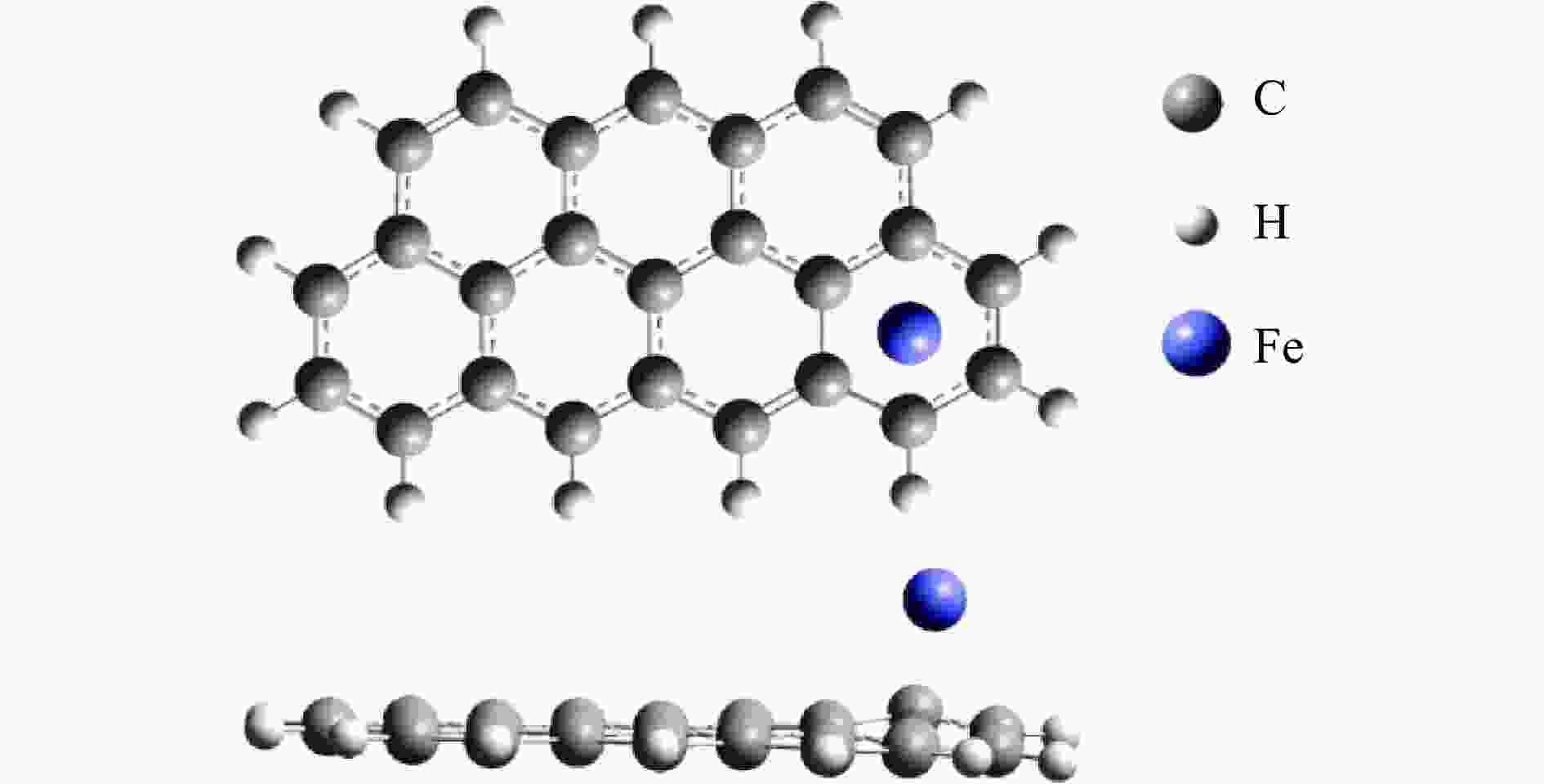
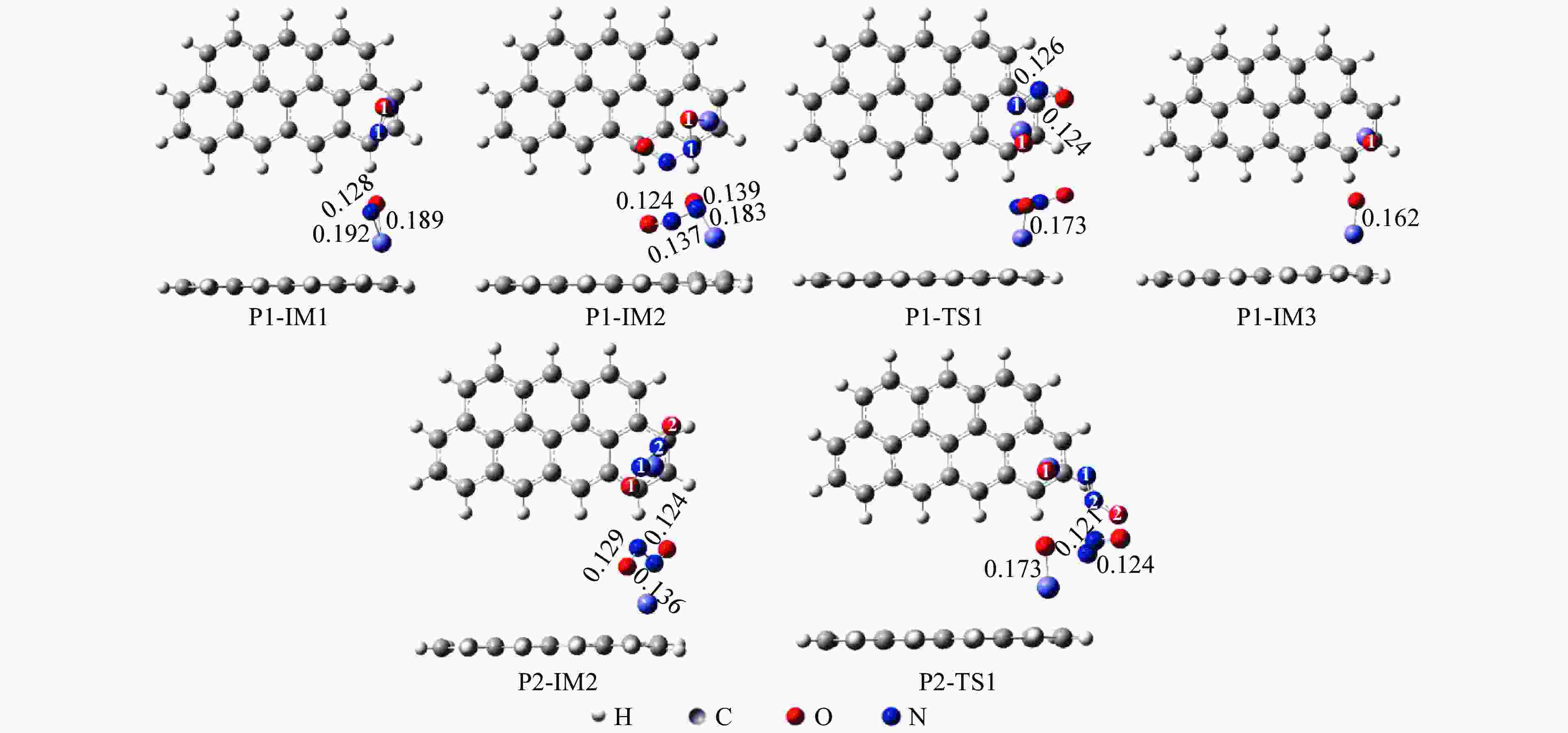
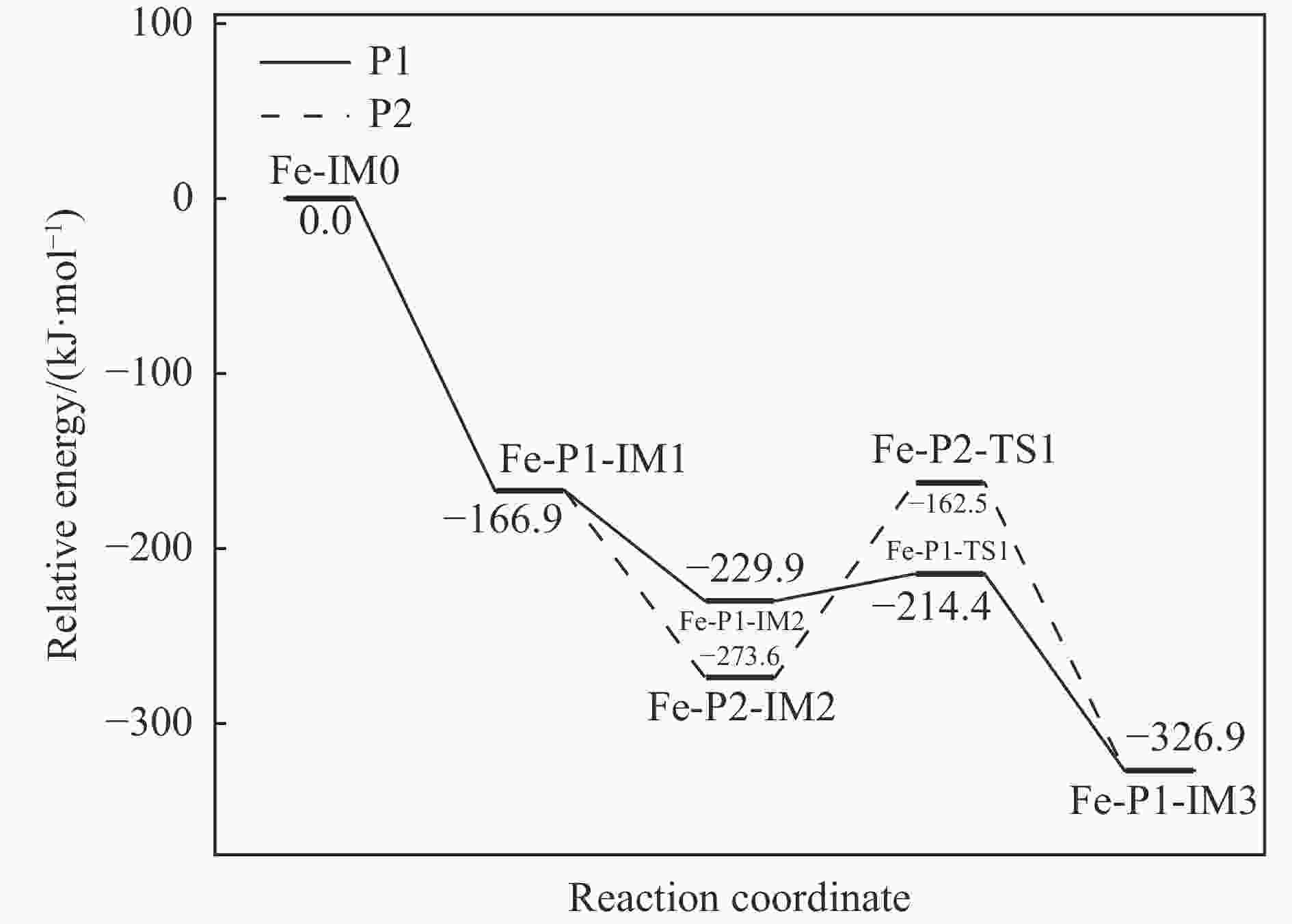
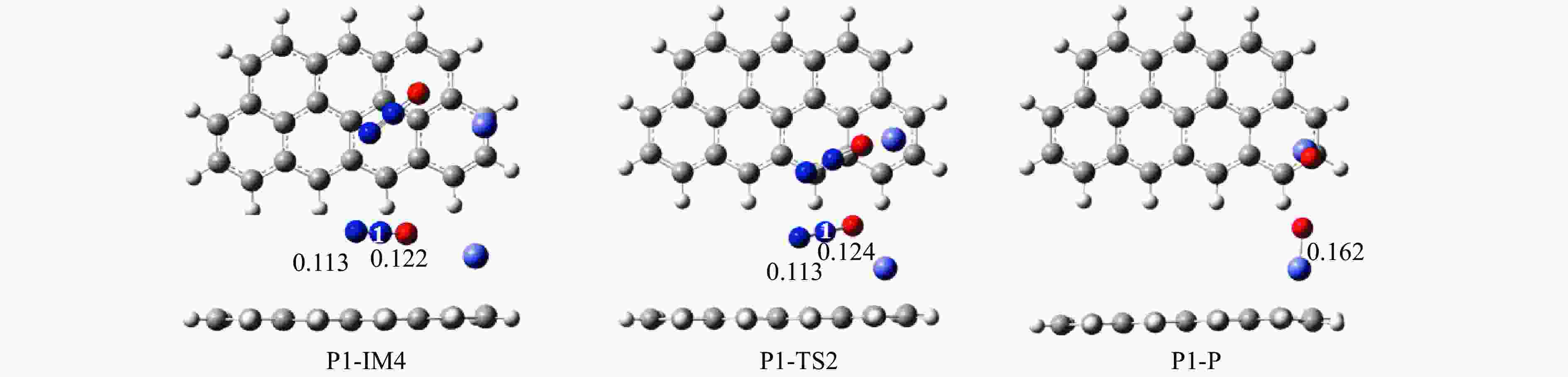
Mechanism of heterogeneous reduction of NO over graphite-supported single-atom Fe catalyst: DFT study
-
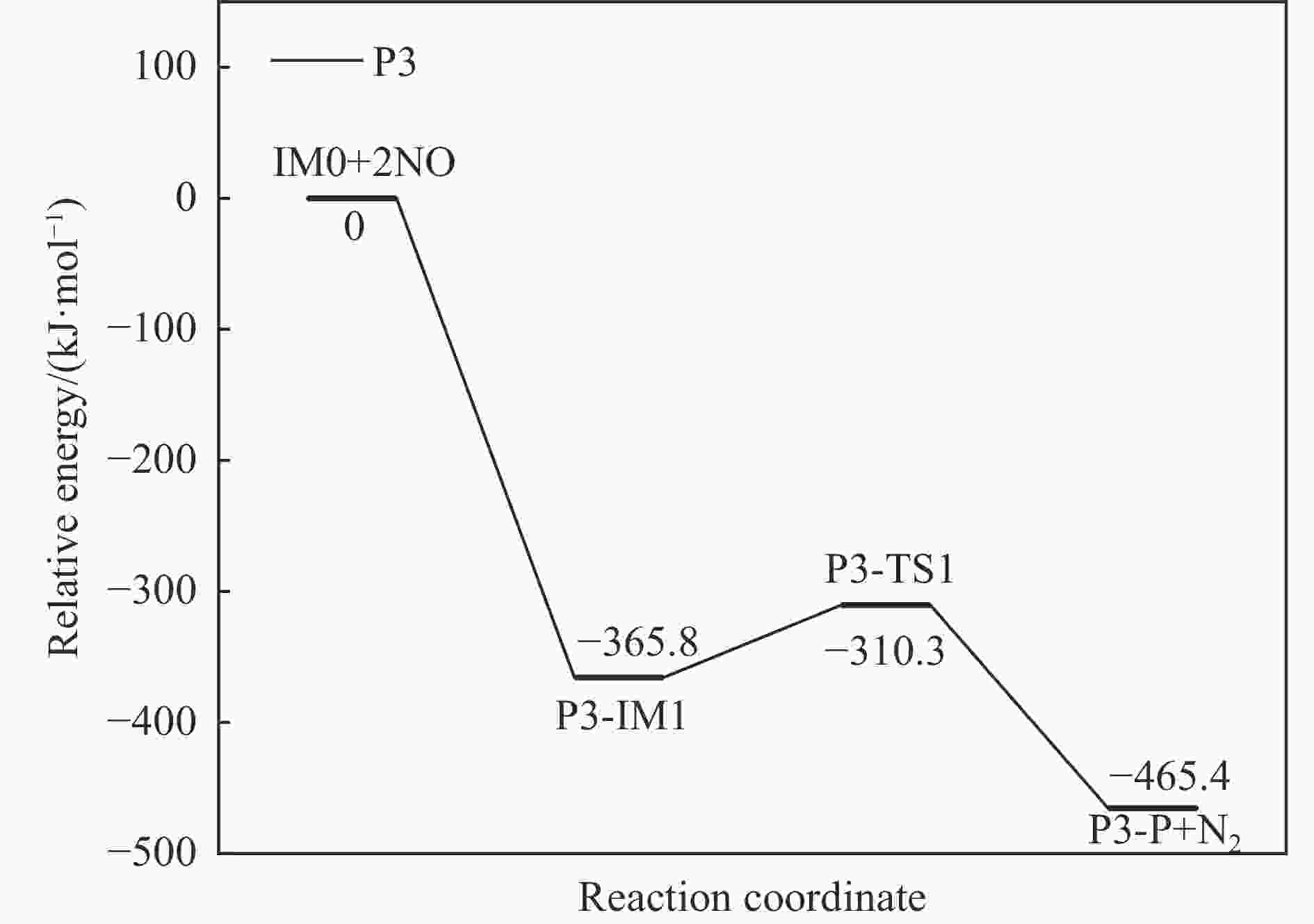
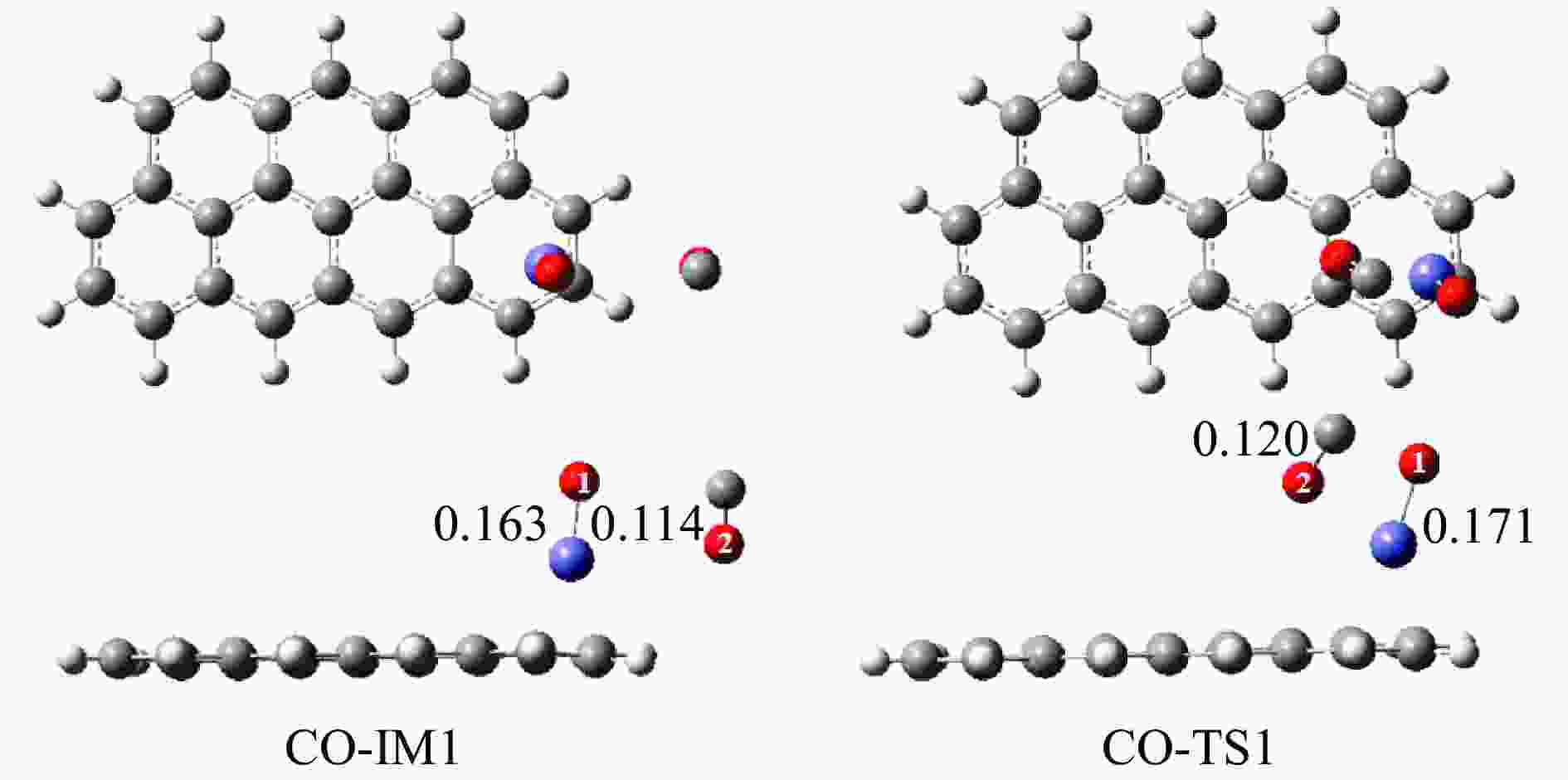
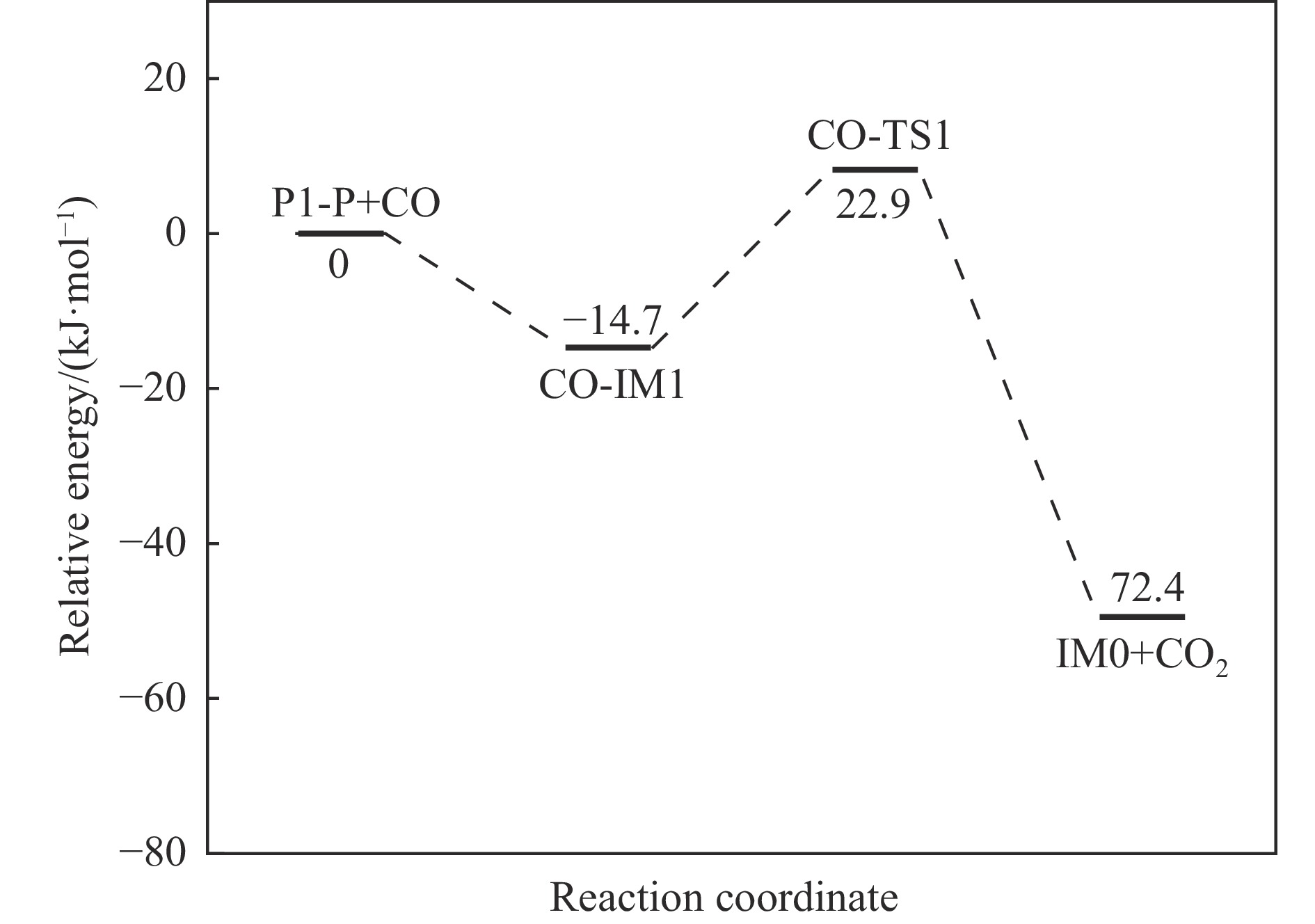
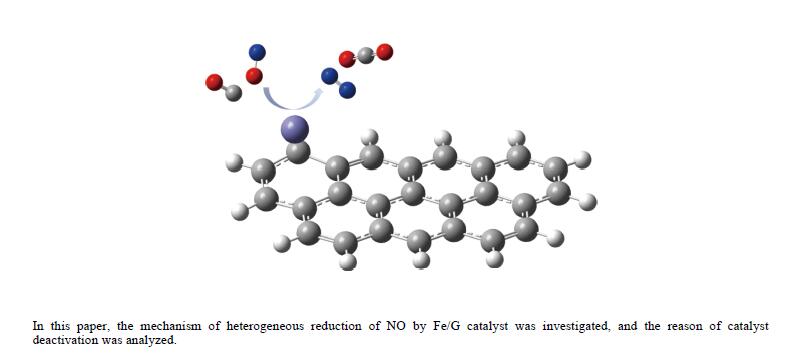
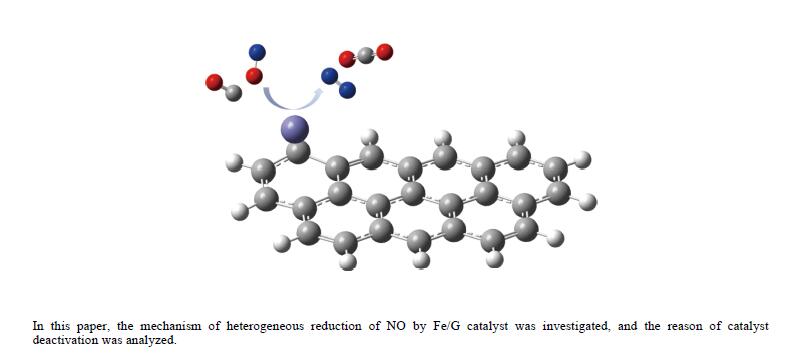
摘要: 基于密度泛函理论和经典过渡态理论,探究了石墨炭负载单原子Fe催化剂(Fe/G)异相还原NO的微观机理,并对催化剂失活原因进行分析。结果表明,基于E-R机理,NO还原反应依次经历了N2O形成与释放、N2形成与释放四个阶段。而基于L-H机理,NO还原反应主要经历了N2形成与释放两个阶段。在E-R机理作用下,NO分子以N,O-down结构吸附在Fe原子上发生的NO还原反应的控速步骤能垒值仅为15.5 kJ/mol,小于其余路径控速步骤能垒值。由能垒角度分析,Fe原子上残留的活性氧被还原的能垒值高于NO还原生成N2的能垒值。NO分解后残留在Fe原子表面的活性氧抑制了NO的吸附与还原,Fe原子活性位的缺失导致催化剂的失活,单原子Fe的存在促进了NO还原反应的进行。由动力学角度分析,随着反应温度的升高,NO还原速率较活性氧转移速率提升更为显著。Abstract: The mechanism of nitrogen oxide (NO) reduction over graphite carbon-supported single-atom iron (Fe) catalyst (Fe/G) was investigated by density functional theory (DFT) and transition state theory (TST). The catalyst deactivation was also analyzed. The results revealed that the NO reduction, based on the Eley-Rideal (E-R) mechanism, underwent four stages including N2O formation and release as well as N2 formation and release. However, the NO reduction only involved two stages according to Langmuir-Hinshelwood (L-H) mechanism: N2 formation and release. Furthermore, for the E-R mechanism, the rate-controlling step was NO reduction, where a NO molecule was adsorbed on an Fe atom with an N, O-down structure with energy barrier of 15.5 kJ/mol, lower than that of other paths. Energy barrier analysis indicated that the energy barrier for the reduction of reactive oxygen species was higher than that for the formation of N2. Reactive oxygen species remaining on the surface of Fe atoms after NO decomposition inhibited the adsorption and reduction of NO, leading to catalyst deactivation due to the absence of active sites. The single-atom Fe species promoted the NO reduction. Kinetic analysis results suggested that, upon increasing the reaction temperature, the NO reduction rate increased more significantly than the reactive oxygen transfer rate.
-
Key words:
- singe-atom Fe catalyst /
- nitrogen oxides /
- density functional theory /
- mechanism /
- deactivation
-
表 1 反应动力学参数
Table 1 Reaction kinetic parameters
Course of reaction Pre-exponential factor A/s−1 Activation energy Ea/(kJ·mol−1) Arrhenius equation P1-IM2→P1-IM3 1.32×1015 19.28 k=1.32×1015e−2318.98/T P2-IM2→P1-IM3 6.68×1012 111.99 k=6.68×1012e−13470.04/T P3-IM1→P3-P+CO2 9.72×1014 61.36 k=9.72×1014e−7380.32/T CO-IM1→IM0+CO2 4.36×1011 37.16 k=4.36×1011e−4469.57/T -
[1] 孙锦昌, 任翠涛, 赵明新, 等. Cu/Hβ催化剂NH3选择性催化还原NO性能研究[J]. 燃料化学学报(中英文),2023,51(6):823−831.SUN Jinchang, REN Cuitao, ZHAO Mingxin, et al. Selective catalytic reduction of NO by Cu/Hβ catalyst NH3[J]. J Fuel Chem Technol,2023,51(6):823−831. [2] WU G, FENG X, ZHANG H, et al. The promotional role of Ni in FeVO4/TiO2 monolith catalyst for selective catalytic reduction of NO x with NH3[J]. Appl Surf Sci,2018,427:24−36. doi: 10.1016/j.apsusc.2017.08.135 [3] ZHOU Y, REN S, WANG M, et al. Mn and Fe oxides co-effect on nanopolyhedron CeO2 catalyst for NH3-SCR of NO[J]. J Energy Inst,2021,99:97−104. doi: 10.1016/j.joei.2021.08.003 [4] LIU S, XUE W, JI Y, et al. Interfacial oxygen vacancies at Co3O4-CeO2 heterointerfaces boost the catalytic reduction of NO by CO in the presence of O2[J]. Appl Catal B: Environ,2023,323:122151. doi: 10.1016/j.apcatb.2022.122151 [5] 吕文婷, 焦卫勇, 秦张峰, 等. 制备条件对Cu-SSZ-13分子筛的形貌及其氨选择性催化还原脱硝性能的影响[J]. 燃料化学学报,2022,50(11):1393−1403.LÜ Wenting, JIAO Weiyong, QIN Zhangfeng, et al. Effect of preparation conditions on the morphology of Cu-SSZ-13 molecular sieve and its denitrification performance by selective catalytic reduction of ammonia[J]. J Fuel Chem Technol,2022,50(11):1393−1403. [6] OTON L F, OLIXEIRA A C, DE ARAUJO J C S, et al. Selective catalytic reduction of NO x by CO (CO-SCR) over metal-supported nanoparticles dispersed on porous alumina[J]. Adv Powder Technol,2020,31:464−476. doi: 10.1016/j.apt.2019.11.003 [7] 张恒, 周皞, 温妮妮, 等. Cu-SAPO-44选择性催化丙烯还原NO性能研究[J]. 燃料化学学报,2022,50(18):1064−1074.ZHANG Heng, ZHOU Hao, WEN Nini, et al. Study on the performance of Cu-SAPO-44 selective catalytic reduction of NO by propylene[J]. J Fuel Chem Technol,2022,50(18):1064−1074. [8] WANG H, LI X, ZHU M, et al. Preparation and evaluation of catalysts of highly dispersed zerovalent iron (Fe0) supported on activated carbon for NO reduction[J]. Fuel,2021,303:121252. doi: 10.1016/j.fuel.2021.121252 [9] YIN P, YAO T, WU Y, et al. Single cobalt atoms with precise N-coordination as superior oxygen reduction reaction catalysts[J]. Angew Chem Int Ed,2016,55:10800−10805. doi: 10.1002/anie.201604802 [10] CUI T, LI L, YE C, et al. Heterogeneous single atom environmental catalysis: Fundamentals, applications, and opportunities[J]. Adv Funct Mater,2021,32:2108381. [11] 张艺严, 陈熙元, 董灵玉, 等. 炭载单原子催化剂在电还原二氧化碳领域的研究进展[J]. 燃料化学学报(中英文),2023,51(11):1617−1632.ZHANG Yiyan, CHEN Xiyuan, DONG Lingyu, et al. Research progress in the field of electroreduction of carbon dioxide by carbon supported single atom catalyst[J]. J Fuel Chem Technol,2023,51(11):1617−1632. [12] QIAO B, WANG A, YANG X, et al. Single-atom catalysis of CO oxidation using Pt1/FeO x[J]. Nat Chem,2011,3:634−641. doi: 10.1038/nchem.1095 [13] 裴永丽, 郭长江, 张宁, 等. 原子层沉积制备纳米催化剂研究进展[J]. 燃料化学学报,2021,49(9):1281−1293.PEI Yongli, GUO Changjiang, ZHANG Ning, et al. Research progress on preparation of nanocatalysts by atomic layer deposition[J]. J Fuel Chem Technol,2021,49(9):1281−1293. [14] JI Y, LIU S, SONG S, et al. Negatively charged single-atom Pt catalyst shows superior SO2 tolerance in NO x reduction by CO[J]. ACS Catal,2022,13:224−236. [15] YUE S, WANG C, XU Z, et al. A theoretical exploration of the effect and mechanism of CO on NO2 heterogeneous reduction over carbonaceous surfaces[J]. Fuel,2021,290:120102. doi: 10.1016/j.fuel.2020.120102 [16] YAMASHITA H, YAMADA H, TOMITA A. Reaction of nitric oxide with metal-loaded carbon in the presence of oxygen[J]. Appl Catal A: Gen,1991,78:L1−L6. doi: 10.1016/0166-9834(91)80101-2 [17] DENG C, SU Y, LI F, et al. Understanding activity origin for the oxygen reduction reaction on bi-atom catalysts by DFT studies and machine-learning[J]. J Mater Chem A,2020,8:24563−24571. doi: 10.1039/D0TA08004G [18] WANG H, LI X, MENG F, et al. Preparation and evaluation of iron nanoparticles embedded CNTs grown on ZSM-5 as catalysts for NO decomposition[J]. Chem Eng J,2020,392:123798. doi: 10.1016/j.cej.2019.123798 [19] ZHANG X, WU H, XIE M, et al. A thorough theoretical exploration of the effect mechanism of Fe on HCN heterogeneous formation from nitrogen-containing char[J]. Fuel,2020,280:118662. doi: 10.1016/j.fuel.2020.118662 [20] WANG H, LI X, WU J, et al. An experimental and density functional theory simulation study of NO reduction mechanisms over Fe(0) supported on graphene with and without CO[J]. Langmuir,2023,39:15369−15379. doi: 10.1021/acs.langmuir.3c02461 [21] ZHANG H, LIU J, SHEN J, et al. Thermodynamic and kinetic evaluation of the reaction between NO (nitric oxide) and char(N) (char bound nitrogen) in coal combustion[J]. Nat Energy,2015,82:312−321. doi: 10.1016/j.energy.2015.01.040 [22] SANTOS J C D S D, FERREIRA D F S, DA SILVA C A B, et al. Transitions in electrical behavior of molecular devices based on 1-D and 2-D graphene-phagraphene-graphene hybrid heterojunctions[J]. Mater Chem Phys,2020,253:123420. doi: 10.1016/j.matchemphys.2020.123420 [23] CHEN N, YANG R T. Ab initio molecular orbital calculation on graphite: Selection of molecular system and model chemistry[J]. Carbon,1998,36:1061−1070. doi: 10.1016/S0008-6223(98)00078-5 [24] HU L, HU X, WU X, et al. Density functional calculation of transition metal adatom adsorption on graphene[J]. Physica B Condens Matter,2010,405:3337−3341. doi: 10.1016/j.physb.2010.05.001 [25] MANADE M, VINES F, ILLAS F. Transition metal adatoms on graphene: A systematic density functional study[J]. Carbon,2015,95:525−534. doi: 10.1016/j.carbon.2015.08.072 [26] BAI Y, BIAN X, WU W. Catalytic properties of CuO/CeO2-Al2O3 catalysts for low concentration NO reduction with CO[J]. Appl Surf Sci,2019,463:435−444. doi: 10.1016/j.apsusc.2018.08.229 [27] LU T, CHEN Q. Shermo: A general code for calculating molecular thermochemistry properties[J]. Comput Theor Chem,2021,1200:113249. doi: 10.1016/j.comptc.2021.113249 [28] 张秀霞, 谢苗, 伍慧喜, 等. 钙对焦炭非均相还原NO的微观作用机理: DFT研究[J]. 燃料化学学报,2020,48(2):163−171.ZHANG Xiuxia, XIE Miao, WU Huixi, et al. The microscopic mechanism of heterogeneous reduction of NO by calcium in coke: A DFT study[J]. J Fuel Chem Technol,2020,48(2):163−171. [29] YANG L, YANG H, LIU Q, et al. Study on the Reaction Performance of Ce- and Co-Modified Mn-Based Catalysts in C3H6-SCR[J]. J Phys Chem C,2023,127:15278−15289. doi: 10.1021/acs.jpcc.3c04279 [30] WANG B, XIONG Y, HAN Y, et al. Preparation of stable and highly active Ni/CeO2 catalysts by glow discharge plasma technique for glycerol steam reforming[J]. Appl Catal B: Environ,2019,249:257−265. doi: 10.1016/j.apcatb.2019.02.074 [31] 张峰, 孙浩, 张建树, 等. Fe的分散程度对煤焦催化加氢气化的影响[J]. 燃料化学学报,2019,47(4):402−410.ZHANG Feng, SUN Hao, ZHANG Jianshu, et al. Effect of Fe dispersion degree on catalytic hydrogasification of coal coke[J]. J Fuel Chem Technol,2019,47(4):402−410. -




 下载:
下载: