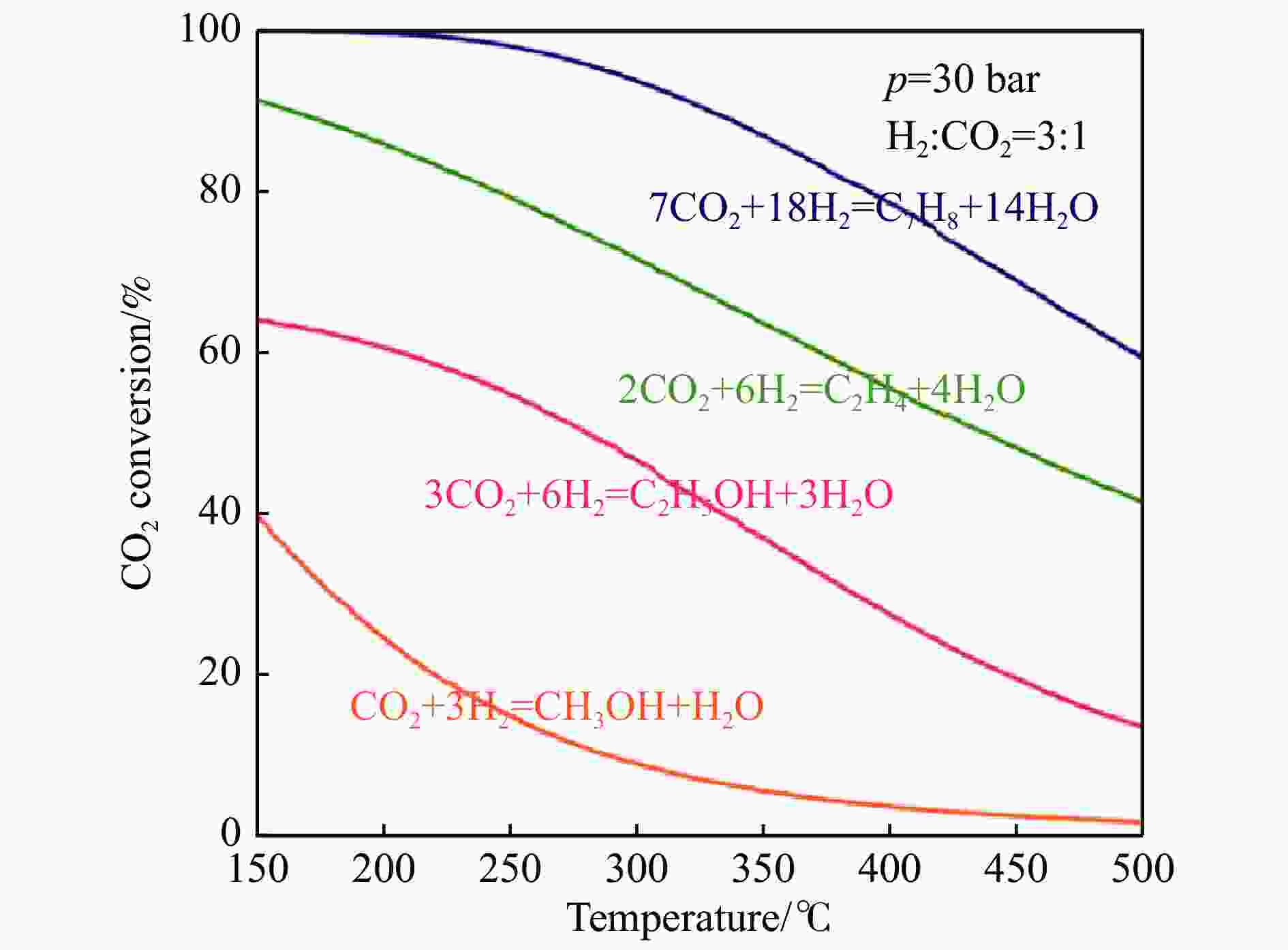
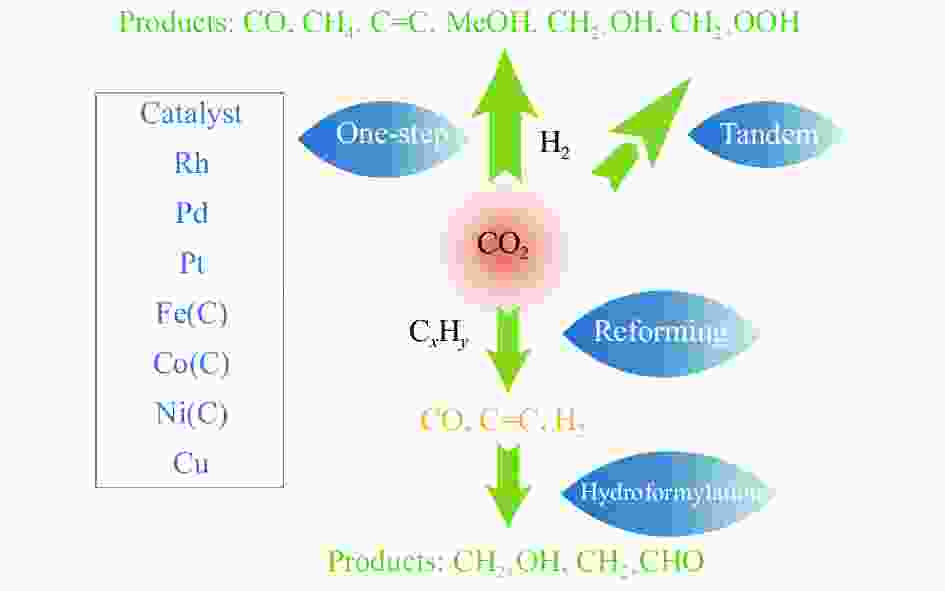
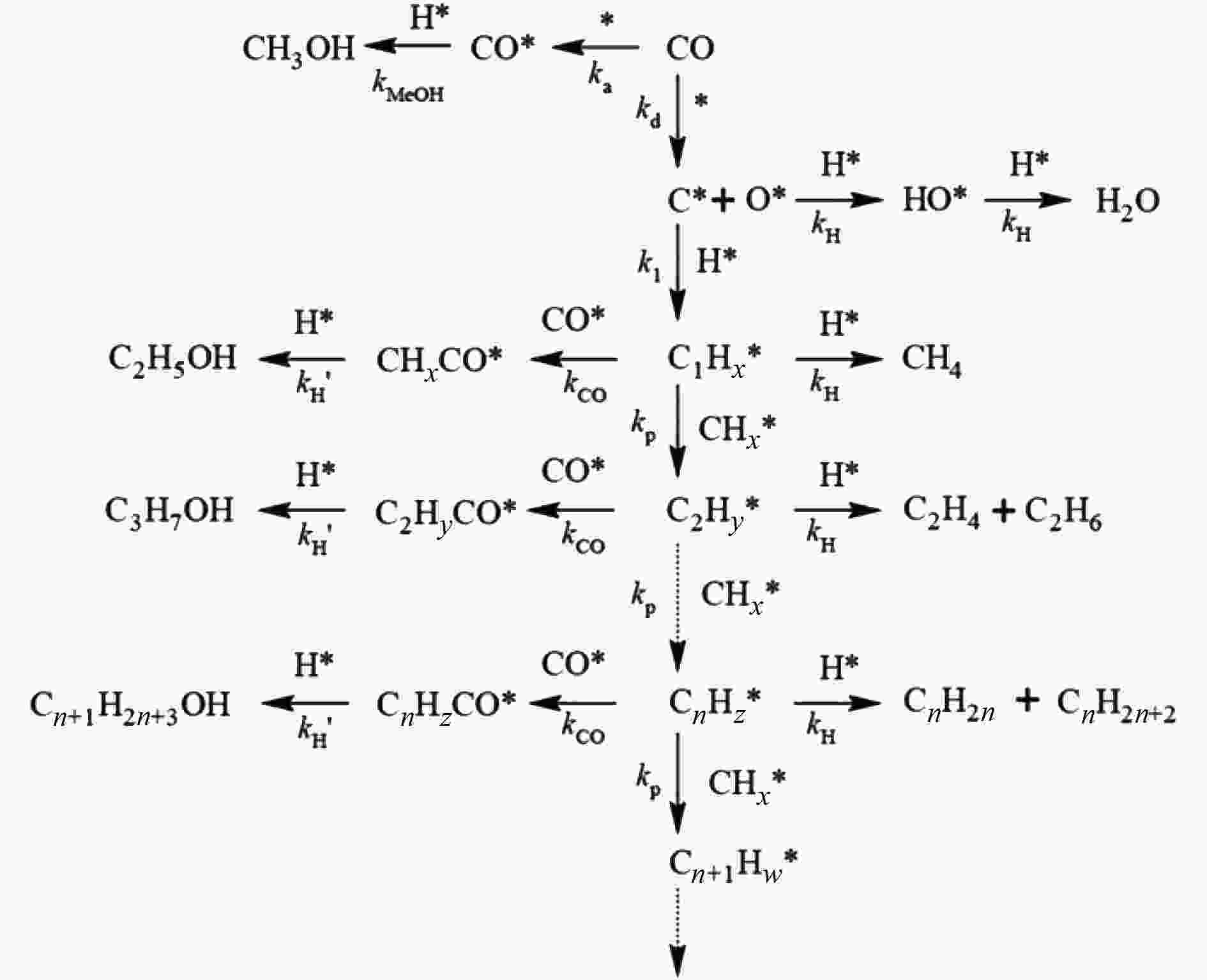
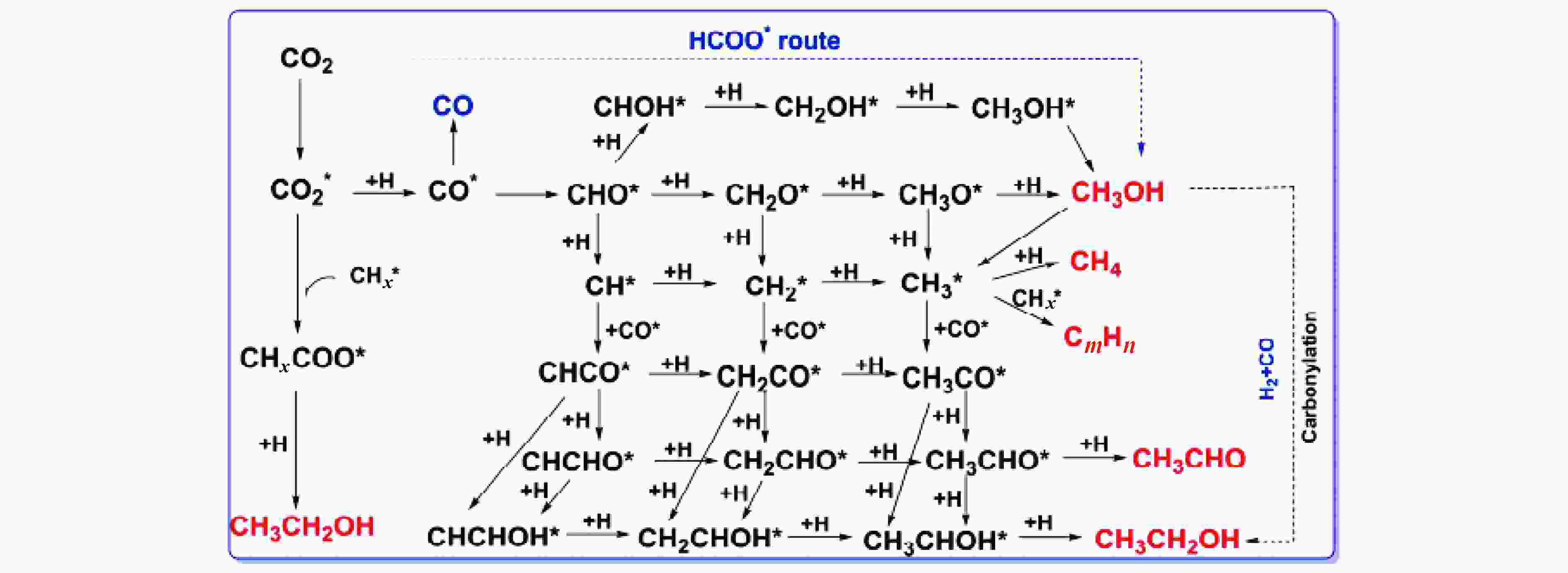
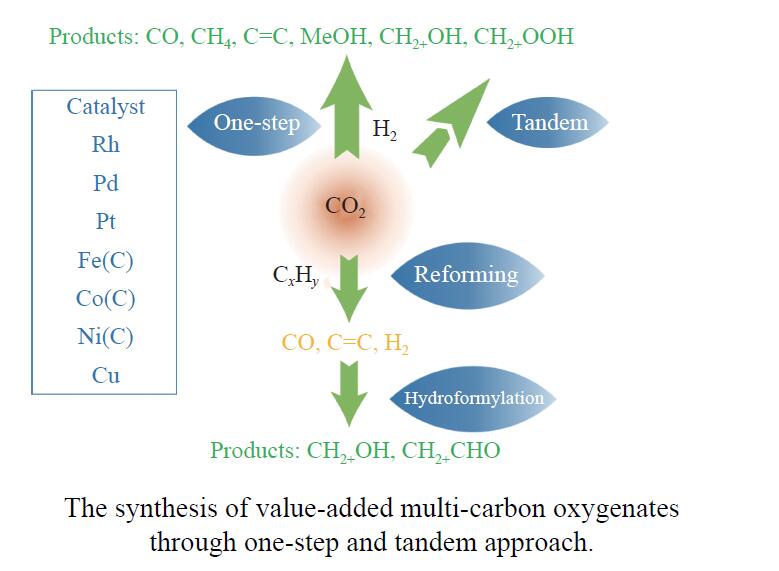
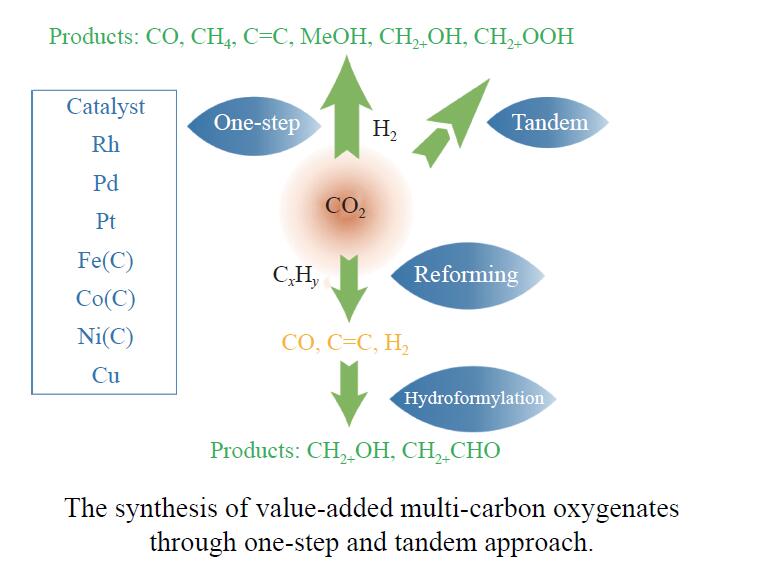
Research progress on CO2 catalytic conversion to value-added oxygenates
-
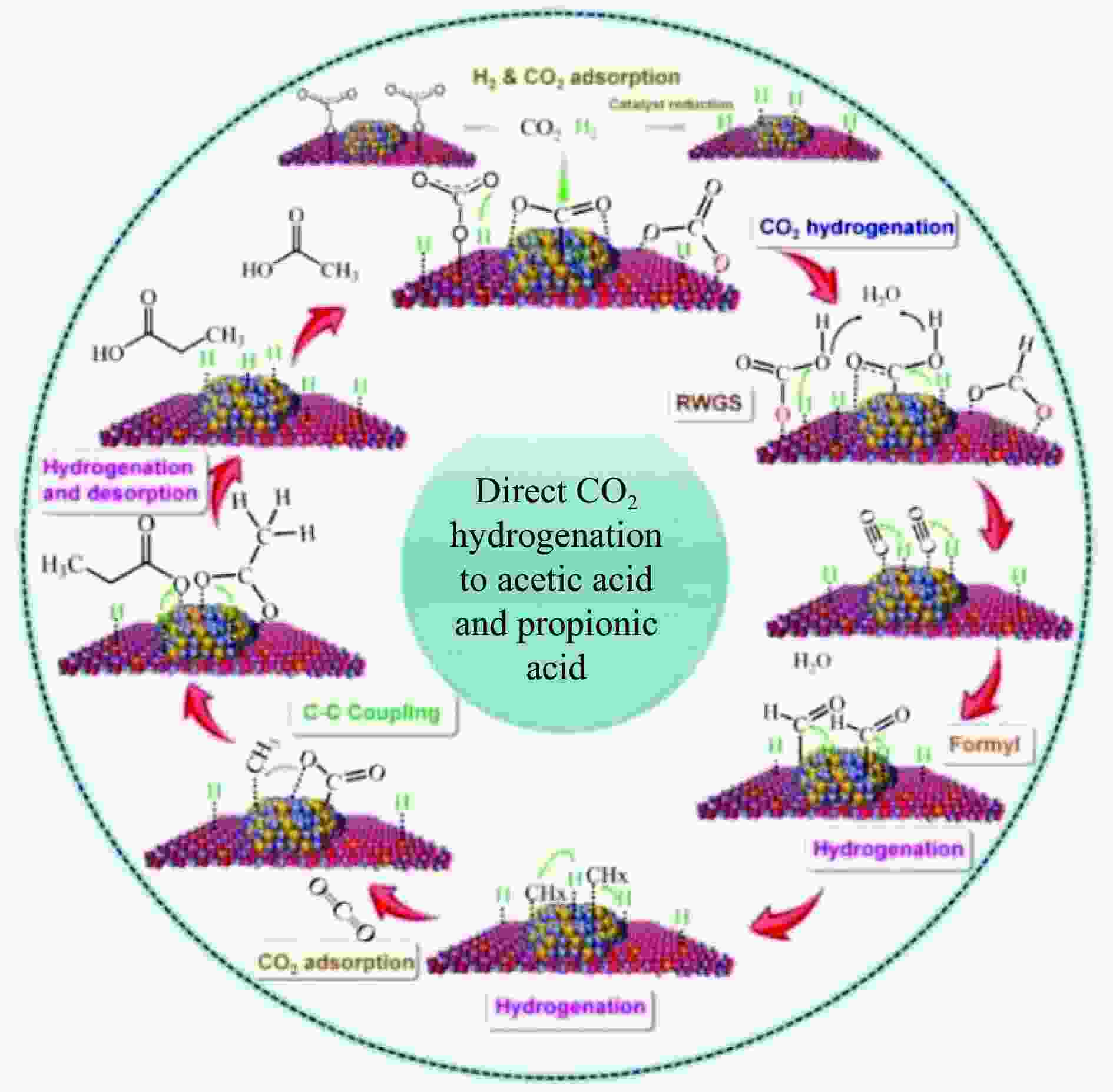
摘要: 将温室气体CO2通过化学反应路径制备高附加值多碳含氧化合物如乙醇、乙酸、丙醛、丙酸、丁醇等具有挑战性。由于C−C偶联反应的复杂性和成键的不可控性,导致合成多碳高值含氧化合物困难。本工作总结了近期在连续流固定床条件下CO2催化合成高附加值多碳含氧化合物的研究进展。首先归纳了CO2加氢路径下可能的反应机理;其次总结了CO2直接加氢(一步法、串联法)、CO2与轻烃重整、CO2氢甲酰化等不同反应路径下具有潜力的催化剂,包括金属碳化物、碱金属修饰的Cu、Fe、Co、Rh等单金属或二元金属制备多碳高值含氧化合物的特点,并进一步阐述了不同催化剂上的作用机制。最后对目前存在的问题和未来可能的解决方案进行了讨论和展望。Abstract: Chemical conversion of greenhouse gas CO2 into value-added oxygenates such as ethanol, acetic acid, propanal, propionic acid, butanol, etc. is challenging due to the complexity of C−C coupling and the uncontrollable bonding. In this review, recent research progresses on the synthesis of multi-carbon oxygenates from CO2 in fixed bed reactor are provided. Firstly, the reaction mechanisms of CO2 hydrogenation are summarized. Then, the potential catalysts applied in one-step or tandem CO2 hydrogenation, dry reforming with light hydrocarbons and hydroformylation were introduced over metal carbides, alkali metal modified single or binary metal catalysts such as Cu, Fe, Co, Rh, etc. The reaction mechanism over different catalysts were further elaborated. Finally, the problems and outlook are discussed.
-
Key words:
- CO2 conversion /
- multi-carbon oxygenates /
- one-step method /
- tandem catalysis
-
表 1 金属碳化物催化剂催化CO2加氢制多碳醇性能比较
Table 1 Catalytic performance comparison for CO2 Hydrogenation to C2+ alcohols over typical metal carbide catalysts
Catalyst Reaction
temp./
℃Reaction
pressure/
MPaH2/
CO2WHSV/
(mL·g−1·h−1)CO2
conversion/
%Selectivity/C%a C2+OH/
ROH/
%C2+OH
STY/
(mg·g−1·h−1)Stability/
hRef. CO CH4+
C2+HxCH3OH C2+OH other
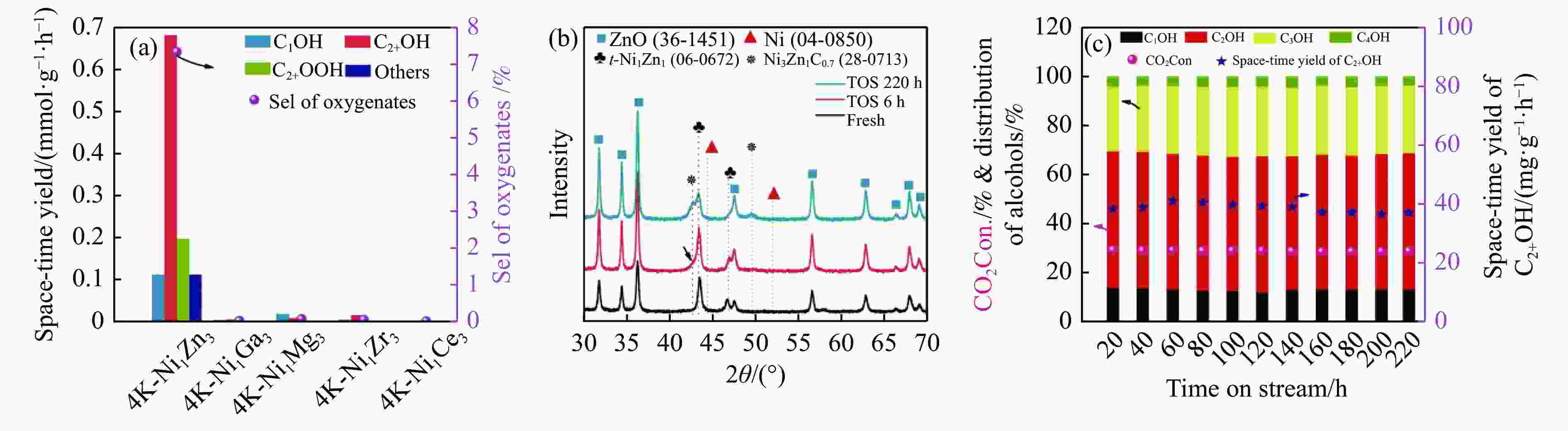
oxy.10Mn1K-FeCb 300 3 3 6000 40.5 33.4 55.9 0.2 10.5 0 92.9 − 100 [31] 4.6K-CuMgZnFe 320 5 3 6000 30.4 30.6 52.4 1.3 15.9 0 89.8 69.6 100 [32] Na-ZnFe@Cb 320 5 3 9000 38.4 7.6 68.1 1.7 22.6 ~ 0 90.5 158.1 528 [33] Na–Co/SiO2 250 5 3 4000 18.82 29.05 61.41 0.85 8.69 0 87.5 − 300 [34] Na–Co/Si3N4 250 5 3 4000 17.75 39.16 51.65 0.75 8.44 0 88.5 − 300 [34] 4K-Ni1Zn3−300b 350 3 3 4800 29.7 47.9 42.5 0.5 6.7 1.3 90 62.7 80 [22] a: C% represents the carbon atoms concentration of given product to the total carbon-containing products, b: C2+OH/ROH fraction (%) was calculated according to the carbon atoms concentration of MeOH to C2+OH. 表 2 金属活性位催化剂催化二氧化碳加氢制乙醇性能比较
Table 2 Catalytic performance comparison for CO2 hydrogenation to C2+ alcohols over different metallic catalysts
Active
metalCatalyst Reaction
temp./
℃Reaction
pressure/
MPaH2/
CO2WHSV/
(mL·g−1·h−1)CO2
conversion/
%Selectivity/C% ROH/
C2+OH/
C%C2+OH
STY/
(mg·g−1·h−1)Ref. CO CH4+
C2+HxCH3OH C2+OH other
oxy.Rh Na-Rh@S-1 250 5 3 6000 10 32 ~ 24.8 ~ 25.2 ~ 18 0 ~ 41.7 72 b [8] Rh-VOx/MCM-41 250 3 3 6000a 12.1 20.1 48.3 7.5 24.1 0 76.3 47.9 [45] RhFeLi/TiO2 250 5 3 6000a 15.7 12.5 53.9 2.2 31.3 0.1 93.4 145.3 [46] Rh-Li/SiO2 240 5 3 6000 7 15.7 63.5 5.2 15.5 0 74.9 33.1 [47] Rh-Fe/SiO2 260 5 3 6000 26.7 19.7 34.7 29.4 16 0 35.2 130.3 [48] Pd Pd2Ce@Si16 240 3 3 3000 5.9 ~ 0 ~ 0 ~ 1 98.7 0 ~ 99 252c [49] Pd/Fe3O4 250 3 3 8000 1.4 ~ 0 ~ 0 2 98 0 98 440c [50] In 2.5K5Co-In2O3 380 4 3 2250 36 81 6.5 1.5 11.2 0 88.1 170 [51] Co Ir/Co(A)-Na2O/SiO2 220 2.1 3 2000a 7.6 38.5 45.3 7.8 7.9 0 50.3 [52] Co/La4Ga2O9 280 3.5 3 3000 9.6 10.8 52.2 13.7 23.3 0 62.9 34.5 [53] Ga(0.4)CuCo 220 3 3 6000 17.8 2.3 45.4 27.5 24.8 0 47.4 1.4 d [54] Fe FeNaS-0.6 320 3 3 8000 32 20.7 66.5 0.16 12.6 0 98.8 78.5 [55] Cu Cu@Na-Beta 300 1.3 3 12000 7.9 30.5 ~ 0 ~ 0 69.5 0 ~ 100 258 [23] CuFe KFeCu/α-ZrO2 320 4 3 3000 30 30 49 1.4 19.6 0 93.3 59.1 [56] a: GHSV of given catalyst, b: C2+OH STY per unit mass of active metal (mmol·g−1·h−1), c: C2+OH STY per unit mass of active metal (mmol·g−1·h−1), d: C2+OH STY per unit mass of catalyst (mmol·g−1·h−1). 表 3 串联催化剂催化CO2加氢制多碳醇性能比较
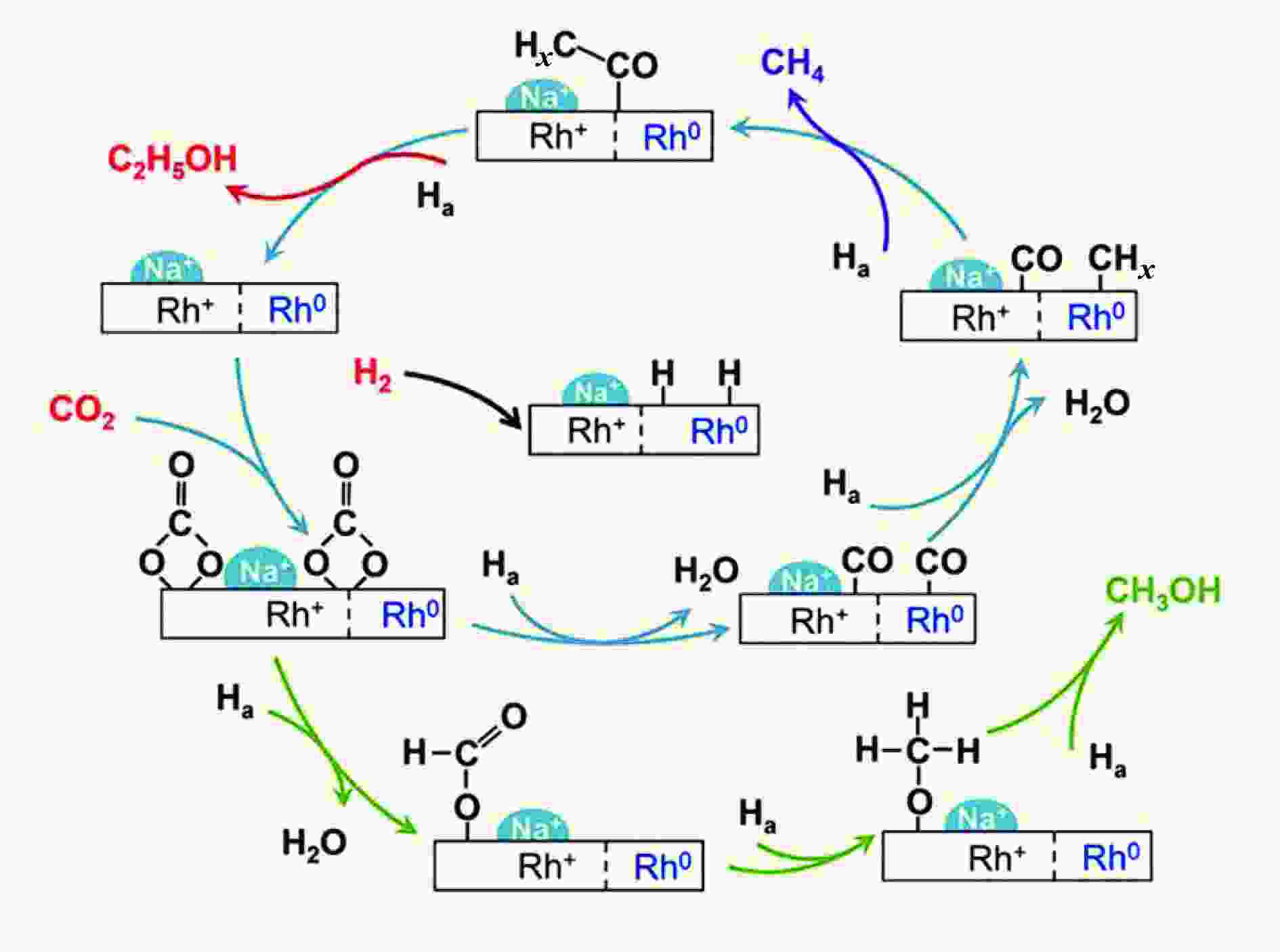
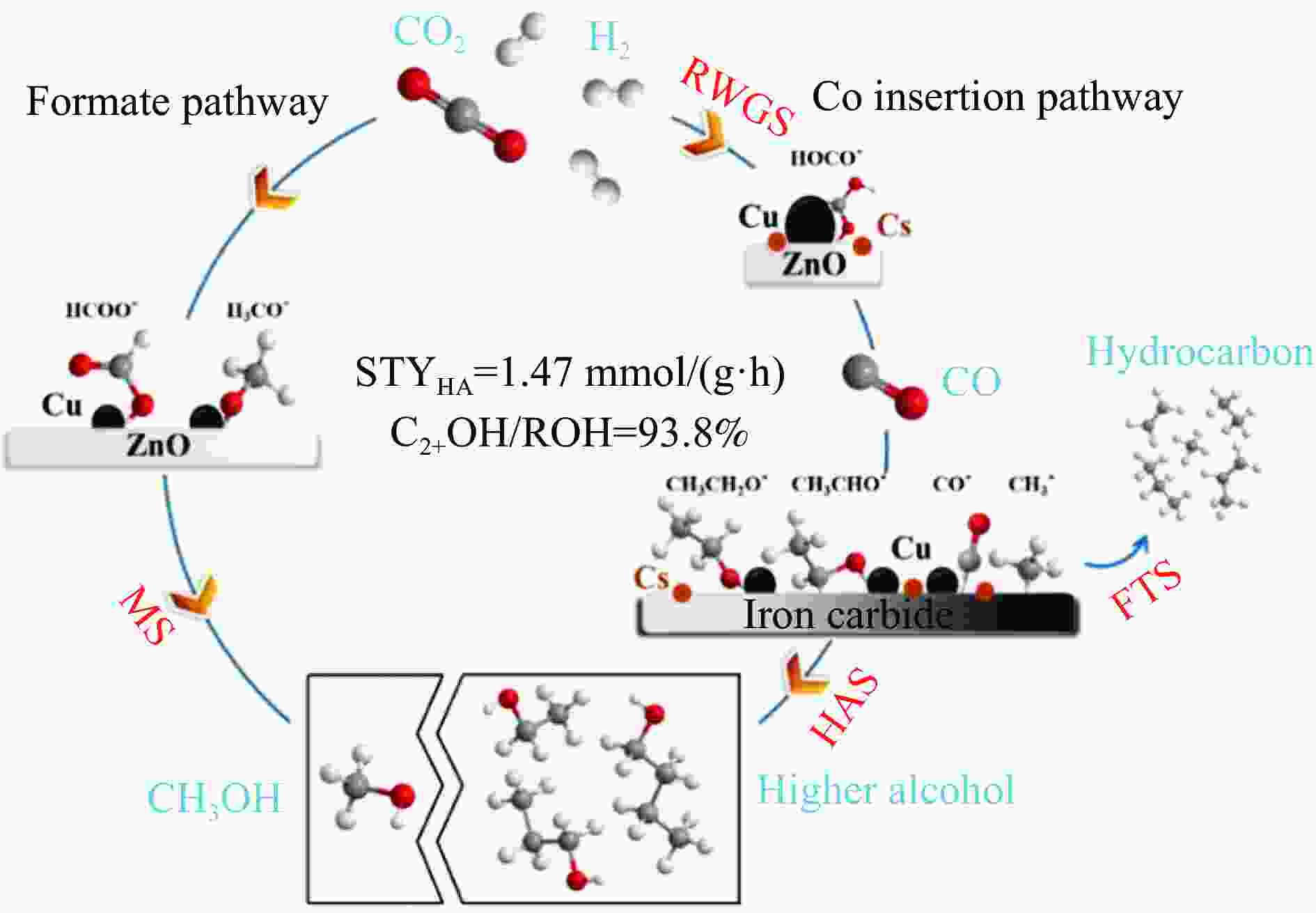
Table 3 Catalytic performance comparison for CO2 hydrogenation to C2+ alcohols over different tandem catalysts
Catalyst Reaction
temp./
℃Reaction
pressure/
MPaH2/
CO2WHSV/
(mL·g−1·h−1)CO2
conversion/
%Selectivity/C% C2+OH/
ROH/
%C2+OH
STY/
(mmol·g−1·h−1)Ref. CO CH4+
C2+HxCH3OH C2+OH other
oxy.Cs-Cu0.8Fe1Zn1 330 5 3 4500 36.6 ~ 20.3 58 0.9 19.8 0 93.8 1.47 [9] NaFe@KCuZnAla 320 5 3 4500 39.2 9.4 49.4 4.2 37 0 ~ 86.5 6.6 [61] MnCuK-Fe5C2/CuZnAlZra 300 3 3 6000 42.1 22.7 60.6 1.2 15.5 0 89.7 3.8 [62] Co2C||CuZnAl 250 5 3 12000 20.7 ~ 21 ~ 57 ~ 3.6 ~ 18.4 0 ~ 84.5 1.6 [63] a: C2+OH/ROH fraction(%) was calculated according to the carbon atoms concentration of MeOH to C2+OH. -
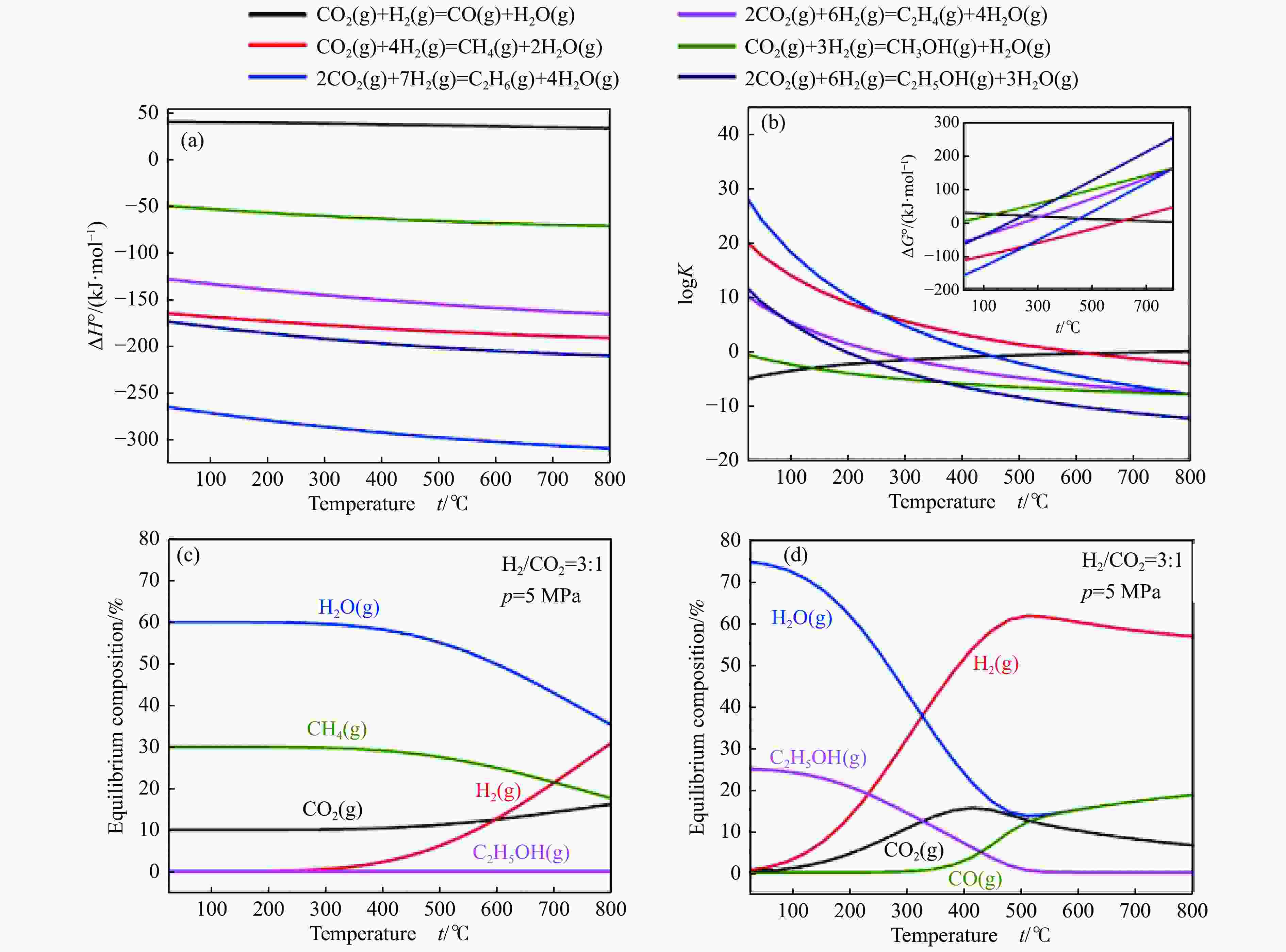
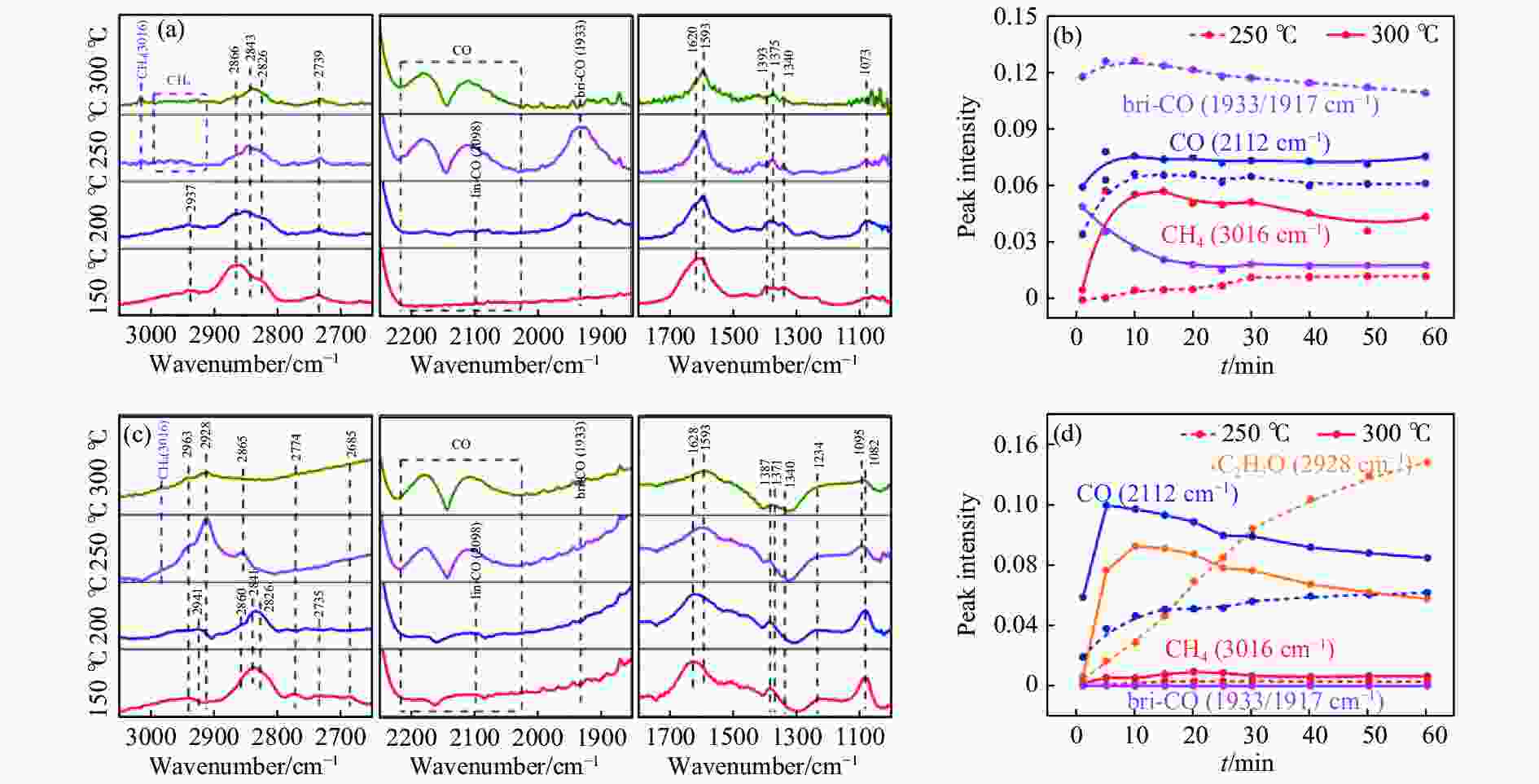
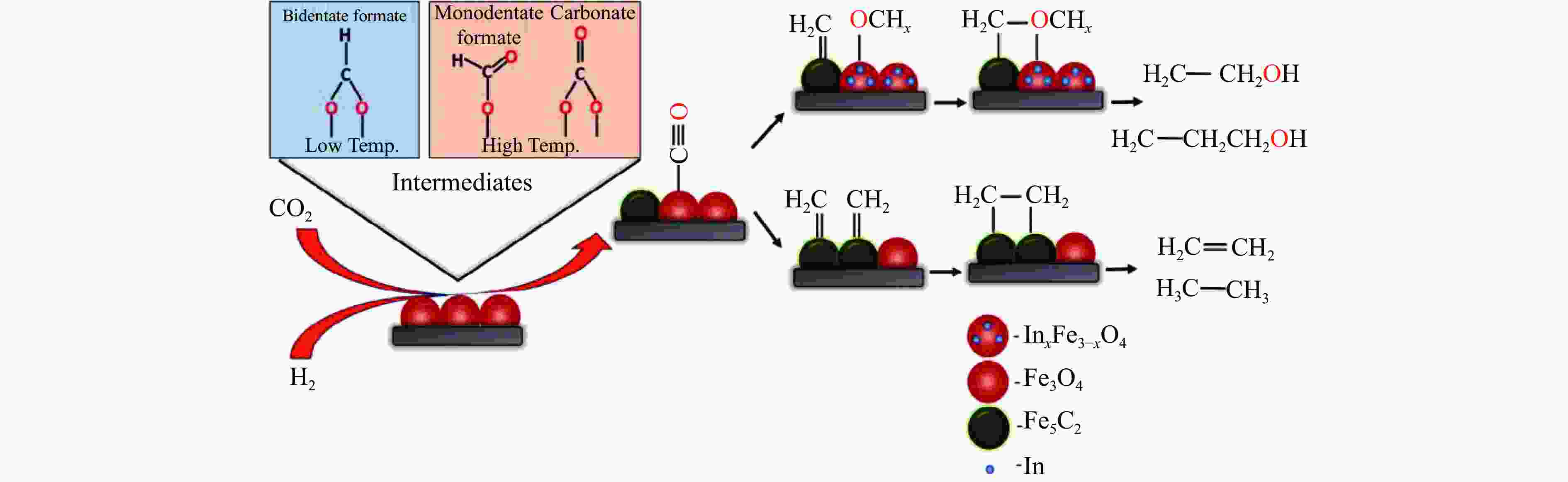
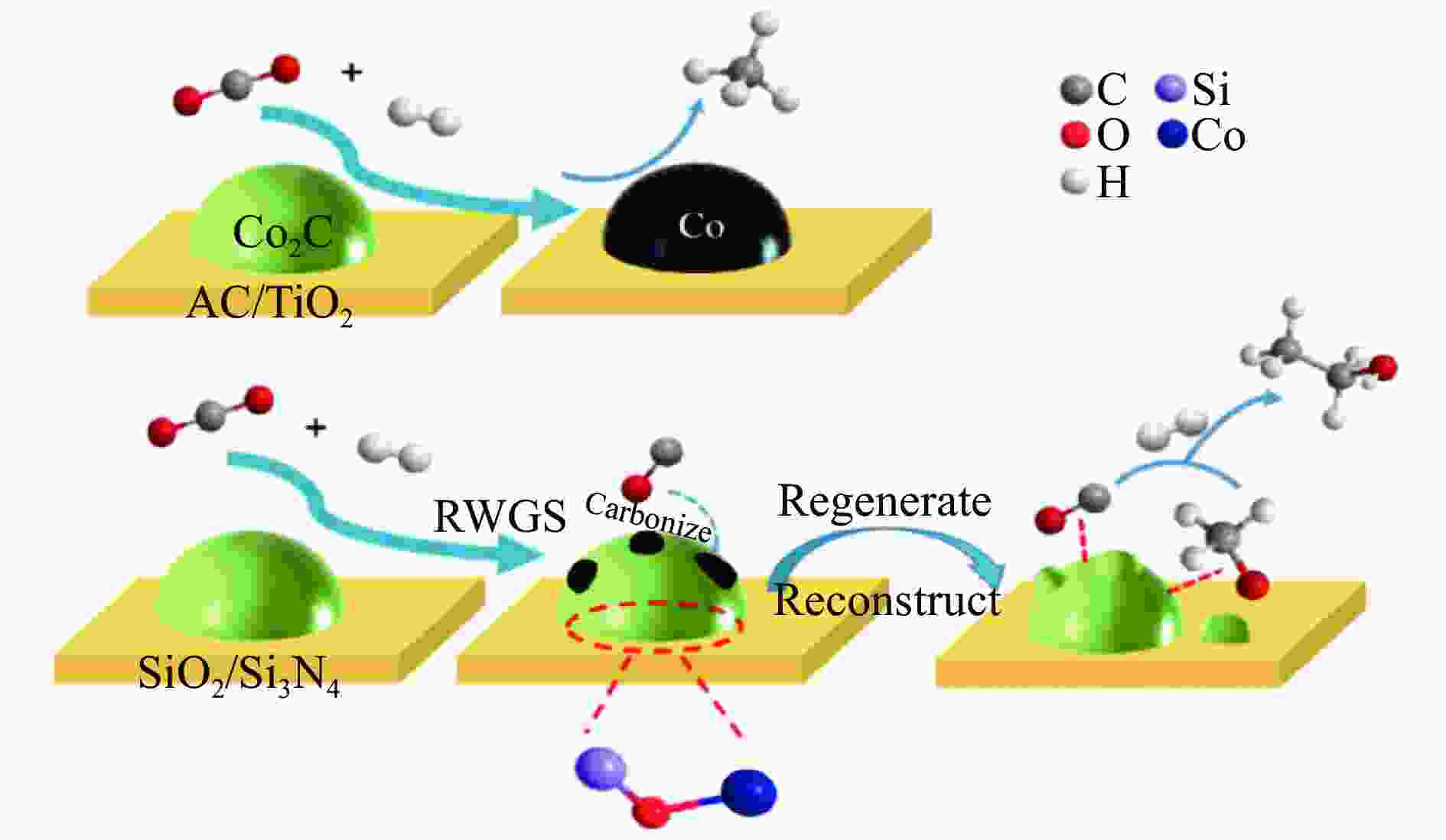
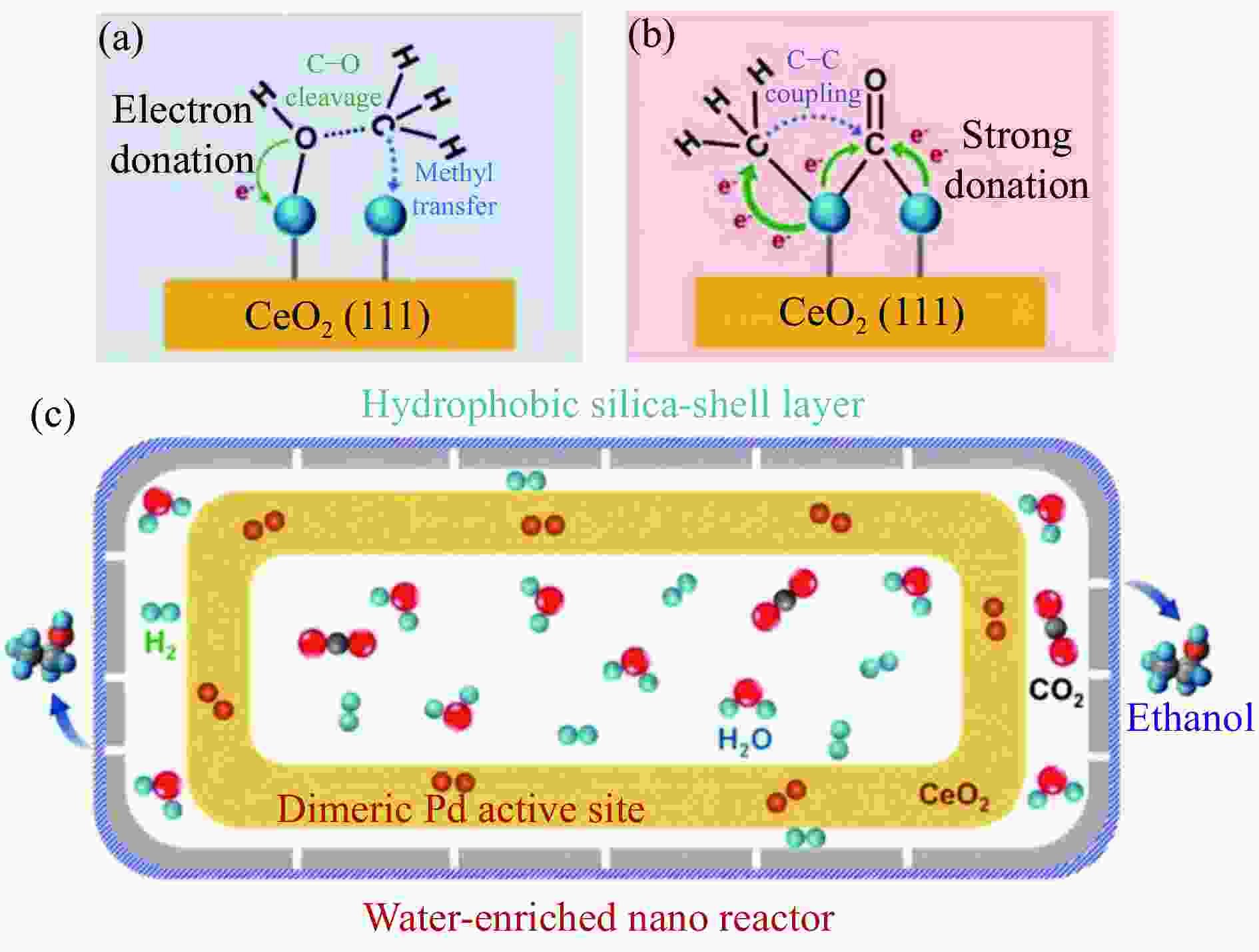
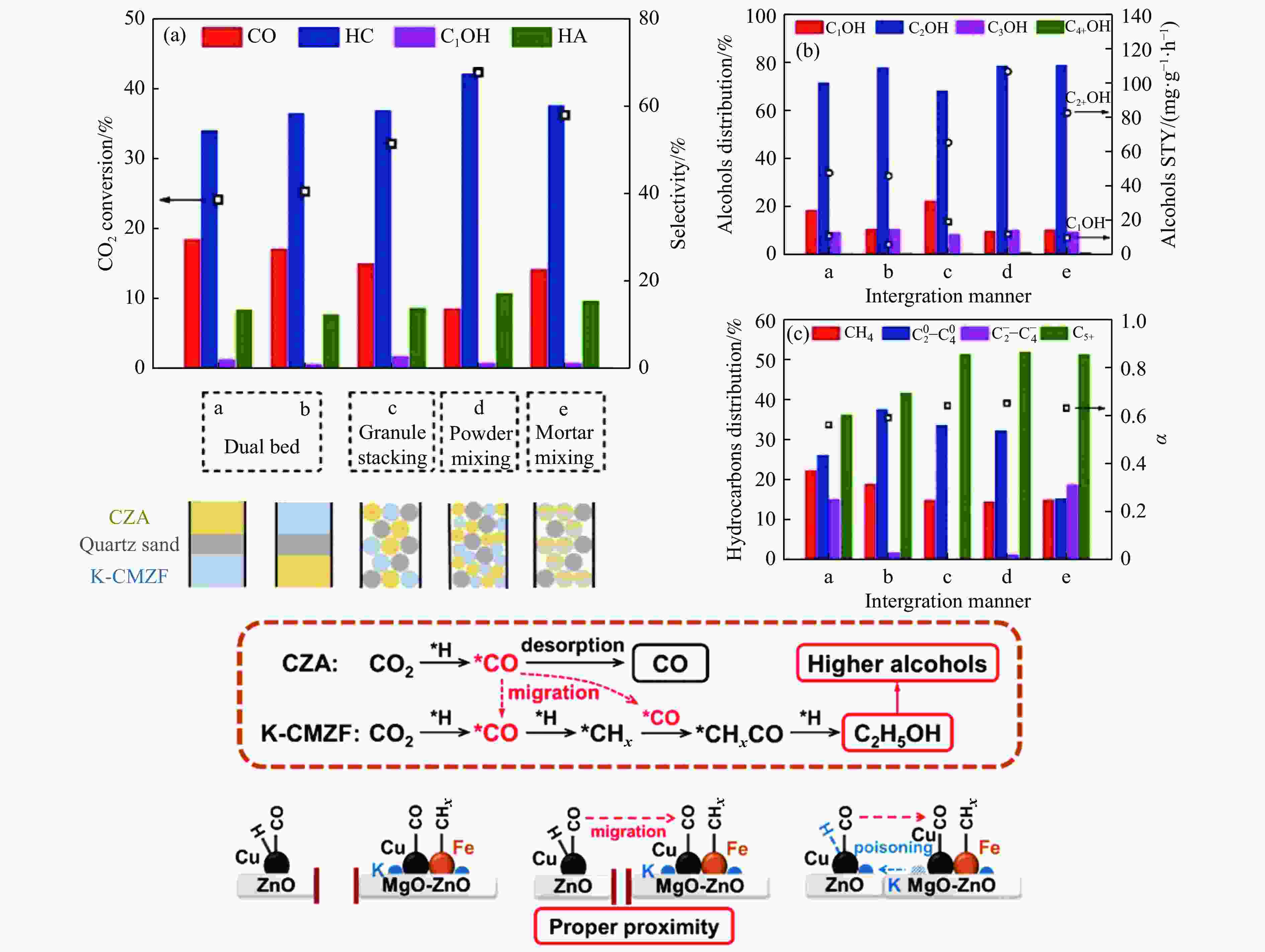
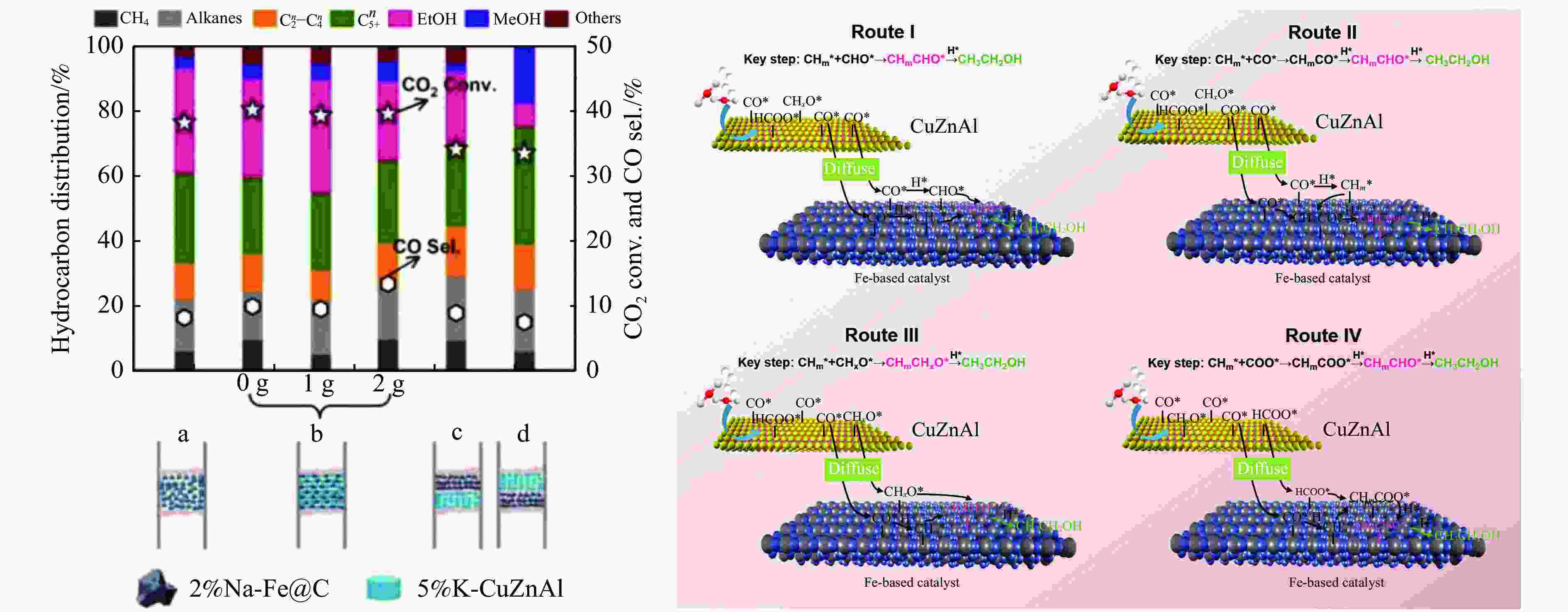
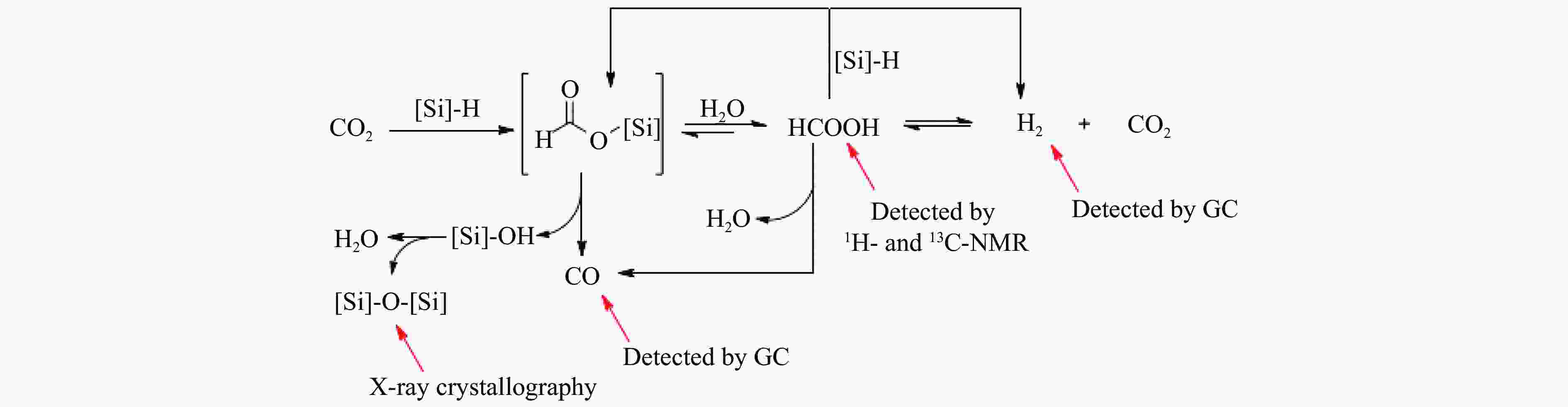
[1] KAMKENG A D, WANG M, HU J, et al. Transformation technologies for CO2 utilisation: Current status, challenges and future prospects[J]. Chem Eng Sci,2021,409:128138. doi: 10.1016/j.cej.2020.128138 [2] 成康, 张庆红, 康金灿, 等. 二氧化碳直接制备高值化学品中的接力催化方法[J]. 中国科学: 化学,2020,50(7):743−755.CHENG Kang, ZHANG Qinghong, KANG Jincan, et al. Relay catalysis in the direct conversion of carbon dioxide to high-value chemicals[J]. Sci Chin Chem,2020,50(7):743−755. [3] 崔国庆, 胡溢玚, 娄颖洁, 等. CO2加氢制醇类催化剂的设计制备及性能研究进展[J]. 化学学报,2023,81(8):1081−1100. doi: 10.6023/A23040126CUI Guoqing, HU Yiyang, LOU Yingjie, et al. Research progress on the design, preparation, and properties of catalysts for CO2 hydrogenation to alcohols[J]. Acta Chim Sin,2023,81(8):1081−1100. doi: 10.6023/A23040126 [4] 张涛, 刘志成, 杨为民. 低碳烷烃与二氧化碳催化转化研究进展[J]. 中国科学: 化学,2021,51(2):154−164.ZHANG Tao, LIU Zhicheng, YANG Weimin, et al. Progress in the catalytic conversion of light alkanes with carbon dioxide[J]. Sci Chin Chem,2021,51(2):154−164. [5] 郭淑佳, 王晗, 秦张峰, 等. 水煤气变换反应作用下的CO和CO2混合物加氢转化制烃和醇——热力学平衡研究(英文)[J]. 燃料化学学报(中英文),2023,51(4):482−491. doi: 10.1016/S1872-5813(23)60346-9GUO Shujia, WANG Han, QIN Zhangfeng, et al. Conversion of the CO and CO2 mixture to alcohols and hydrocarbons by hydrogenation under the influence of the water-gas shift reaction, a thermodynamic consideration[J]. J Fuel Chem Technol,2023,51(4):482−491. doi: 10.1016/S1872-5813(23)60346-9 [6] XU D, YANG H, HONG X, et al. Tandem catalysis of direct CO2 hydrogenation to higher alcohols[J]. ACS Catal,2021,11(15):8978−8984. doi: 10.1021/acscatal.1c01610 [7] WEI J, YAO R, HAN Y, et al. Towards the development of the emerging process of CO2 heterogenous hydrogenation into high-value unsaturated heavy hydrocarbons[J]. Chem Soc Rev,2021,50(19):10764−10805. doi: 10.1039/D1CS00260K [8] ZHANG F, ZHOU W, XIONG X, et al. Selective hydrogenation of CO2 to ethanol over sodium-modified rhodium nanoparticles embedded in zeolite silicalite-1[J]. J Phys Chem C,2021,125(44):24429−24439. doi: 10.1021/acs.jpcc.1c07862 [9] XU D, DING M, HONG X, et al. Selective C2+ alcohol synthesis from direct CO2 hydrogenation over a Cs-promoted Cu-Fe-Zn catalyst[J]. ACS Catal,2020,10(9):5250−5260. doi: 10.1021/acscatal.0c01184 [10] MA Z, POROSOFF M D. Development of tandem catalysts for CO2 hydrogenation to olefins[J]. ACS Catal,2019,9(3):2639−2656. doi: 10.1021/acscatal.8b05060 [11] MARTÍN N, CIRUJANO F G. Multifunctional heterogeneous catalysts for the tandem CO2 hydrogenation-Fischer Tropsch synthesis of gasoline[J]. J CO2 Util,2022,65:102176. doi: 10.1016/j.jcou.2022.102176 [12] XIE C, CHEN C, YU Y, et al. Tandem catalysis for CO2 hydrogenation to C2–C4 hydrocarbons[J]. Nano Lett,2017,17(6):3798−3802. doi: 10.1021/acs.nanolett.7b01139 [13] NEZAM I, ZHOU W, GUSMãO G S, et al. Direct aromatization of CO2 via combined CO2 hydrogenation and zeolite-based acid catalysis[J]. J CO2 Util,2021,45:101405. doi: 10.1016/j.jcou.2020.101405 [14] 王晓星, 段永鸿, 张俊峰, 等. 串联催化剂上CO2催化转化制备高附加值烃类研究进展[J]. 燃料化学学报,2022,50(5):538−563. doi: 10.1016/S1872-5813(21)60181-0WANG Xiaoxing, DUAN Yongming, ZHANG Junfeng, et al. Catalytic conversion of CO2 into high value-added hydrocarbons over tandem catalyst[J]. J Fuel Chem Technol,2022,50(5):538−563. doi: 10.1016/S1872-5813(21)60181-0 [15] ZHANG F, CHEN K, JIANG Q. Selective transformation of methanol to ethanol in the presence of syngas over composite catalysts[J]. ACS Catal,2022,12(14):8451−8461. doi: 10.1021/acscatal.2c01725 [16] XIE Z, XU Y, XIE M, et al. Reactions of CO2 and ethane enable CO bond insertion for production of C3 oxygenates[J]. Nat Commun,2020,11(1):1887. doi: 10.1038/s41467-020-15849-x [17] XIE Z, GUO H, HUANG E, et al. Catalytic tandem CO2–ethane reactions and hydroformylation for C3 oxygenate production[J]. ACS Catal,2022,12(14):8279−8290. doi: 10.1021/acscatal.2c01700 [18] LUK H T, MONDELLI C, MITCHELL S, et al. Role of carbonaceous supports and potassium promoter on higher alcohols synthesis over copper-iron catalysts[J]. ACS Catal,2018,8(10):9604−9618. doi: 10.1021/acscatal.8b02714 [19] KHAN W U, BAHARUDIN L, CHOI J, et al. Recent progress in CO hydrogenation over bimetallic catalysts for higher alcohol synthesis[J]. ChemCatChem,2021,13(1):111−120. doi: 10.1002/cctc.202001436 [20] AO M, PHAM G H, SUNARSO J, et al. Active centers of catalysts for higher alcohol synthesis from syngas: A review[J]. ACS Catal,2018,8(8):7025−7050. doi: 10.1021/acscatal.8b01391 [21] XU X, DOESBURG E, SCHOLTEN J. Synthesis of higher alcohols from syngas-recently patented catalysts and tentative ideas on the mechanism[J]. Catal Today,1987,2(1):125−170. doi: 10.1016/0920-5861(87)80002-0 [22] WANG J, WANG T, XI Y, et al. In-situ-formed potassium-modified nickel-zinc carbide boosts production of higher alcohols beyond CH4 in CO2 hydrogenation[J]. Angew Chem Int Ed,2023,e202311335. [23] DING L, SHI T, GU J, et al. CO2 hydrogenation to ethanol over Cu@ Na-Beta[J]. Chem,2020,6(10):2673−2689. doi: 10.1016/j.chempr.2020.07.001 [24] SIBI M G, VERMA D, SETIYADI H C, et al. Synthesis of monocarboxylic acids via direct CO2 conversion over Ni-Zn intermetallic catalysts[J]. ACS Catal,2021,11(13):8382−8398. doi: 10.1021/acscatal.1c00747 [25] LI J, WANG L, CAO Y, et al. Recent advances on the reduction of CO2 to important C2+ oxygenated chemicals and fuels[J]. Chin J Chem Eng,2018,26(11):2266−2279. doi: 10.1016/j.cjche.2018.07.008 [26] XU D, WANG Y, DING M, et al. Advances in higher alcohol synthesis from CO2 hydrogenation[J]. Chem,2021,7(4):849−881. doi: 10.1016/j.chempr.2020.10.019 [27] LI S, YANG J, SONG C, et al. Iron carbides: Control synthesis and catalytic applications in COx hydrogenation and electrochemical HER[J]. Adv Mater,2019,31(50):1901796. doi: 10.1002/adma.201901796 [28] FAN T, LIU H, SHAO S, et al. Cobalt catalysts enable selective hydrogenation of CO2 toward diverse products: recent progress and perspective[J]. J Phys Chem Lett,2021,12(43):10486−10496. doi: 10.1021/acs.jpclett.1c03043 [29] GALHARDO T S, BRAGA A H, ARPINI B H, et al. Optimizing active sites for high CO selectivity during CO2 hydrogenation over supported nickel catalysts[J]. J Am Chem Soc,2021,143(11):4268−4280. doi: 10.1021/jacs.0c12689 [30] BADDOUR F G, ROBERTS E J, TO A T, et al. An exceptionally mild and scalable solution-phase synthesis of molybdenum carbide nanoparticles for thermocatalytic CO2 hydrogenation[J]. J Am Chem Soc,2020,142(2):1010−1019. doi: 10.1021/jacs.9b11238 [31] HUANG J, ZHANG G, ZHU J, et al. Boosting the production of higher alcohols from CO2 and H2 over Mn-and K-modified iron carbide[J]. Ind Eng Chem Res,2022,61(21):7266−7274. doi: 10.1021/acs.iecr.2c00720 [32] XU D, DING M, HONG X, et al. Mechanistic aspects of the role of K promotion on Cu-Fe-based catalysts for higher alcohol synthesis from CO2 hydrogenation[J]. ACS Catal,2020,10(24):14516−14526. doi: 10.1021/acscatal.0c03575 [33] WANG Y, WANG W, HE R, et al. Carbon-based electron buffer layer on ZnOx-Fe5C2-Fe3O4 boosts ethanol synthesis from CO2 hydrogenation[J]. Angew Chem Int Ed,2023,e202311786. [34] ZHANG S, LIU X, SHAO Z, et al. Direct CO2 hydrogenation to ethanol over supported Co2C catalysts: Studies on support effects and mechanism[J]. J Catal,2020,382:86−96. doi: 10.1016/j.jcat.2019.11.038 [35] GOUD D, CHURIPARD S R, BAGCHI D, et al. Strain-enhanced phase transformation of iron oxide for higher alcohol production from CO2[J]. ACS Catal,2022,12(18):11118−11128. doi: 10.1021/acscatal.2c03183 [36] ZHANG S, WU Z, LIU X, et al. Tuning the interaction between Na and Co2C to promote selective CO2 hydrogenation to ethanol[J]. Appl Catal B: Environ,2021,293:120207. doi: 10.1016/j.apcatb.2021.120207 [37] KUSAMA H, OKABE K, SAYAMA K, et al. Alcohol synthesis by catalytic hydrogenation of CO2 over Rh-Co/SiO2[J]. Appl Organomet Chem,2000,14(12):836−840. doi: 10.1002/1099-0739(200012)14:12<836::AID-AOC97>3.0.CO;2-C [38] BAI S, SHAO Q, WANG P, et al. Highly active and selective hydrogenation of CO2 to ethanol by ordered Pd-Cu nanoparticles[J]. J Am Chem Soc,2017,139(20):6827−6830. doi: 10.1021/jacs.7b03101 [39] HE Z, QIAN Q, MA J, et al. Water-enhanced synthesis of higher alcohols from CO2 hydrogenation over a Pt/Co3O4 catalyst under milder conditions[J]. Angew Chem Int Ed,2016,55(2):737−741. doi: 10.1002/anie.201507585 [40] TATSUMI T, MURAMATS A, TOMINAGA H-O. Alcohol synthesis from CO2/H2 on silica-supported molybdenum catalysts[J]. Chem Lett,1985,14(5):593−594. doi: 10.1246/cl.1985.593 [41] PAN X, FAN Z, CHEN W, et al. Enhanced ethanol production inside carbon-nanotube reactors containing catalytic particles[J]. Nat Mater,2007,6(7):507−511. doi: 10.1038/nmat1916 [42] 郑晋楠, 安康, 王嘉明, 等. Co/La-Ga-O复合氧化物用于催化二氧化碳加氢制乙醇[J]. 燃料化学学报.,2019,47(6):697−708. doi: 10.1016/S1872-5813(19)30031-3ZHENG Jinnan, AN Kang, WANG Jiaming, et al. Direct synthesis of ethanol via CO2 hydrogenation over the Co/La-Ga-O composite oxide catalyst[J]. J Fuel Chem Technol,2019,47(6):697−708. doi: 10.1016/S1872-5813(19)30031-3 [43] LI S G, GUO H J, ZHANG H R, et al. The reverse water-gas shift reaction and the synthesis of mixed alcohols over K/Cu-Zn catalyst from CO2 hydrogenation[J]. Adv Mater Res,2013,772:275−280. doi: 10.4028/www.scientific.net/AMR.772.275 [44] YANG H, GENG X, YANG Y, et al. Mechanisms of CO2 hydrogenative conversion on supported Ni/ZrO2 catalyst[J]. Appl Surf Sci,2022,600:154151. doi: 10.1016/j.apsusc.2022.154151 [45] WANG G, LUO R, YANG C, et al. Active sites in CO2 hydrogenation over confined VOx-Rh catalysts[J]. Sci Chin Chem,2019,62:1710−1719. doi: 10.1007/s11426-019-9590-6 [46] YANG C, MU R, WANG G, et al. Hydroxyl-mediated ethanol selectivity of CO2 hydrogenation[J]. Chem Sci,2019,10(11):3161−3167. doi: 10.1039/C8SC05608K [47] KUSAMA H, OKABE K, SAYAMA K, et al. CO2 hydrogenation to ethanol over promoted Rh/SiO2 catalysts[J]. Catal Today,1996,28(3):261−266. doi: 10.1016/0920-5861(95)00246-4 [48] KUSAMA H, OKABE K, SAYAMA K, et al. Ethanol synthesis by catalytic hydrogenation of CO2 over Rh-FeSiO2 catalysts[J]. Energy,1997,22(2-3):343−348. doi: 10.1016/S0360-5442(96)00095-3 [49] CHEN J, ZHA Y, LIU B, et al. Rationally designed water enriched nano reactor for stable CO2 hydrogenation with near 100% ethanol selectivity over diatomic palladium active sites[J]. ACS Catal,2023,13:7110−7121. doi: 10.1021/acscatal.3c00586 [50] CAPARRÓS F J, SOLER L, ROSSELL M D, et al. Remarkable carbon dioxide hydrogenation to ethanol on a palladium/iron oxide single-atom catalyst[J]. ChemCatChem,2018,10(11):2365−2369. doi: 10.1002/cctc.201800362 [51] WITOON T, NUMPILAI T, NIJPANICH S, et al. Enhanced CO2 hydrogenation to higher alcohols over K-Co promoted In2O3 catalysts[J]. Chem Eng Sci,2022,431:133211. doi: 10.1016/j.cej.2021.133211 [52] OKABE K, YAMADA H, HANAOKA T, et al. CO2 hydrogenation to alcohols over highly dispersed Co/SiO2 catalysts derived from acetate[J]. Chem Lett,2001,30(9):904−905. doi: 10.1246/cl.2001.904 [53] AN K, ZHANG S, WANG J, et al. A highly selective catalyst of Co/La4Ga2O9 for CO2 hydrogenation to ethanol[J]. J Energy Chem,2021,56:486−495. doi: 10.1016/j.jechem.2020.08.045 [54] ZHANG G, FAN G, ZHENG L, et al. Ga-promoted CuCo-based catalysts for efficient CO2 hydrogenation to ethanol: The key synergistic role of Cu-CoGaOx interfacial sites[J]. ACS Appl Mater Interfaces,2022,14(31):35569−35580. doi: 10.1021/acsami.2c07252 [55] YAO R, WEI J, GE Q, et al. Monometallic iron catalysts with synergistic Na and S for higher alcohols synthesis via CO2 hydrogenation[J]. Appl Catal B: Environ,2021,298:120556. doi: 10.1016/j.apcatb.2021.120556 [56] LIU T, XU D, SONG M, et al. K–ZrO2 interfaces boost CO2 hydrogenation to higher alcohols[J]. ACS Catal,2023,13(7):4667−4674. doi: 10.1021/acscatal.3c00074 [57] ZHENG K, LI Y, LIU B, et al. Ti-doped CeO2 stabilized single-atom rhodium catalyst for selective and stable CO2 hydrogenation to ethanol[J]. Angew Chem Int Ed,2022,61(44):e202210991. doi: 10.1002/anie.202210991 [58] HAN S, FAN D, CHEN N, et al. Efficient conversion of syngas into ethanol by tandem catalysis[J]. ACS Catal,2023,13(16):10651−10660. doi: 10.1021/acscatal.3c01577 [59] KHATAMIRAD M, KONRAD M, GENTZEN M, et al. Silica-supported catalyst system Rh-Mn-Ir-Li-Ti in syngas to ethanol reaction: Reactivity trends and performance optimization[J]. ChemCatChem,2023,15(1):e202201104. doi: 10.1002/cctc.202201104 [60] XIE J, OLSBYE U. The oxygenate-mediated conversion of COx to hydrocarbons-on the role of zeolites in tandem catalysis[J]. Chem Rev,2023,123(20):11775−11816. doi: 10.1021/acs.chemrev.3c00058 [61] WANG Y, WANG K, ZHANG B, et al. Direct conversion of CO2 to ethanol boosted by intimacy-sensitive multifunctional catalysts[J]. ACS Catal,2021,11(18):11742−11753. doi: 10.1021/acscatal.1c01504 [62] HUANG J, ZHANG G, WANG M, et al. The synthesis of higher alcohols from CO2 hydrogenation over Mn-Cu-K modified Fe5C2 and CuZnAlZr tandem catalysts[J]. Front Energy Res,2023,10:995800. doi: 10.3389/fenrg.2022.995800 [63] ZHANG S, HUANG C, SHAO Z, et al. Revealing and regulating the complex reaction mechanism of CO2 hydrogenation to higher alcohols on multifunctional tandem catalysts[J]. ACS Catal,2023,13(5):3055−3065. doi: 10.1021/acscatal.2c06245 [64] JIANG X, NIE X, GUO X, et al. Recent advances in carbon dioxide hydrogenation to methanol via heterogeneous catalysis[J]. Chem Rev,2020,120(15):7984−8034. doi: 10.1021/acs.chemrev.9b00723 [65] TAN G, WU Y, SHI Y, et al. Syngas-free highly regioselective rhodium-catalyzed transfer hydroformylation of alkynes to α, β-unsaturated aldehydes[J]. Angew Chem Int Ed,2019,131(22):7518−7522. doi: 10.1002/ange.201902553 [66] NEVES Â C, CALVETE M J, PINHO E MELO T M, et al. Immobilized catalysts for hydroformylation reactions: A versatile tool for aldehyde synthesis[J]. Eur J Inorg Chem,2012,2012(32):6309−6320. doi: 10.1002/ejoc.201200709 [67] REN X, ZHENG Z, ZHANG L, et al. Rhodium-complex-catalyzed hydroformylation of olefins with CO2 and hydrosilane[J]. Angew Chem Int Ed,2017,56(1):310−313. doi: 10.1002/anie.201608628 -




 下载:
下载: