Mechanism of catalytic decomposition of NO by Cu-ZSM-5
-
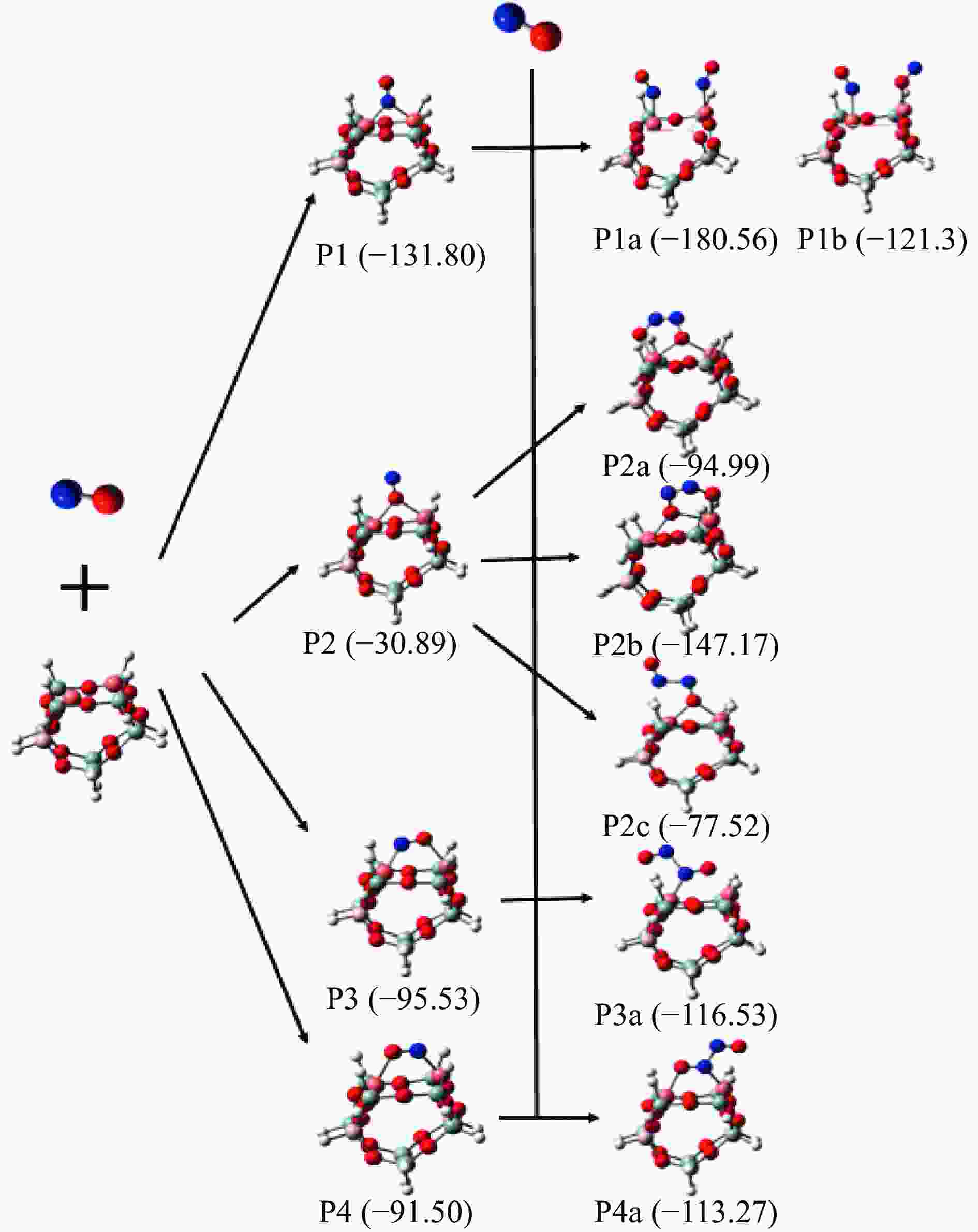
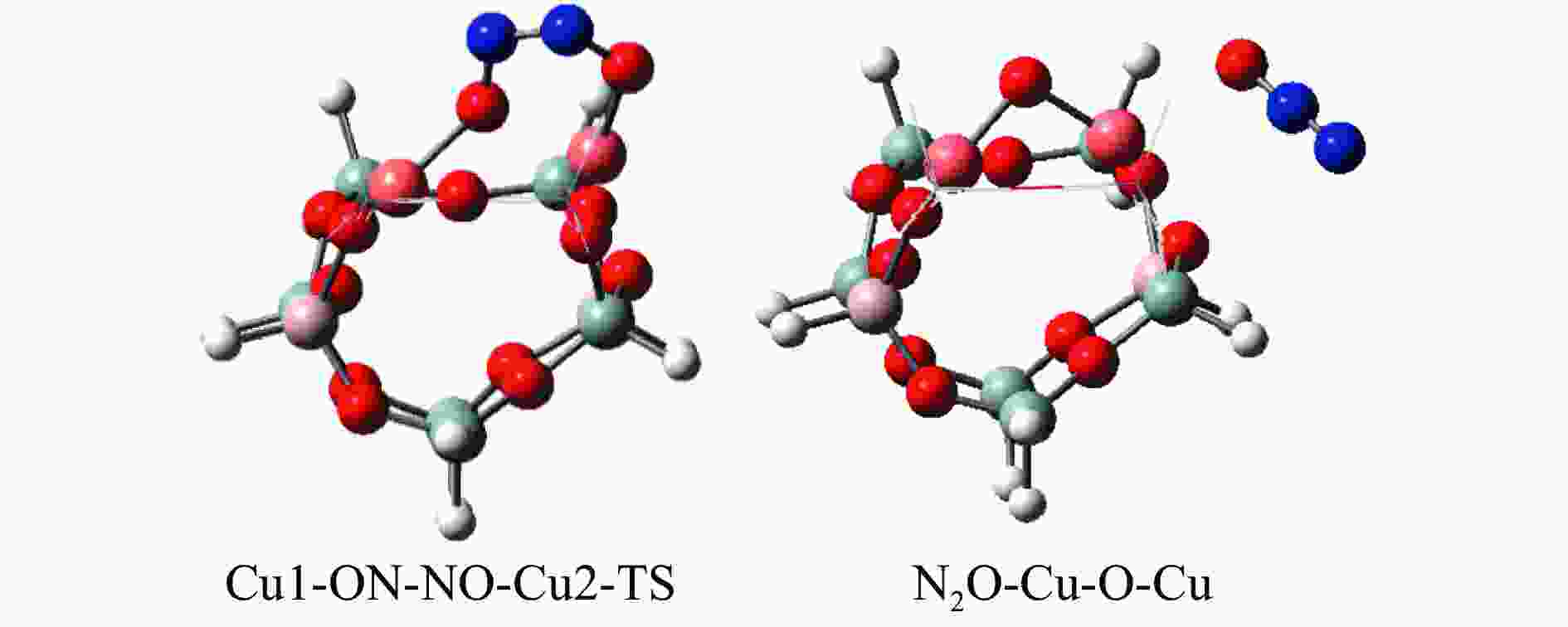
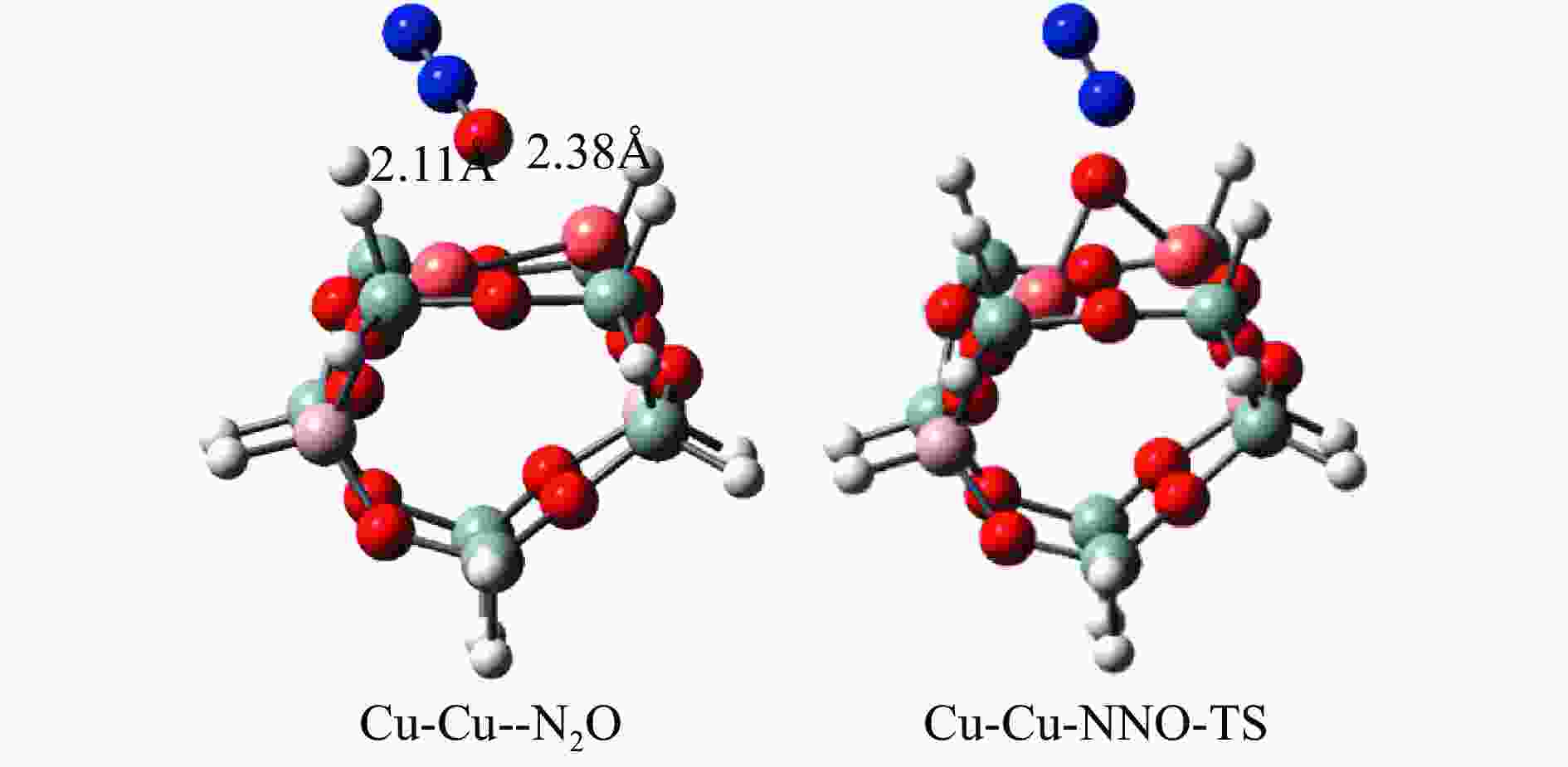
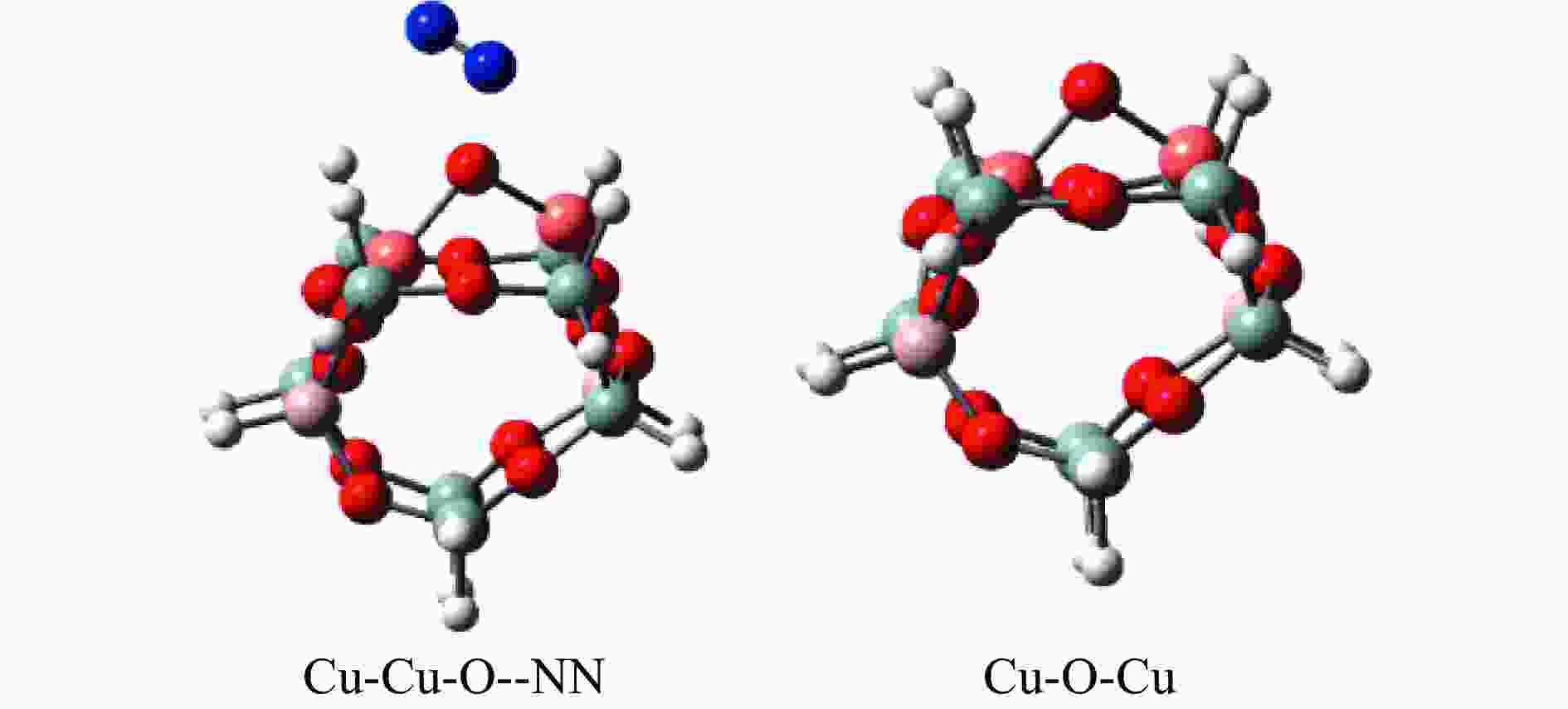
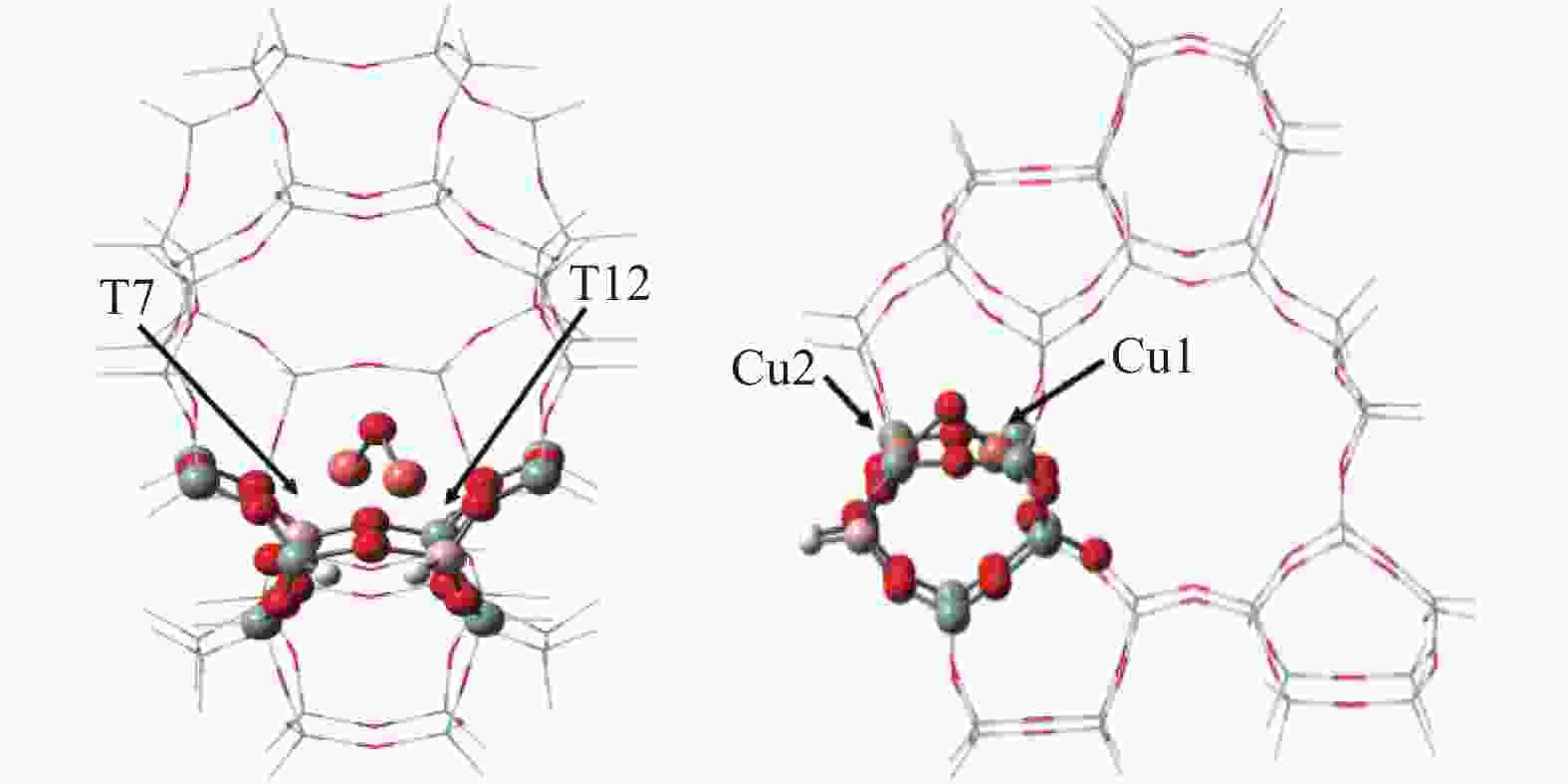
摘要: Cu-ZSM-5催化分解NO具有潜在的应用前景。为揭示NO在Cu-ZSM-5催化剂的催化分解机理,基于密度泛函模拟了NO在Cu-ZSM-5催化剂中短距离Cu+对上的吸附,并提出副产物N2O、NO2辅助催化分解NO的反应路径。计算结果表明,双核铜氧物种是Cu基催化剂的重要活性中心。催化分解NO过程中,副产物NO2在双核铜氧物种上的分解需要的活化能最高(为171.39 kJ/mol),N2O分解需要86.92 kJ/mol的活化能垒,表明NO2在活性位的分解难于N2O的分解。N2、O2的解析分别吸收28.43、100.78 kJ/mol的热量,限速步骤为O2的脱附。NO既作为反应物,同时又是催化过程中Cu-ZSM-5催化剂活性中心实现氧化还原循环的关键还原剂。Abstract: Catalytic decomposition of NO by Cu-ZSM-5 has potential application. In order to reveal the catalytic decomposition mechanism of NO over Cu-ZSM-5, the adsorption of NO over short-range Cu+ pairs in Cu-ZSM-5 was simulated based on density functional theory. The reaction pathways of NO decomposition assisted by the by-products N2O and NO2 were also proposed. The results showed that the double nuclear copper-oxygen species was an important active centre. During the reaction, the highest activation energy (171.39 kJ/mol) was required for the decomposition of the by-product NO2 on the binuclear copper-oxygen species. While that for the decomposition of N2O was 86.92 kJ/mol, suggesting that the decomposition of NO2 was more difficult. The desorption energy of N2 and O2 were 28.43 and 100.78 kJ/mol, respectively. The rate determining step was O2 desorption. NO acted both as a reactant and a key reductant for the redox cycle of the active centre of Cu-ZSM-5 during the process.
-
Key words:
- Cu-ZSM-5 /
- catalytic decomposition /
- binuclear copper-oxygen species /
- redox cycle
-
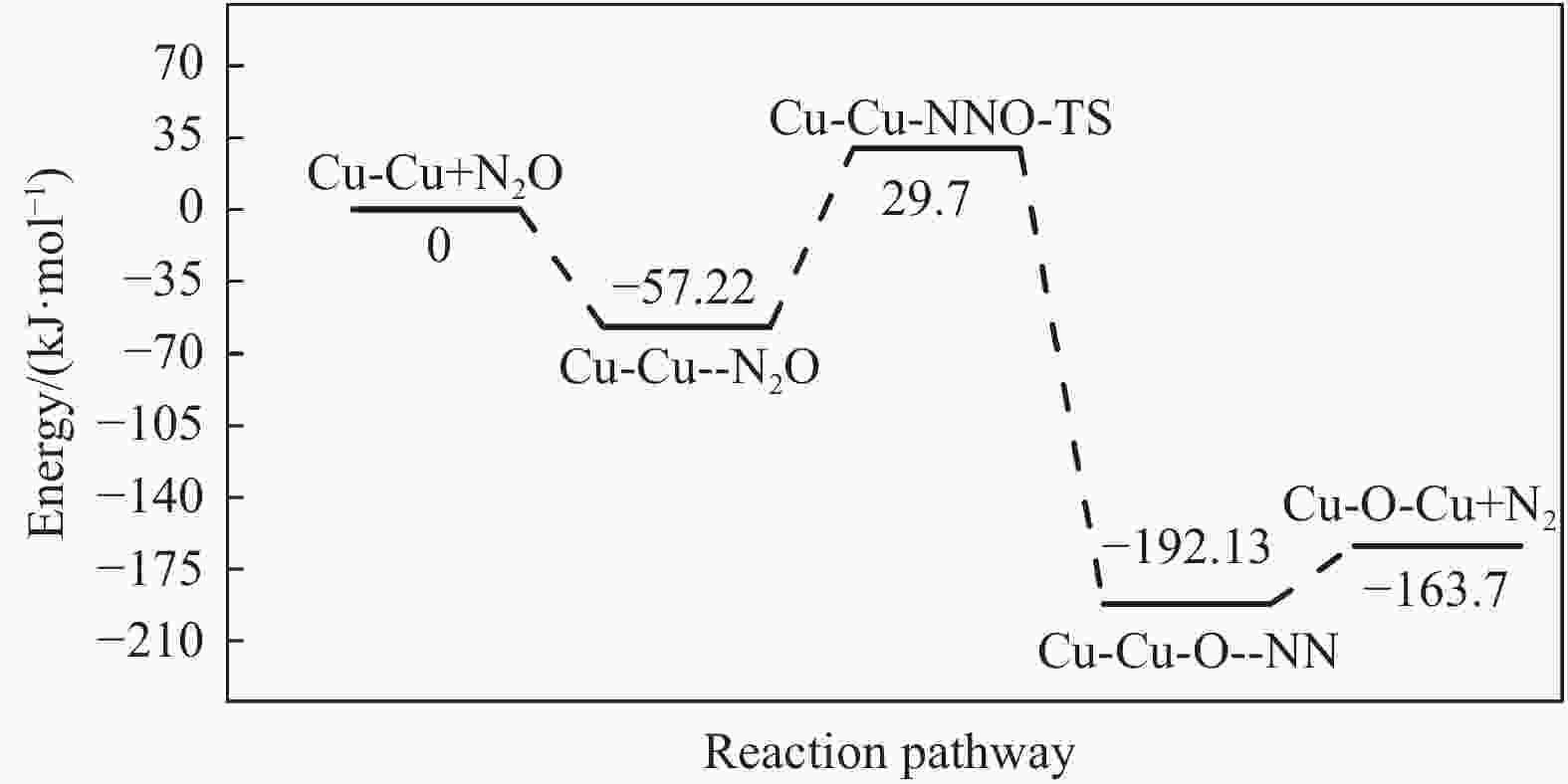
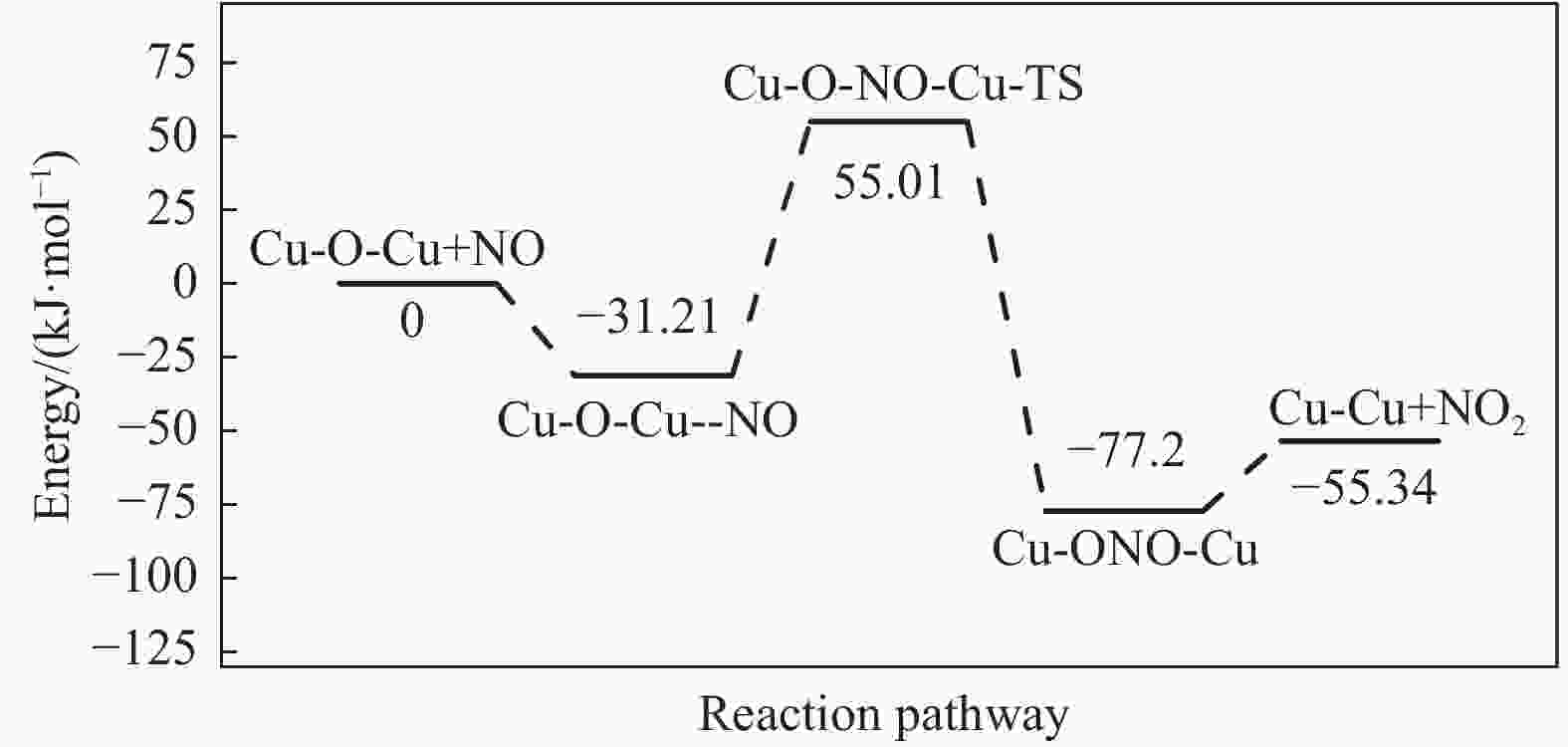
表 1 NO在Cu-ZSM-5上催化分解的能垒
Table 1 Energy barrier for the catalytic decomposition of NO on Cu-ZSM-5
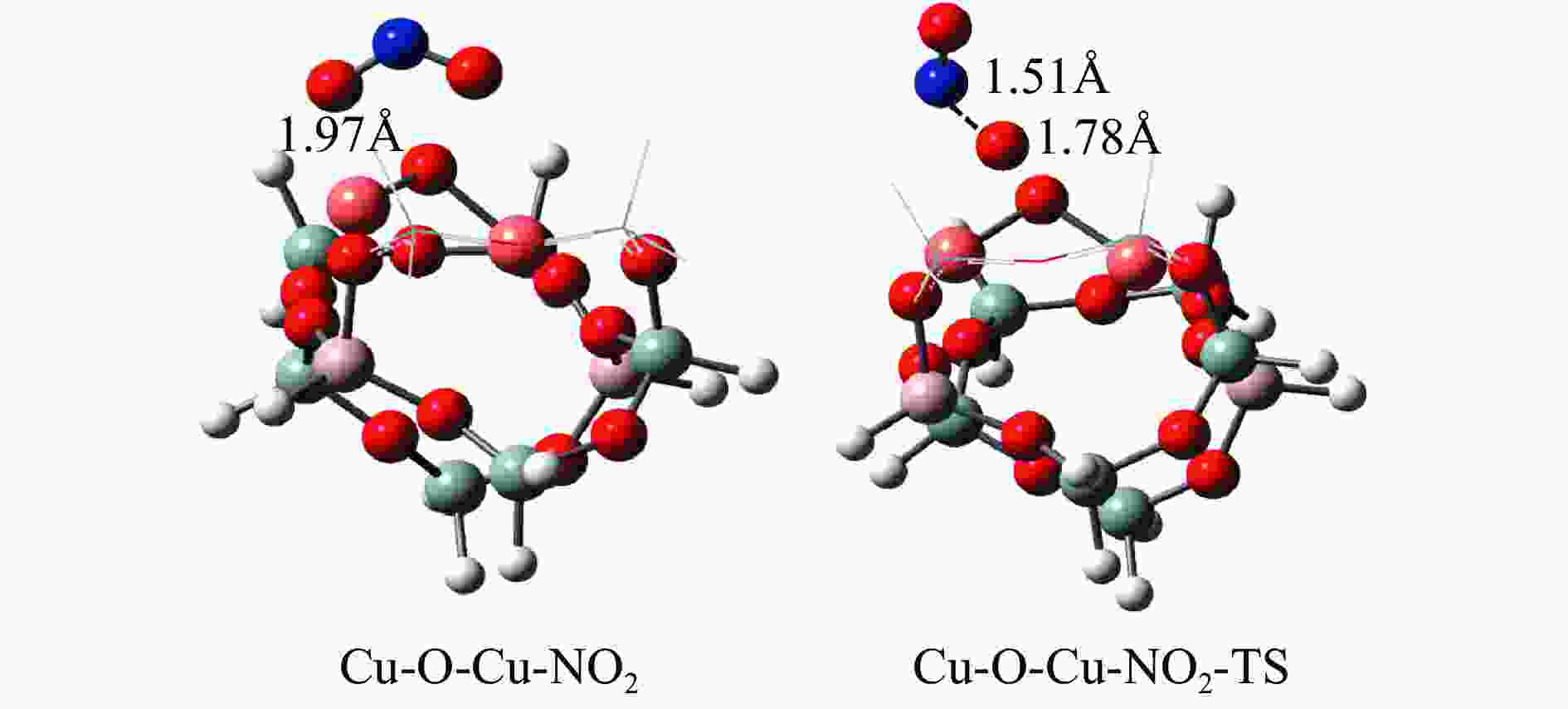
Elementary step Barrier/(kJ·mol−1) Cu-Cu+2NO→Cu-O-Cu+N2O 16.55 Cu-Cu+N2O→Cu-O-Cu+N2 86.92 Cu-O-Cu+NO→Cu-Cu+NO2 86.22 Cu-O-Cu+NO2→Cu-NO3-Cu→Cu-Cu+NO+O2 171.39 -
[1] 张霄玲. 低温锰系催化剂制备及烟气脱硫脱硝性能研究[D]. 北京: 中国科学院大学, 2020.ZHANG Xiaoling. Synthesis of manganese based catalyst for desulfurization and denitrification from low-temperature flue gas[D]. Beijing: University of Chinese Academy of Sciences. 2020.) [2] GOU X, ZHANG K, LIU L S, et al. Study on noble metal catalyst for selective catalytic reduction of NO x at low temperature[J]. Appl Mech Mater,2013,448:885−889. [3] IWAMOTO M, FURUKAWA H, MINE Y, et al. Copper(II)ion-exchanged ZSM-5 zeolites as highly active catalysts for direct and continuous decomposition of nitrogen monoxide[J]. J Chem Soc, Chem Commun,1986,16(16):1272−1273. [4] IWAMOTO M, YAHIRO H, TANDA K, et al. Removal of nitrogen monoxide through a novel catalytic process. 1. decomposition on excessively copper-ion-exchanged ZSM-5 zeolites[J]. J Phys Chem B,1991,22(34):3727−3730. [5] SPOTO G, ZECCHINA A, BORDIGA S, et al. Cu(I)-ZSM-5 zeolites prepared by reaction of H-ZSM-5 with gaseous CuCl: Spectroscopic characterization and reactivity towards carbon monoxide and nitric oxide[J]. Appl Catal B: Environ,1994,3(2/3):151−172. doi: 10.1016/0926-3373(93)E0032-7 [6] VALYON J, HALL W K. Studies of the surface species formed from NO on copper zeolites[J]. J Phys Chem,1993,97(6):1204−1212. doi: 10.1021/j100108a016 [7] MORPURGO S, MORETTI G, BOSSA M. A computational study on the mechanism of NO decomposition catalyzed by Cu-ZSM-5: A comparison between single and dimeric Cu+ active sites[J]. J Mol Catal A: Chem,2012,358:134−144. [8] COSTA P D, MODÉN B, MEITZNER G D, et al. Spectroscopic and chemical characterization of active and inactive Cu species in NO decomposition catalysts based on Cu-ZSM-5[J]. Phys Chem Chem Phys,2002,4(18):4590−4601. doi: 10.1039/B203700A [9] SENGUPTA D, ADAMS J B, SCHNEIDER W F, et al. Theoretical analysis of N2O to N2 conversion during the catalytic decomposition of NO by Cu-Zeolites[J]. Catal Lett,2001,74(3):193−199. [10] SOLANS-MONFORT X, BRANCHADELL V, SODUPE M. On the NO decomposition by Cu−ZSM-5 through the ZCu(NO2)(NO) or ZCu(N2O3) intermediates[J]. J Phys Chem B,2002,106(6):1372−1379. doi: 10.1021/jp0130620 [11] LIU X, YANG Z Y, LI Y P, et al. Theoretical study of N2O decomposition mechanism over binuclear Cu-ZSM-5 zeolites[J]. J Mol Catal A: Chem, 2015, 396: 181−187. [12] SMEETS P J, GROOTHAERT M H, VAN TEEFFELEN R M, et al. Direct NO and N2O decomposition and NO-assisted N2O decomposition over Cu-zeolites: Elucidating the influence of the CuCu distance on oxygen migration[J]. J Catal,2007,245(2):358−368. doi: 10.1016/j.jcat.2006.10.017 [13] LI G, VASSILEV P, SANCHEZ S M, et al. Stability and reactivity of copper oxo-clusters in ZSM-5 zeolite for selective methane oxidation to methanol[J]. J Catal,2016,338:305−312. doi: 10.1016/j.jcat.2016.03.014 [14] 高丛茹. Cu-ZSM-5催化N2O分解和NOx还原过程机理研究[D]. 北京: 北京化工大学, 2023.GAO Congru. Study on the mechanism of Cu-ZSM-5 catalyzed N2O decomposition and NOx reduction[D]. Beijing: Beijing University of Chemical Technology, 2023.) [15] 何俊龙. 第一性原理研究NO在3d过渡金属掺杂石墨烯上的催化还原反应[D]. 上海: 东华大学, 2021.HE Junlong. The first principles calculation of NO catalytic reduction on 3d transition metal-doped graphene[D]. Shanghai: Donghua University, 2021.) [16] 霍培英. 铜钴二元合金团簇吸附特性及NO分解机理的密度泛函理论研究[D]. 镇江: 江苏科技大学, 2019.HUO Peiying. Density functional theory study on adsorption properties and NO decomposition mechanism of Cu-Co bimetallic clusters[D]. Zhenjiang: Jiangsu University of Science and Technology, 2019.) [17] 尤丽霞, 刘子忠, 刘红霞. NO自促还原反应机理的密度泛函研究[J]. 宝鸡文理学院学报(自然科学版),2016,36(1):30−35.YOU Lixia, LIU Zizhong, LIU Hongxia. A density functional study on the self-promoting reduction reaction mechanism of NO[J]. J Baoji Univ Arts Sci (Nat Sci Ed),2016,36(1):30−35. [18] 王焕然. Fe-C催化剂协同CO催化还原NO研究[D]. 鞍山: 辽宁科技大学, 2023.WANG Huanran. A fundamental study of NO reduction over Fe-C catalysts with CO as the reductant[D]. Anshan: University of Science and Technology Liaoning, 2023.) [19] LI P Y, LU F, YUAN F L, et al. Effect of surface copper species on NO+CO reaction over xCuO-Ce0.9Zr0.1O2 catalysts: In situ DRIFTS studies[J]. Catal,2016,6(8):124. [20] LIU L, YU Q, ZHU J, et al. Effect of MnO x modification on the activity and adsorption of CuO/Ce0.67Zr0.33O2 catalyst for NO reduction[J]. J Colloid Interface Sci,2010,349(1):246−255. doi: 10.1016/j.jcis.2010.05.044 [21] ZHANG X, CHENG X, MA C, et al. Effects of the Fe/Ce ratio on the activity of CuO/CeO2-Fe2O3 catalysts for NO reduction by CO[J]. Catal Sci Technol,2018,8(13):3336−3345. doi: 10.1039/C8CY00709H [22] WANG J, XIA H, JU X, et al. Catalytic performance of different types of iron zeolites in N2O decomposition[J]. Chin J Catal,2013,34(5):876−888. doi: 10.1016/S1872-2067(12)60555-5 [23] MENG T, REN N, MA Z. Silicalite-1@Cu-ZSM-5 core-shell catalyst for N2O decomposition[J]. J Mol Catal A: Chem,2015,404-405:233−239. [24] 刘清雅, 刘振宇, 李成岳. NH3在选择性催化还原NO过程中的吸附与活化[J]. 催化学报,2006,27(7):636−646. doi: 10.3321/j.issn:0253-9837.2006.07.022LIU Qingya, LIU Zhenyu, LI Chengyue. Adsorption and activation of NH3 during selective catalytic reduction of NO by NH3[J]. Chin J Catal,2006,27(7):636−646. doi: 10.3321/j.issn:0253-9837.2006.07.022 -




 下载:
下载: