Highly effective MFe2O4 (M=Zn, Mg, Cu and Mn) spinel catalysts for Fischer-Tropsch synthesis
-
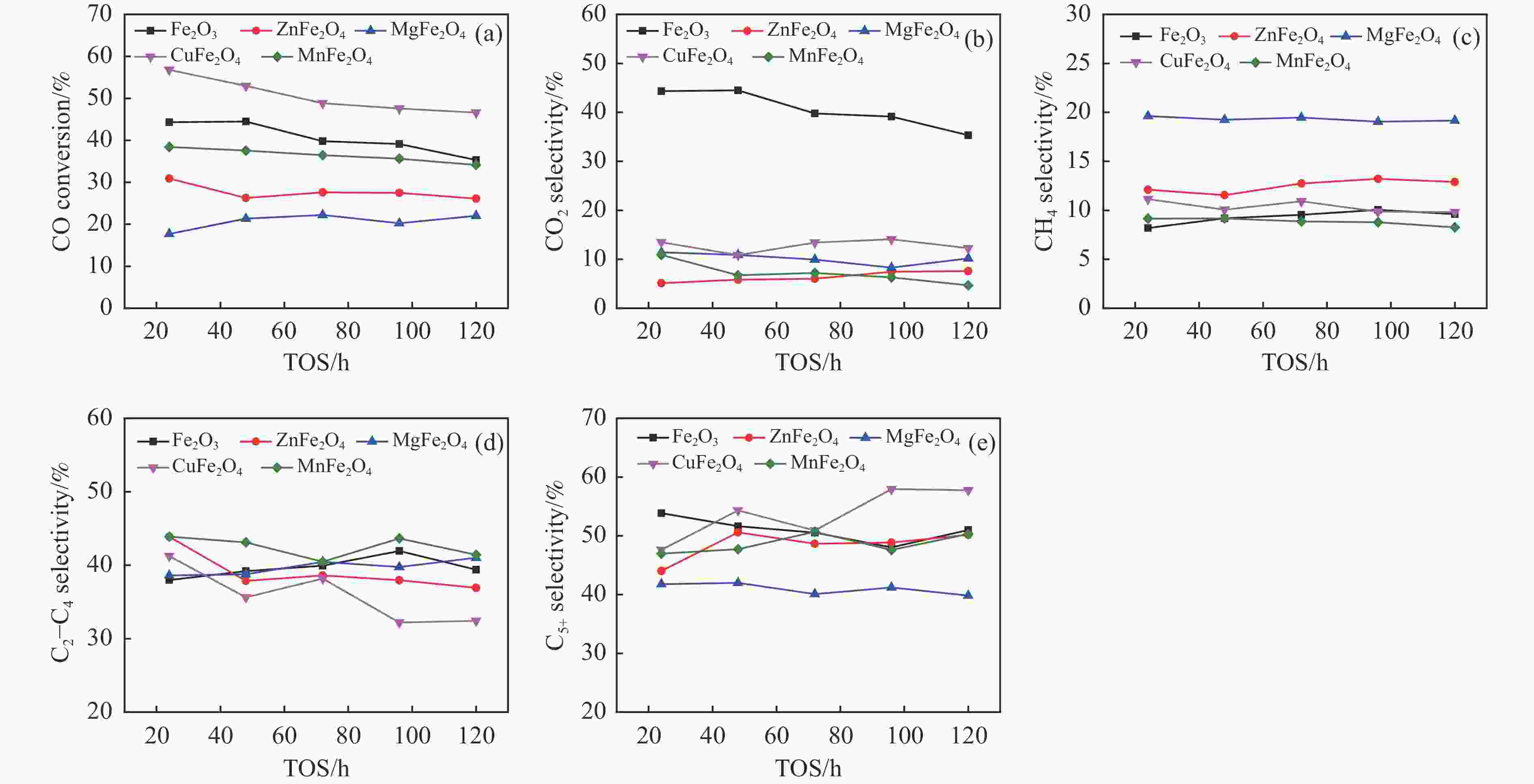
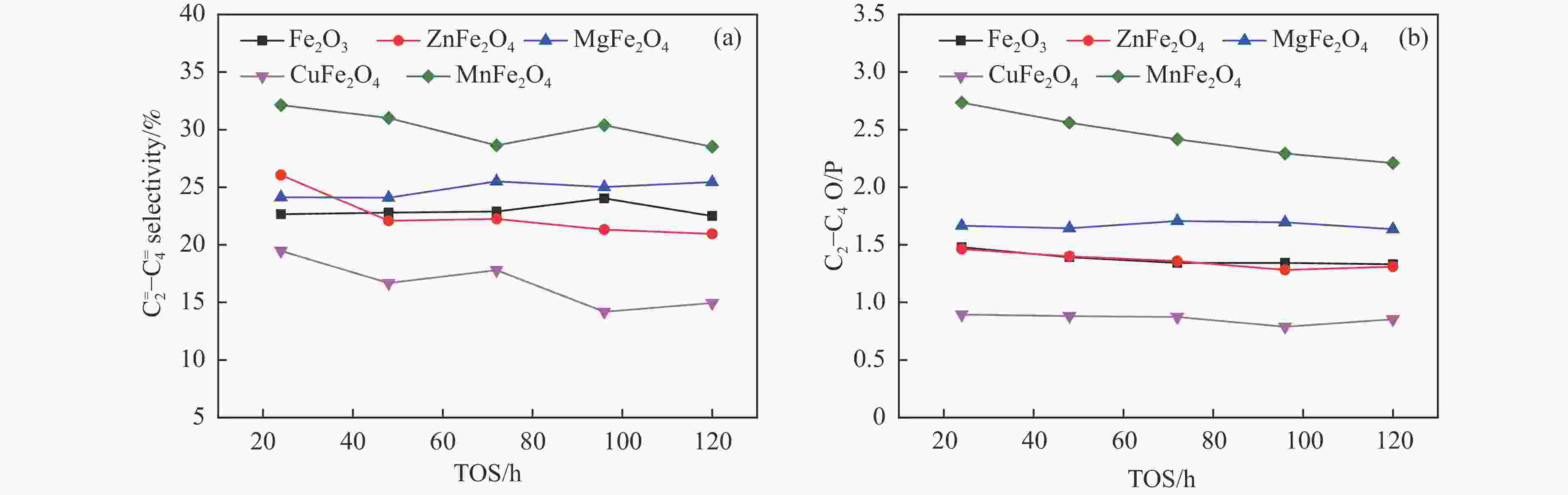
摘要: 一系列尖晶石催化剂,包括 ZnFe2O4、MgFe2O4、CuFe2O4和MnFe2O4被用于费托合成反应(Fischer-Tropsch synthesis, FTS)。Zn、Mg、Cu和Mn很容易与Fe形成尖晶石。其中,在前处理和反应过程中,Zn和Mg能够显著维持尖晶石结构,使得CO转化率较低。在反应过程中,Cu和Mn有利于碳化铁的生成,导致CuFe2O4和MnFe2O4对FTS性能影响显著。ZnFe2O4对烃分布和C2−C4 烯/烷比影响很小。MgFe2O4的C5+选择性较低,同时由于Mg的碱性作用,从而提高了$ {\mathrm{C}}_2^=-{\mathrm{C}}_4^=$选择性和C2−C4烯/烷比。Cu可以促进催化剂的碳化,从而使CuFe2O4具有较高的活性。同时,CuFe2O4可以显著提高C5+选择性。此外,Cu可以促进H2的解离和活化,从而有利于烯烃的二次加氢,降低$ {\mathrm{C}}_2^=-{\mathrm{C}}_4^=$选择性和C2−C4烯/烷比。虽然Mn在反应过程中会促进催化剂的碳化,但MnFe2O4对碳链的长短影响很小。然而,Mn能促进少量ε-Fe2C的生成,这是导致MnFe2O4具有较高$ {\mathrm{C}}_2^=-{\mathrm{C}}_4^=$选择性和C2−C4烯/烷比的原因。同时,所有尖晶石催化剂都具有较低的二氧化碳选择性,符合当前的绿色环保发展要求。Abstract: A series of spinel catalysts, including ZnFe2O4, MgFe2O4, CuFe2O4, and MnFe2O4, were prepared and applied to the Fischer-Tropsch synthesis (FTS). Zn, Mg, Cu and Mn easily form spinels with Fe. Among them, Zn and Mg can significantly maintain the spinel structure during the pretreatment and reaction, resulting in a low CO conversion. Cu and Mn are beneficial to the formation of iron carbide during the reaction, resulting in an apparent influence on FTS performance. ZnFe2O4 has little effect on the hydrocarbon distribution and the olefin/paraffin (O/P) ratio of C2−C4. MgFe2O4 exhibits low selectivity for C5+ hydrocarbons, and the selectivity of $ {\mathrm{C}}_2^=-{\mathrm{C}}_4^=\;$ and the O/P ratio of C2−C4 in the product are increased due to the alkaline effect of Mg. Cu can promote the carbonization of the catalyst, so that CuFe2O4 has higher activity. Meanwhile, CuFe2O4 can significantly improve the selectivity of C5+ hydrocarbons. Moreover, Cu can promote the dissociation and activation of H2, which is beneficial to the secondary hydrogenation of olefins, thereby reducing the selectivity of $ {\mathrm{C}}_2^=-{\mathrm{C}}_4^=\;$ and the O/P ratio of C2−C4. Mn promotes carbonization during the reaction, but MnFe2O4 has little effect on the chain growth of hydrocarbon. However, Mn can promote the generation of a certain amount of ε-Fe2C, which may explain the higher selectivity of $ {\mathrm{C}}_2^=-{\mathrm{C}}_4^=\;$ and the O/P ratio of C2−C4 for MnFe2O4. All spinel catalysts exhibit low CO2 selectivity, which meets the current green environmental protection requirements.
-
Key words:
- Fischer-Tropsch synthesis /
- spinel /
- phase structure /
- O/P
-
Figure 5 Mössbauer spectra of the spent spinel catalysts (The blue and green sextets are assigned to A sites and B sites of spinel or Fe3O4, the magenta, olive and violet sextets are assigned to χ-Fe5C2, the cyan sextet is assigned to ε-Fe2C and the yellow doublet is assigned to superparamagnetic Fe2+ and Fe3+)
Table 1 Textural properties of the fresh catalysts
Catalyst SBET/(m2·g−1) vpa/(cm3·g−1) dpb/nm dc/nm Fe2O3 58.65 0.15 8.01 30.00 ZnFe2O4 41.92 0.13 8.20 11.50 MgFe2O4 86.35 0.13 4.32 6.20 CuFe2O4 34.29 0.09 7.63 10.00 MnFe2O4 90.58 0.14 5.25 8.80 a: BJH adsorption pore volume; b: BJH adsorption average pore size; c: Crystallite size calculated by Scherrer equation according to XRD. -
[1] ZHANG Q H, KANG J C, WANG Y. Development of novel catalysts for Fischer-Tropsch synthesis: Tuning the product selectivity[J]. ChemCatChem,2010,2(9):1030−1058. doi: 10.1002/cctc.201000071 [2] LIN T J, AN Y L, YU F, et al. Advances in selectivity control for Fischer-Tropsch synthesis to fuels and chemicals with high carbon efficiency[J]. ACS Catal,2022,12(19):12092−12112. doi: 10.1021/acscatal.2c03404 [3] ZHAI P, SUN G, ZHU Q J, et al. Fischer-Tropsch synthesis nanostructured catalysts: Understanding structural characteristics and catalytic reaction[J]. Nanotechnol Rev,2013,2(5):547−576. doi: 10.1515/ntrev-2013-0025 [4] TSUBAKI N, FUJIMOTO K. Product control in Fischer-Tropsch synthesis[J]. Fuel Process Technol,2000,62(2/3):173−186. doi: 10.1016/S0378-3820(99)00122-8 [5] FLORY P J. Molecular size distribution in linear condensation polymers[J]. J Am Chem Soc,1936,58:1877−1885. doi: 10.1021/ja01301a016 [6] LI Y W, ZHANG X, WEI M. New development in Fe/Co catalysts: Structure modulation and performance optimization for syngas conversion[J]. Chin J Catal,2018,39(8):1329−1346. doi: 10.1016/S1872-2067(18)63100-6 [7] VASILEV A A, IVANTSOV M I, DZIDZIGURI E L, et al. Size effect of the carbon-supported bimetallic Fe-Co nanoparticles on the catalytic activity in the Fischer-Tropsch synthesis[J]. Fuel, 2022, 310 : 122455. [8] LI J F, CHENG X F, ZHANG C H, et al. Alkalis in iron-based Fischer-Tropsch synthesis catalysts: Distribution, migration and promotion[J]. J Chem Technol Biotechnol,2017,92(6):1472−1480. doi: 10.1002/jctb.5152 [9] PENDYALA V R R, GRAHAM U M, JACOBS G, et al. Fischer-Tropsch synthesis: Deactivation as a function of potassium promoter loading for precipitated iron catalyst[J]. Catal Lett,2014,144(10):1704−1716. doi: 10.1007/s10562-014-1336-z [10] LI J F, ZHANG C H, CHENG X F, et al. Effects of alkaline-earth metals on the structure, adsorption and catalytic behavior of iron-based Fischer-Tropsch synthesis catalysts[J]. Appl Catal A: Gen,2013,464:10−19. [11] CHONCO Z H, LODYA L, CLAEYS M, et al. Copper ferrites: A model for investigating the role of copper in the dynamic iron-based Fischer-Tropsch catalyst[J]. J Catal,2013,308:363−373. doi: 10.1016/j.jcat.2013.08.012 [12] ZHAO M, CUI Y, SUN J C, et al. Modified iron catalyst for direct synthesis of light olefin from syngas[J]. Catal Today,2018,316:142−148. doi: 10.1016/j.cattod.2018.05.018 [13] LI S, LI A, KRISHNAMOORTHY S, et al. Effects of Zn, Cu, and K promoters on the structure and on the reduction, carburization, and catalytic behavior of iron-based Fischer-Tropsch synthesis catalysts[J]. Catal Lett,2001,77(4):197−205. doi: 10.1023/A:1013284217689 [14] SHI B F, ZHANG Z P, LIU Y T, et al. Promotional effect of Mn-doping on the structure and performance of spinel ferrite microspheres for CO hydrogenation[J]. J Catal,2020,381:150−162. doi: 10.1016/j.jcat.2019.10.034 [15] YANG Z X, ZHANG Z P, LIU Y T, et al. Tuning direct CO hydrogenation reaction over Fe-Mn bimetallic catalysts toward light olefins: Effects of Mn promotion[J]. Appl Catal B: Environ, 2021, 285 . [16] CANNAS C, FALQUI A, MUSINU A, et al. CoFe2O4 nanocrystalline powders prepared by citrate-gel methods: Synthesis, structure and magnetic properties[J]. J Nanopart Res,2006,8(2):255−267. doi: 10.1007/s11051-005-9028-7 [17] SHI B F, ZHANG Z P, ZHA B B, et al. Structure evolution of spinel Fe-M-II (M=Mn, Fe, Co, Ni) ferrite in CO hydrogeneration[J]. Mol Catal,2018,456:31−37. doi: 10.1016/j.mcat.2018.06.019 [18] CASULA M F, CONCAS G, CONGIU F, et al. Characterization of stoichiometric nanocrystalline spinel ferrites dispersed on porous silica aerogel[J]. J Nanosci Nanotechnol,2011,11(11):10136−10141. doi: 10.1166/jnn.2011.4975 [19] LIANG M S, KANG W K, XIE K C. Comparison of reduction behavior of Fe2O3, ZnO and ZnFe2O4 by TPR technique[J]. J Nat Gas Chem,2009,18(1):110−113. doi: 10.1016/S1003-9953(08)60073-0 [20] MA L J, CHEN L S, CHEN S Y. Study on the characteristics and activity of Ni-Cu-Zn ferrite for decomposition of CO2[J]. Mater Chem Phys,2009,114(2/3):692−696. doi: 10.1016/j.matchemphys.2008.10.050 [21] GE X, LI M S, SHEN J Y. The reduction of Mg-Fe-O and Mg-Fe-Al-O complex oxides studied by temperature-programmed reduction combined with in situ Mössbauer spectroscopy[J]. J Solid State Chem,2001,161(1):38−44. doi: 10.1006/jssc.2001.9264 [22] WANG C, ZHU H, ZHANG J, et al. Tuning Fischer-Tropsch synthesis product distribution toward light olefins over nitrided Fe-Mn bimetallic catalysts[J]. Fuel,2023,343:127977. doi: 10.1016/j.fuel.2023.127977 [23] DE SMIT E, CINQUINI F, BEALE A M, et al. Stability and reactivity of ϵ-χ-θ iron carbide catalyst phases in Fischer-Tropsch synthesis: Controlling μC[J]. J Am Chem Soc,2010,132(42):14928−14941. doi: 10.1021/ja105853q [24] PENDYALA V R R, JACOBS G, MOHANDAS J C, et al. Fischer-Tropsch synthesis: Effect of water over iron-based catalysts[J]. Catal Lett,2010,140(3/4):98−105. doi: 10.1007/s10562-010-0452-7 [25] SATTERFIELD C N, HANLON R T, TUNG S E, et al. Effect of water on the iron-catalyzed Fischer-Tropsch synthesis[J]. Ind Eng Chem Prod Res Dev,1986,25(3):407−414. doi: 10.1021/i300023a007 [26] DRY M E, SHINGLES T, BOTHA C. Factors influencing the formation of carbon on iron Fischer-Tropsch catalysts: I. The influence of promoters[J]. J Catal,1970,17(3):341−346. doi: 10.1016/0021-9517(70)90109-0 [27] DE SMIT E, WECKHUYSEN B M. The renaissance of iron-based Fischer-Tropsch synthesis: on the multifaceted catalyst deactivation behaviour[J]. Chem Soc Rev,2008,37(12):2758−2781. doi: 10.1039/b805427d [28] XU Y F, LI X Y, GAO J H, et al. A hydrophobic FeMn@Si catalyst increases olefins from syngas by suppressing C1 by-products[J]. Science,2021,371(6529):610−613. doi: 10.1126/science.abb3649 [29] YU X F, ZHANG J L, WANG X, et al. Fischer-Tropsch synthesis over methyl modified Fe2O3@SiO2 catalysts with low CO2 selectivity[J]. Appl Catal B: Environ,2018,232:420−428. doi: 10.1016/j.apcatb.2018.03.048 [30] GALVIS H M T, DE JONG K P. Catalysts for production of lower olefins from synthesis gas: A review[J]. ACS Catal,2013,3(9):2130−2149. doi: 10.1021/cs4003436 [31] GONG W B, YE R P, DING J, et al. Effect of copper on highly effective Fe-Mn based catalysts during production of light olefins via Fischer-Tropsch process with low CO2 emission[J]. Appl Catal B: Environ, 2020, 278 . [32] LI T Z, WANG H L, YANG Y, et al. Effect of manganese on the catalytic performance of an iron-manganese bimetallic catalyst for light olefin synthesis[J]. J Energy Chem,2013,22(4):624−632. doi: 10.1016/S2095-4956(13)60082-0 [33] ABBOT J, CLARK N J, BAKER B G. Effects of sodium, aluminium and manganese on the Fischer-Tropsch synthesis over alumina-supported iron catalysts[J]. Appl Catal,1986,26(1/2):141−153. [34] BUKUR D B, MUKESH D, PATEL S A. Promoter effects on precipitated iron catalysts for Fischer-Tropsch synthesis[J]. Ind Eng Chem Res,1990,29(2):194−204. doi: 10.1021/ie00098a008 -




 下载:
下载: