumina catalyst[J]. J Taiwan Inst Chem Eng,2017,79:97−102. doi: 10.1016/j.jtice.2017.03.028 [80] VOLKOV A, GUSTAFSON K P J, TAI C W, et al. Mild deoxygenation of aromatic ketones and aldehydes over Pd/C using polymethylhydrosiloxane as the reducing agent[J]. Angew Chem Int Ed,2015,54(17):5122−5126. doi: 10.1002/anie.201411059 [81] LI A Y, SEGALLA A, LI C J, et al. Mechanochemical metal-free transfer hydrogenation of carbonyls using polymethylhydrosiloxane as the hydrogen source[J]. ACS Sustainable Chem Eng,2017,5(12):11752−11760. doi: 10.1021/acssuschemeng.7b03298 [82] ZHAO W F, WU W B, LI H, et al. Quantitative synthesis of 2, 5-bis (hydroxymethyl) furan from biomass-derived 5-hydroxymethylfurfural and sugars over reusable solid catalysts at low temperatures[J]. Fuel,2018,217:365−369. doi: 10.1016/j.fuel.2017.12.069 [83] LONG J X, ZHAO W F, XU Y F, et al. Carbonate-catalyzed room-temperature selective reduction of biomass-derived 5-hydroxymethylfurfural into 2, 5-bis (hydroxymethyl) furan[J]. Catalysts,2018,8(12):633. doi: 10.3390/catal8120633 [84] LI G, SUN Z, YAN Y, et al. Direct transformation of HMF into 2, 5-diformylfuran and 2, 5-dihydroxymethylfuran without an external oxidant or reductant[J]. ChemSusChem,2017,10(3):494−498. doi: 10.1002/cssc.201601322 [85] KANG E S, CHAE D W, KIM B, et al. Efficient preparation of DHMF and HMFA from biomass-derived HMF via a cannizzaro reaction in ionic liquids[J]. J Ind Eng Chem,2012,18(1):174−177. doi: 10.1016/j.jiec.2011.11.020 [86] SUBBIAH S, SIMEONOV S P, ESPERANÇA J M S S, et al. Direct transformation of 5-hydroxymethylfurfural to the building blocks 2, 5-dihydroxymethylfurfural (DHMF) and 5-hydroxymethyl furanoic acid (HMFA) via cannizzaro reaction[J]. Green Chem,2013,15(10):2849−2853. doi: 10.1039/c3gc40930a [87] KWON Y, DE JONG E, RAOUFMOGHADDAM S, et al. Electrocatalytic hydrogenation of 5-hydroxymethylfurfural in the absence and presence of glucose[J]. ChemSusChem,2013,6(9):1659−1667. doi: 10.1002/cssc.201300443 [88] KWON Y, BIRDJA Y Y, RAOUFMOGHADDAM S, et al. Electrocatalytic hydrogenation of 5-hydroxymethylfurfural in acidic solution[J]. ChemSusChem,2015,8(10):1745−1751. doi: 10.1002/cssc.201500176 [89] ROYLANCE J J, KIM T W, CHOI K S. Efficient and selective electrochemical and photoelectrochemical reduction of 5-hydroxymethylfurfural to 2, 5-bis (hydroxymethyl) furan using water as the hydrogen source[J]. ACS Catal,2016,6(3):1840−1847. doi: 10.1021/acscatal.5b02586 [90] ZHANG L, ZHANG F, MICHEL JR F C, et al. Efficient electrochemical hydrogenation of 5-hydroxymethylfurfural to 2, 5-bis (hydroxymethyl) furan on Ag-displaced nanotextured Cu catalysts[J]. ChemElectroChem,2019,6(18):4739−4749. doi: 10.1002/celc.201900640 [91] CHADDERDON X H, CHADDERDON D J, PFENNIG T, et al. Paired electrocatalytic hydrogenation and oxidation of 5-(hydroxymethyl) furfural for efficient production of biomass-derived monomers[J]. Green Chem,2019,21(22):6210−6219. doi: 10.1039/C9GC02264C [92] ZHANG Z L, HUANG K, QIU X Y, et al. Operando generated copper-based catalyst enabling efficient electrosynthesis of 2,5-bis (hydroxymethyl) furan[J]. Fundam Res,2023,3(5):763−769. [93] JI K Y, XU M, XU S M, et al. Electrocatalytic hydrogenation of 5-hydroxymethylfurfural promoted by a Ru1Cu single-atom alloy catalyst[J]. Angew Chem Int Ed,2022,61(37):e202209849. doi: 10.1002/anie.202209849 [94] CHEN S, WOJCIESZAK R, DUMEIGNIL F, et al. How catalysts and experimental conditions determine the selective hydroconversion of furfural and 5-hydroxymethylfurfural[J]. Chem Rev,2018,118(22):11023−11117. doi: 10.1021/acs.chemrev.8b00134 -




 下载:
下载: