Study on copper-based oxygen carrier catalytic power plant flue gas deoxidation
-
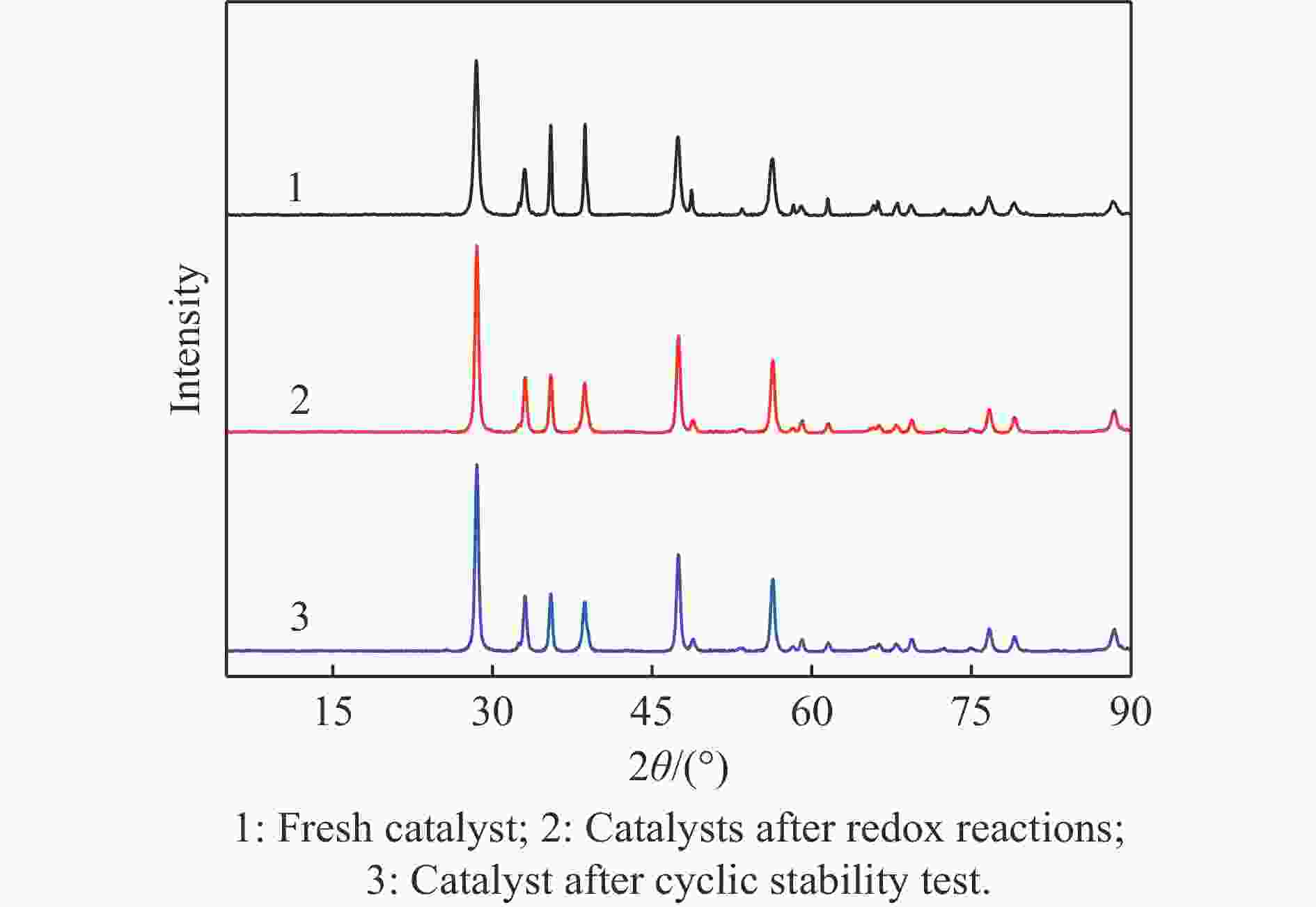
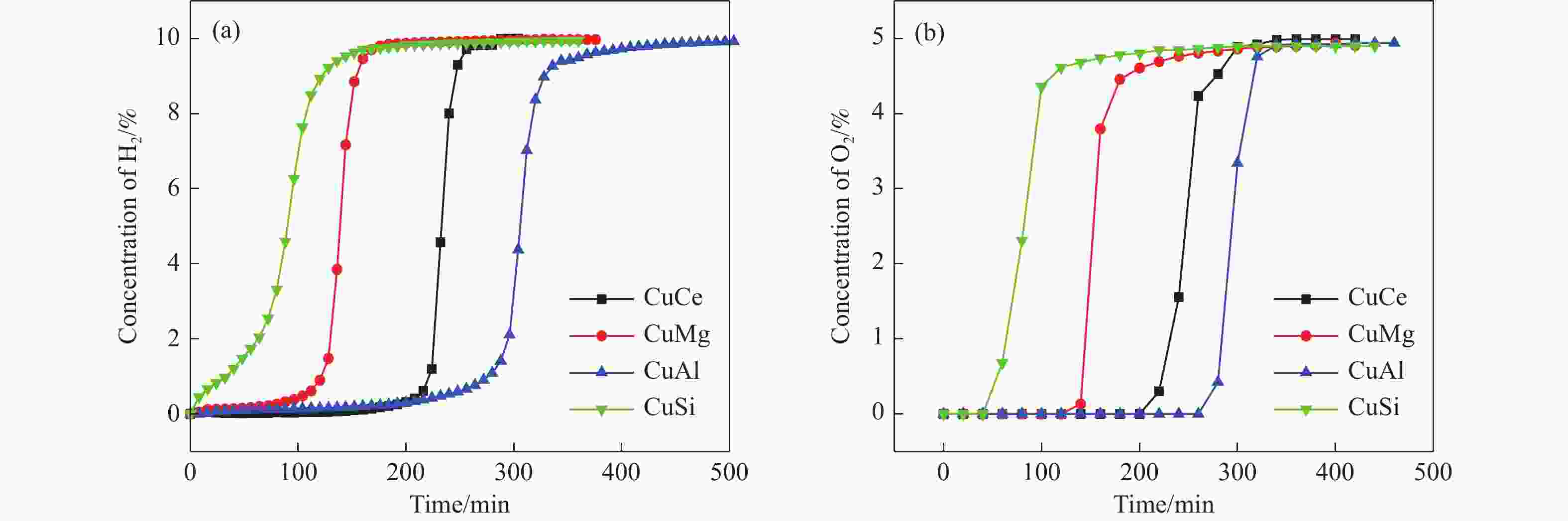
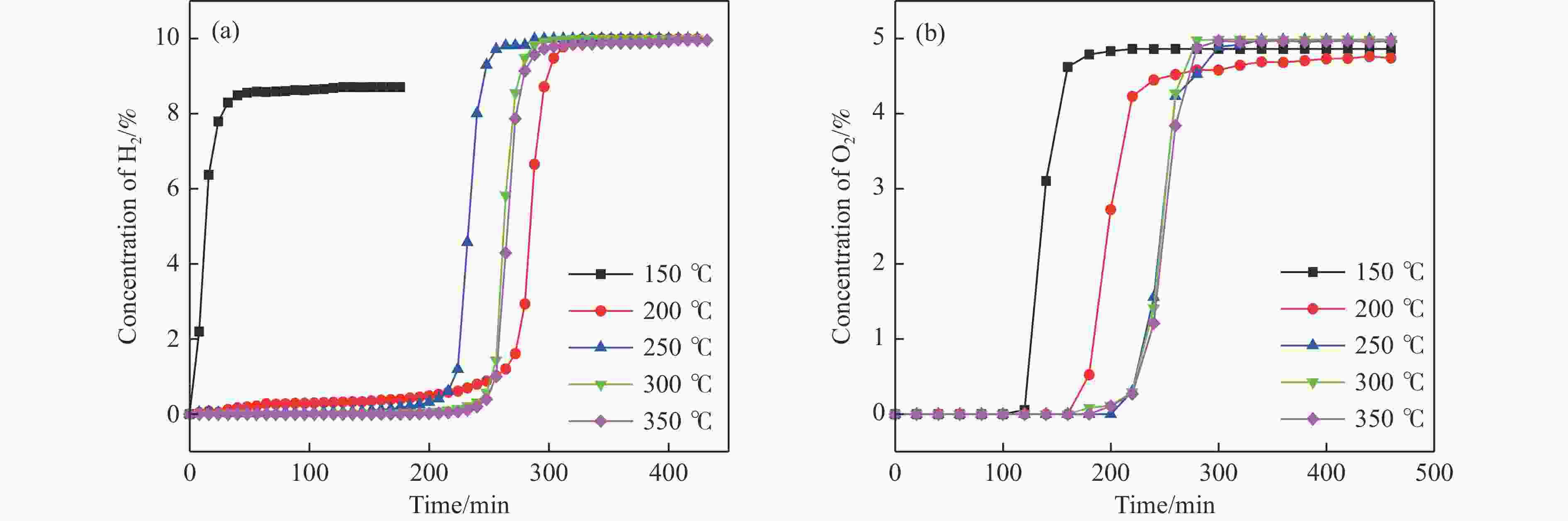
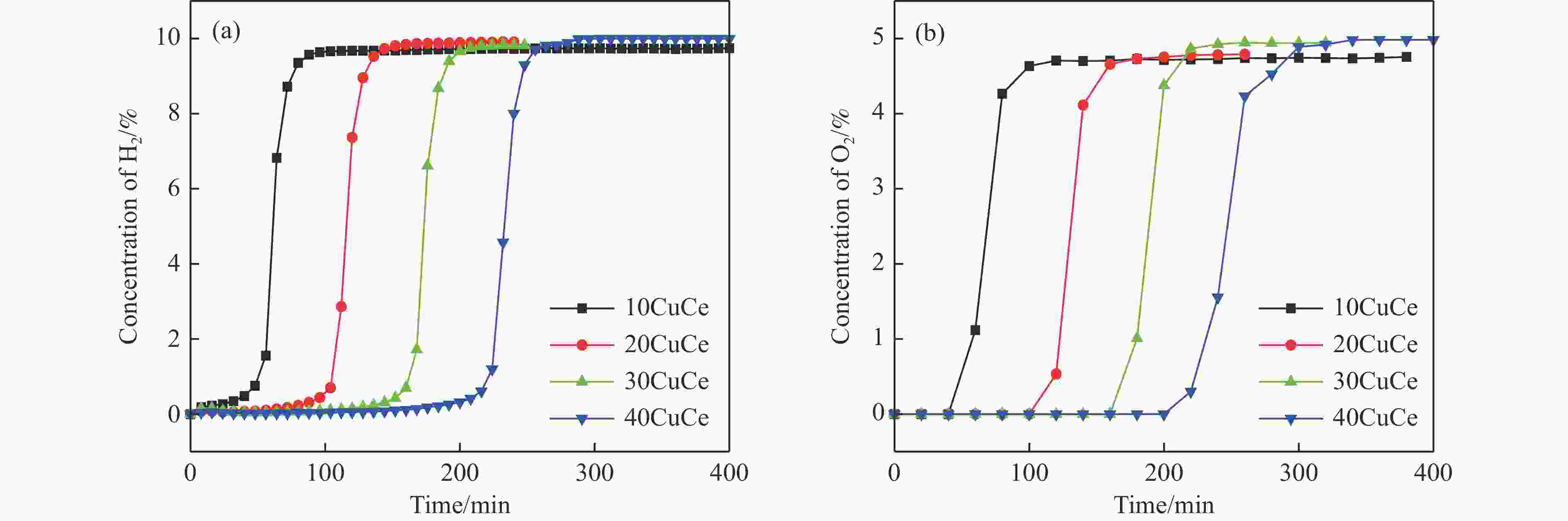
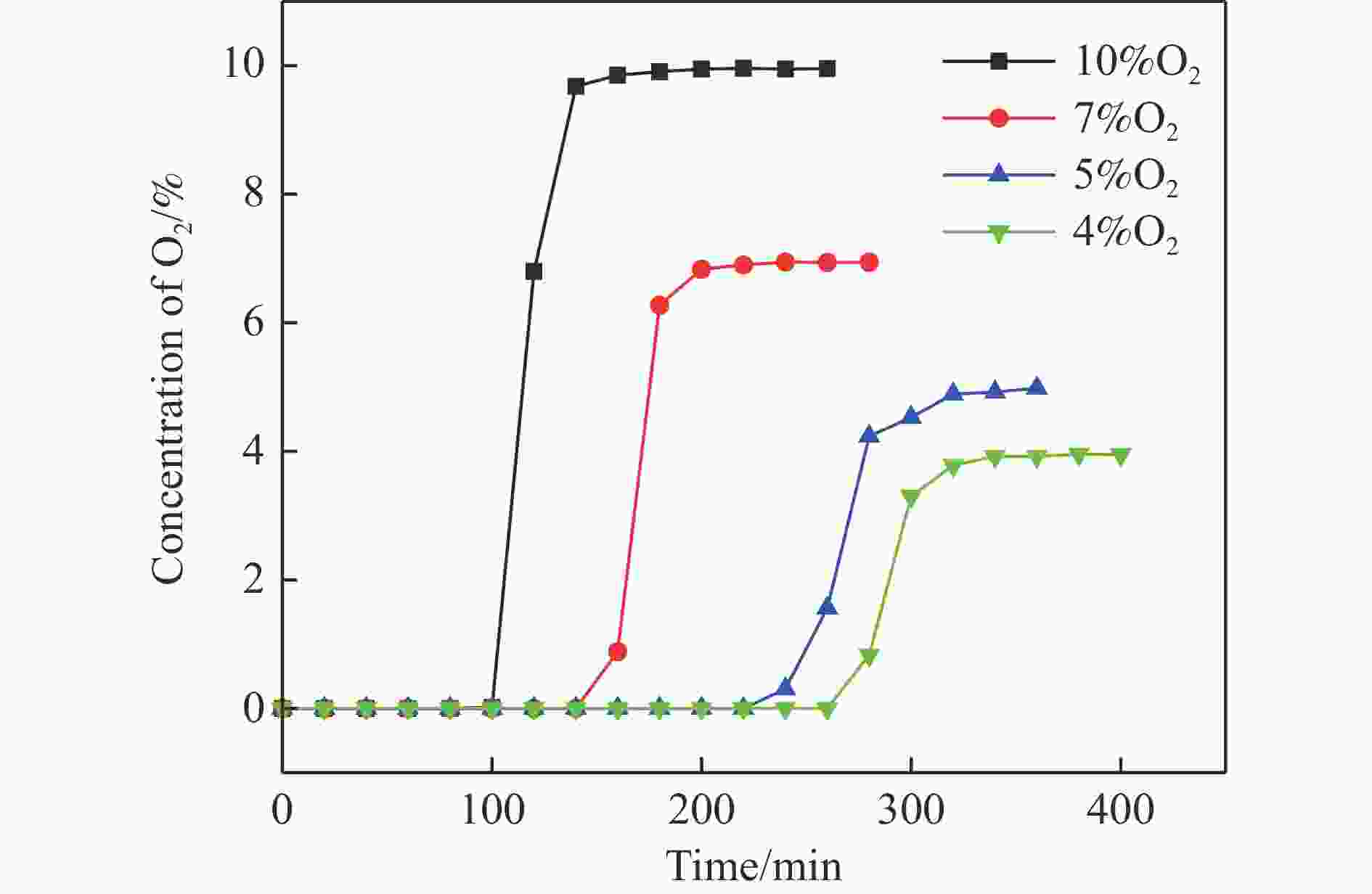
摘要: 电厂烟气主要成分为N2、CO2和部分O2,将电厂烟气注入矿井采空区可实现CO2封存,并替代注氮气防治遗煤自燃,但是烟气中的O2是造成遗煤自燃的因素之一。因此,亟待开发一种经济有效的催化剂来脱除电厂烟气中的O2。本研究采用共沉淀法,通过调变载体和负载量可控制备了铜基催化剂和系列xCuO/CeO2催化剂,利用BET、XRD、ICP、TEM、H2-TPR和XPS等手段对催化剂进行了表征,并建立催化剂结构与催化电厂烟气脱氧性能之间的构效关系。结果表明,CeO2的加入提高了CuO的分散性、增加了催化剂的氧空位,提高了催化剂的活性和还原氧化性能,Cu-Ce界面结构的协同效应促进了还原氧化过程,表现出良好的活性和循环稳定性。30CuO/CeO2由于其CuO颗粒尺寸最小、分散性最高、氧空位浓度最高,表现出较优的催化电厂烟气脱氧性能。本研究为开发低成本可循环使用、高活性和高稳定性的脱氧催化剂提供了参考。Abstract: The main components of power plant flue gas are N2, CO2 and part O2. Injecting power plant flue gas into mine goaf can achieve CO2 storage and replace nitrogen injection to prevent spontaneous combustion of left coal. However, O2 in flue gas is one of the factors causing spontaneous combustion of left coal. Therefore, it is urgent to develop an economical and effective catalyst to remove O2 from power plant flue gas. In this study, four types of copper-based catalysts were prepared using a controllable modulating carrier and loading capacity through co-precipitation method. Additionally, a series of xCuO/CeO2 catalystswere synthesized. The catalysts were characterized using BET analysis, XRD analysis, ICP analysis, TEM analysis, H2-TPR and XPS analysis to establish a structure-activity relationship between catalyst structure and deoxidation performance for catalytic power plant flue gases. The results showed that the addition of CeO2 improved the dispersion of CuO, increased the oxygen vacancy of the catalyst, and improved the activity and reduction oxidation performance of the catalyst. Moreover, the synergistic effect of Cu-Ce interface structure promoted the reduction oxidation process, showing good activity and cycle stability. Among xCuO/CeO2 catalysts, 30CuO/CeO2 showed the best catalytic deoxidation performance due to its smallest CuO particle size, highest dispersion and highest oxygen vacancy concentration. The results of this study provide a reference for the development of low cost, recyclable, high activity and high stability deoxidation catalysts.
-
Key words:
- chemical-looping combustion /
- CuO/CeO2 /
- redox /
- interface structure /
- oxygen vacancies
-
表 1 催化剂的物理参数
Table 1 Physical parameters of the catalyst
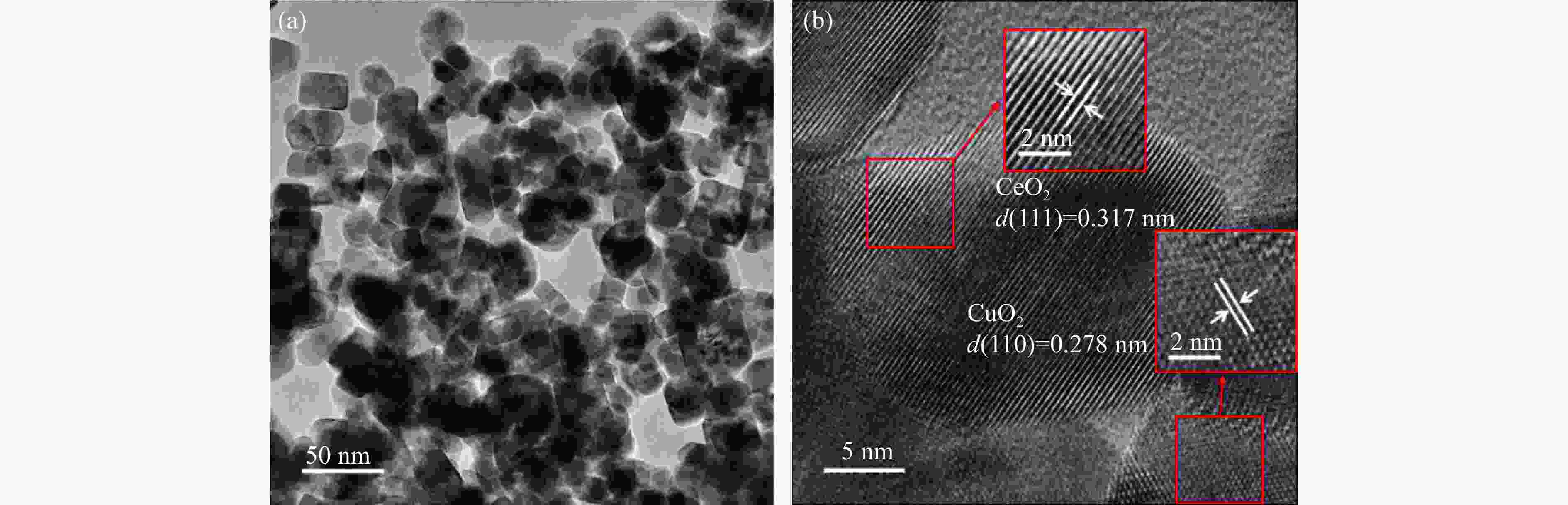
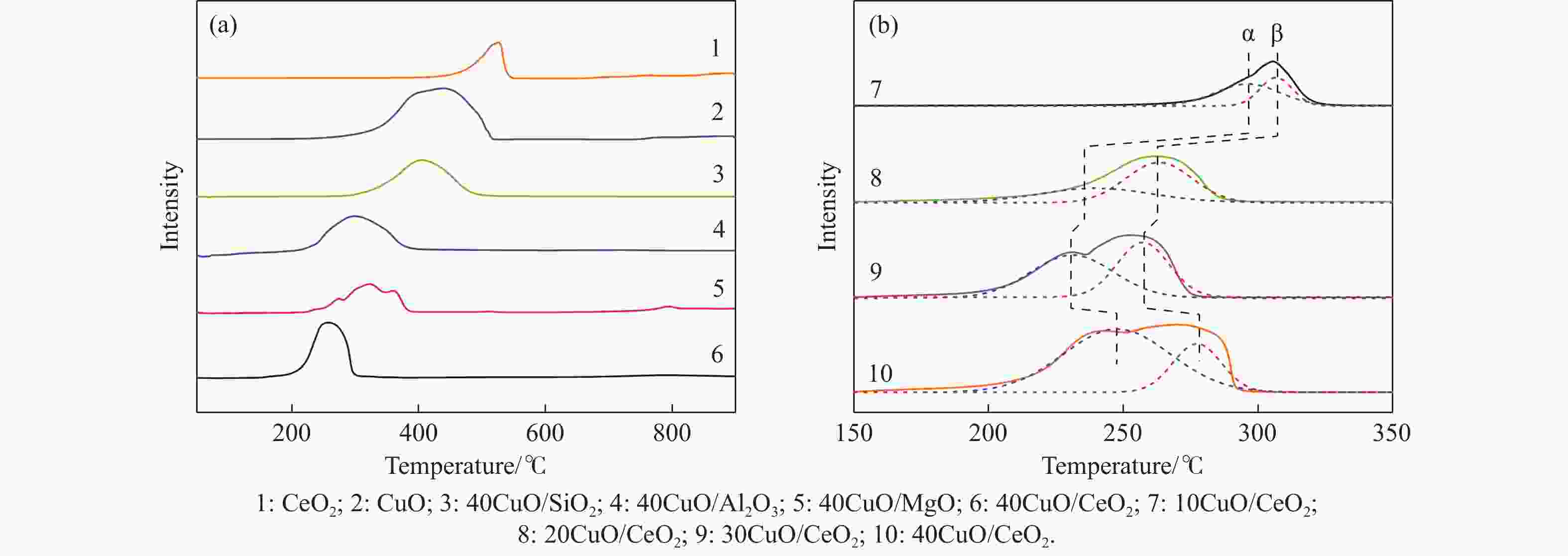
Catalyst dCuO/nm Abefore/(m2·g−1) Aafter/(m2·g−1) vbefore/(cm3·g−1) vafter/(cm3·g−1) Cu w/% CuO 40 1.929 1.897 0.005 0.005 − 40CuO/MgO 23 10.393 10.048 0.047 0.044 40.493 40CuO/Al2O3 11 21.790 21.304 0.110 0.062 41.508 40CuO/SiO2 13 18.155 18.096 0.103 0.098 40.464 40CuO/CeO2 17 15.796 8.477 0.101 0.060 39.112 30CuO/CeO2 15 21.380 17.451 0.140 0.109 30.412 20CuO/CeO2 16 19.159 15.374 0.052 0.112 20.019 10CuO/CeO2 19 18.439 14.877 0.123 0.096 9.984 表 2 xCuO/CeO2的XPS表征
Table 2 XPS results of xCuO/CeO2 catalyst
Catalyst Ce3+/(Ce3++Ce4+)/% 10CuO/CeO2 13.54 20CuO/CeO2 16.78 30CuO/CeO2 23.19 40CuO/CeO2 15.02 -
[1] 荆蕊, 王雪峰, 乔玲, 等. 电厂烟气注入采空区防灭火技术的研究进展[J]. 煤炭工程,2021,53(11):125−130.JING Rui, WANG Xuefeng , QIAO Ling, et al. Research status and future prospect of power plant flue gas sealed in goaf[J]. Coal Eng,2021,53(11):125−130. [2] 黄戈, 王继仁, 邓存宝, 等. 电厂烟道气预防遗煤自燃的合理含氧量模拟研究[J]. 中国安全科学学报,2017,27(3):42−47.HUANG Ge, WANG Jiren, DENG Cunbao, et al. Numerical simulation of reasonable oxygen content in flue gas for preventing residual coal spontaneous combustion[J]. China Saf Sci J,2017,27(3):42−47. [3] COLUSSI S, TROVARELLI A, GROPPI G, et al. The effect of CeO2 on the dynamics of Pd-PdO transformation over Pd/Al2O3 combustion catalysts[J]. Catal Commun,2007,8(8):1263−1266. doi: 10.1016/j.catcom.2006.11.020 [4] 张艳, 张永发, 张国杰. 含氧煤层气脱氧过程中硫化物的脱氧特性[J]. 煤炭转化,2009,32(1):68−71. doi: 10.3969/j.issn.1004-4248.2009.01.015ZHANG Yan, ZHANG Yongfa, ZHANG Guojie. Deoxygenation characteristic of sulfide oxidation in the process of oxygen-bearing coal mine methane[J]. Coal Convers,2009,32(1):68−71. doi: 10.3969/j.issn.1004-4248.2009.01.015 [5] RYDEN M, LYNGFELT A, MATTISSON T. Chemical-looping combustion and chemical-looping reforming in a circulating fluidized-bed reactor using Ni-based oxygen carriers[J]. Energy Fuels,2008,22(4):2585−2597. doi: 10.1021/ef800065m [6] KWAK B, PARK N, RYU H, et al. Reduction and oxidation performance evaluation of manganese-based iron, cobalt, nickel, and copper bimetallic oxide oxygen carriers for chemical-looping combustion[J]. Appl Therm Eng,2018,128:1273−1281. doi: 10.1016/j.applthermaleng.2017.09.111 [7] MATTISSON T, JERNDAL E, LINDERHOLM C, et al. Reactivity of a spray-dried NiO/NiAl2O4 oxygen carrier for chemical-looping combustion[J]. Chem Eng Sci,2011,66(20):4636−4644. doi: 10.1016/j.ces.2011.06.025 [8] KOLBITSCH P, JOHANNES B, PROLL T, et al. Comparison of two ni-based oxygen carriers for chemical looping combustion of natural gas in 140 kW continuous looping operation[J]. Ind Eng Chem Res,2009,48(11):5542−5547. doi: 10.1021/ie900123v [9] TIJANI M, AQSHA A, MAHINPEY N. Synthesis and study of metal-based oxygen carriers (Cu, Co, Fe, Ni) and their interaction with supported metal oxides (Al2O3, CeO2, TiO2, ZrO2) in a chemical looping combustion system[J]. Energy,2017,138:873−882. doi: 10.1016/j.energy.2017.07.100 [10] 杨浩, 郑华艳, 常瑜, 等. 以共沉淀法为基础的铜基催化剂制备新技术的研究进展[J]. 化工进展,2014,(2):379−386. doi: 10.3969/j.issn.1000-6613.2014.02.020YANG Hao, ZHENG Huayan, CHANG Yu, et al. Research progress of preparation of copper-based catalyst by coprecipitation[J]. Chem Ind Eng Prog,2014,(2):379−386. doi: 10.3969/j.issn.1000-6613.2014.02.020 [11] LUO M, SONG Y, LU J, et al. Identification of CuO species in high surface area CuO−CeO2 Catalysts and their catalytic activities for CO oxidation[J]. J Phys Chem C,2007,111(34):12686−12692. doi: 10.1021/jp0733217 [12] 陈国星, 李巧灵, 魏育才, 等. 镍促进CuO-CeO2催化剂的结构表征及低温CO氧化活性[J]. 催化学报,2013,34(2):322−329. doi: 10.1016/S1872-2067(11)60468-3CHEN Guoxing, LI Qiaoling, WEI Yucai, et al. Low temperature Co oxidation on Ni-promoted CuO-CeO2 catalysts[J]. Chin J Catal,2013,34(2):322−329. doi: 10.1016/S1872-2067(11)60468-3 [13] 杨志强, 毛东森, 郭强胜, 等. 制备方法对CuO/CeO2-ZrO2催化CO低温氧化活性的影响[J]. 物理化学学报,2010,26(12):3278−3284. doi: 10.3866/PKU.WHXB20101210YANG Zhiqiang, MAO Dongsen, GUO Qiangsheng, et al. Effect of preparation method on the activity of CuO/CeO2-ZrO2 catalysts for low temperature CO oxidation[J]. Acta Phys -Chim Sin,2010,26(12):3278−3284. doi: 10.3866/PKU.WHXB20101210 [14] ZOU Z, MENG M, ZHA Y. Surfactant-assisted synthesis, characterizations, and catalytic oxidation mechanisms of the mesoporous MnOx-CeO2and Pd/MnOx-CeO2catalysts used for CO and C3H8 oxidation[J]. J Phys Chem C,2010,114(1):468−477. doi: 10.1021/jp908721a [15] QI L, YU Q, DAI Y, et al. Influence of cerium precursors on the structure and reducibility of mesoporous CuO-CeO2 catalysts for CO oxidation[J]. Appl Catal B: Environ,2012,119:308−320. [16] ZENG S, LIU K, ZHANG L, et al. Deactivation analyses of CeO2/CuO catalysts in the preferential oxidation of carbon monoxide[J]. J Power Sources,2014,261:46−54. doi: 10.1016/j.jpowsour.2014.03.043 [17] ZHAO Q , LIU Q, ZHENG Y, et al. Enhanced catalytic performance for volatile organic compound oxidation over in-situ growth of MnOx on Co3O4 nanowire[J]. Chemosphere, 2020, 244 (C): 125−532. [18] WANG X, WANG S, WANG S, et al. The preparation of Au/CeO2 catalysts and their activities for low-temperature CO oxidation[J]. Catal Letters,2006,112(1/2):115−119. doi: 10.1007/s10562-006-0173-0 [19] GUO Z, SEOL M, KIM M, et al. Hollow CuO nanospheres uniformly anchored on porous Si nanowires: preparation and their potential use as electrochemical sensors[J]. Nanoscale,2012,4(23):7525. doi: 10.1039/c2nr32556j [20] REDDY B, BHARALI P, SAIKIA P, et al. Structural Characterization and catalytic activity of nanosized Ce x M1− x O2(M = Zr and Hf) mixed oxides[J]. J Phys Chem C,2008,112(31):11729−11737. doi: 10.1021/jp802674m [21] QIN J, LU J, CAO M, et al. Synthesis of porous CuO-CeO2 nanospheres with an enhanced low-temperature CO oxidation activity[J]. Nanoscale,2010,2(12):2739. doi: 10.1039/c0nr00446d [22] GHOLAMI Z, LUO G. Low-Temperature selective catalytic reduction of NO by CO in the presence of O2 over Cu: Ce catalysts supported by multiwalled carbon nanotubes[J]. Ind Eng Chem Res,2018,57(27):8871−8883. doi: 10.1021/acs.iecr.8b01343 [23] JOHNSTON-PECK A, SENANAYAKE S. , PLATA J, et al. Nature of the mixed-oxide interface in ceria-titania catalysts: Clusters, chains, and nanoparticles[J]. J Phys Chem C,2013,117(28):14463−14471. doi: 10.1021/jp3125268 [24] MUNOZ-BATISTA M, GOMEZ-CEREZO M, KUBACKA A, et al. Role of interface contact in CeO2-TiO2 photocatalytic composite materials[J]. ACS Catal,2014,4(1):63−72. doi: 10.1021/cs400878b [25] TROGADAS P, PARRONDO J, RAMANI V. CeO2 surface oxygen vacancy concentration governs in situ free radical scavenging efficacy in polymer electrolytes[J]. ACS Appl Mater Interfaces,2012,4(10):5098−5102. doi: 10.1021/am3016069 [26] KUMAR A, BABU S, KARAKOTI A, et al. Luminescence properties of europium-doped cerium oxide nanoparticles: Role of vacancy and oxidation states[J]. Langmuir,2009,25(18):10998−11007. doi: 10.1021/la901298q [27] BERA P, PRIOLKAR K, SARODE P. Structural investigation of combustion synthesized CuCeO2 catalysts by exafs and other physical techniques: Formation of a Ce1− x Cu x O2− δ solid solution[J]. Chem Mater,2002,14(8):3591−3601. doi: 10.1021/cm0201706 [28] WAN H, LI D, DAI Y, et al. Catalytic behaviors of CuO supported on Mn2O3 modified γ-Al2O3 for NO reduction by CO[J]. J Mol Catal A Chem,2010,332(1/2):32−44. doi: 10.1016/j.molcata.2010.08.016 [29] DI MONTE R, KASPAR J, FORNASIERO P, et al. NO reduction by CO over Pd/Ce0.6Zr0.4O2 Al2O3 catalysts: In situ FT-IR studies of NO and CO adsorption[J]. Inorganica Chim Acta,2002,334:318−326. doi: 10.1016/S0020-1693(02)00800-9 [30] QI G, YANG R. Characterization and FT-IR studies of MnOx−CeO2 catalyst for low-temperature selective catalytic reduction[J]. J Phys Chem B,2004,40(18):15738−15747. -





 下载:
下载: