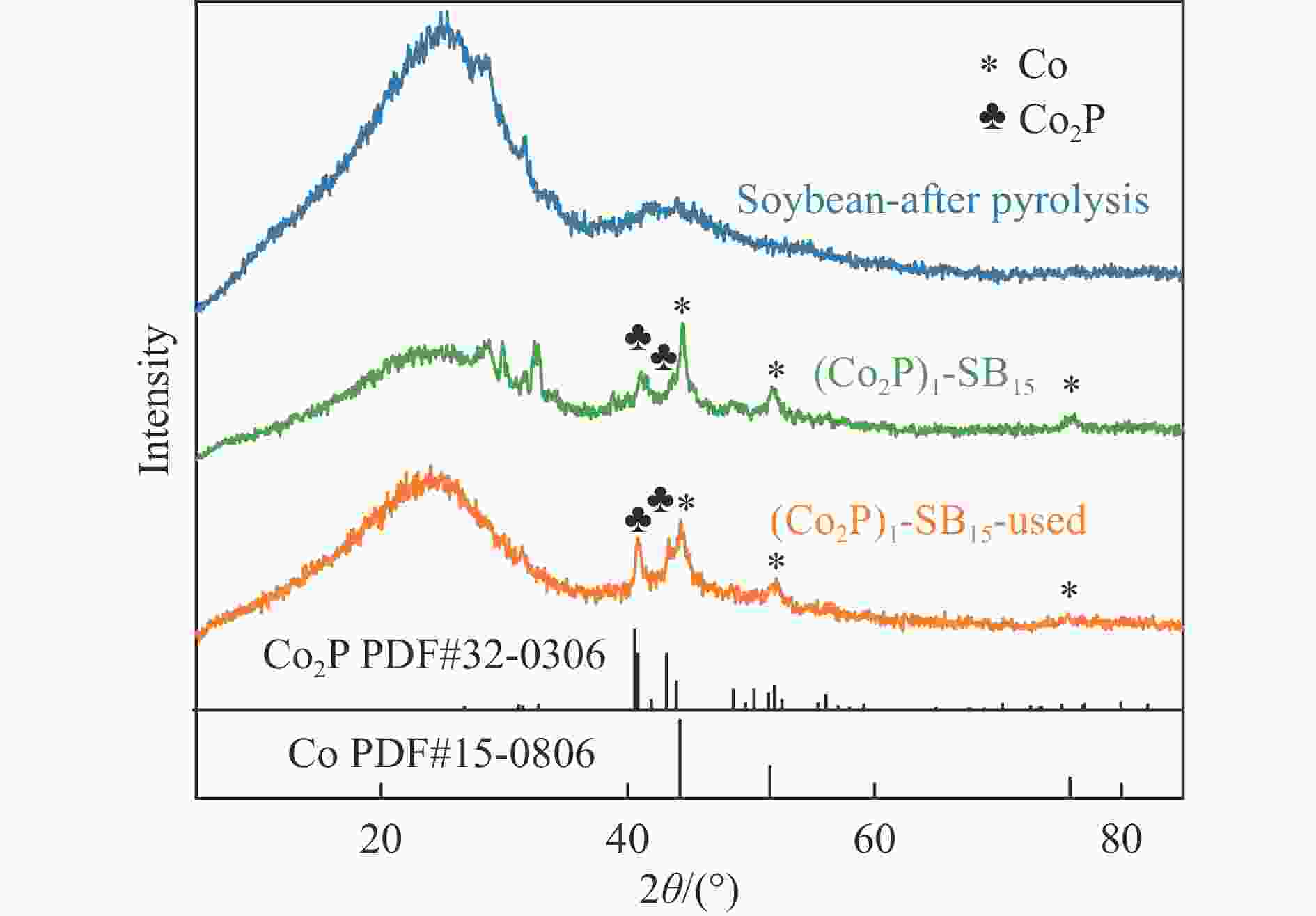
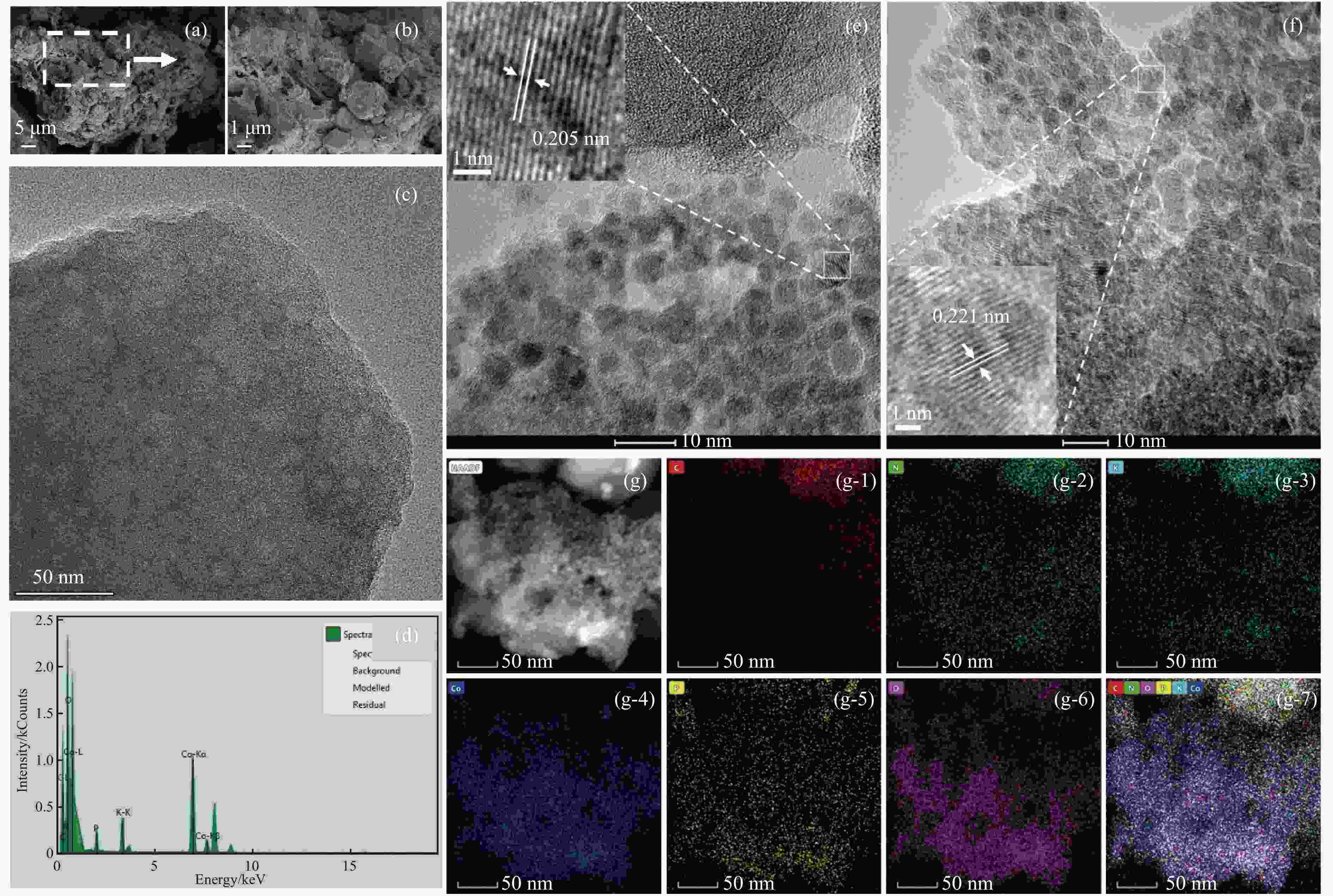
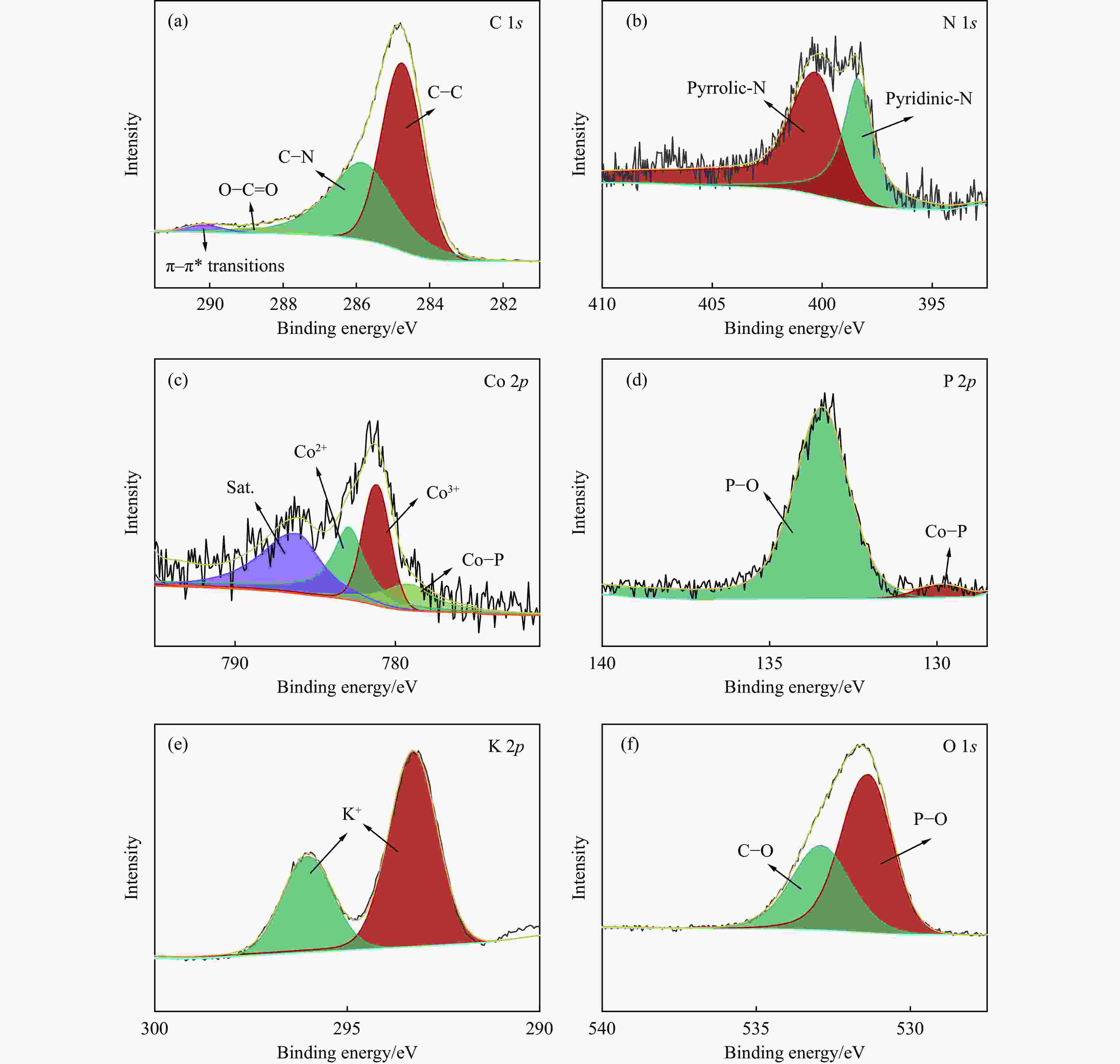
Multi-site Co2P catalyst derived from soybean biomass for dehydrogenation of formic acid
-
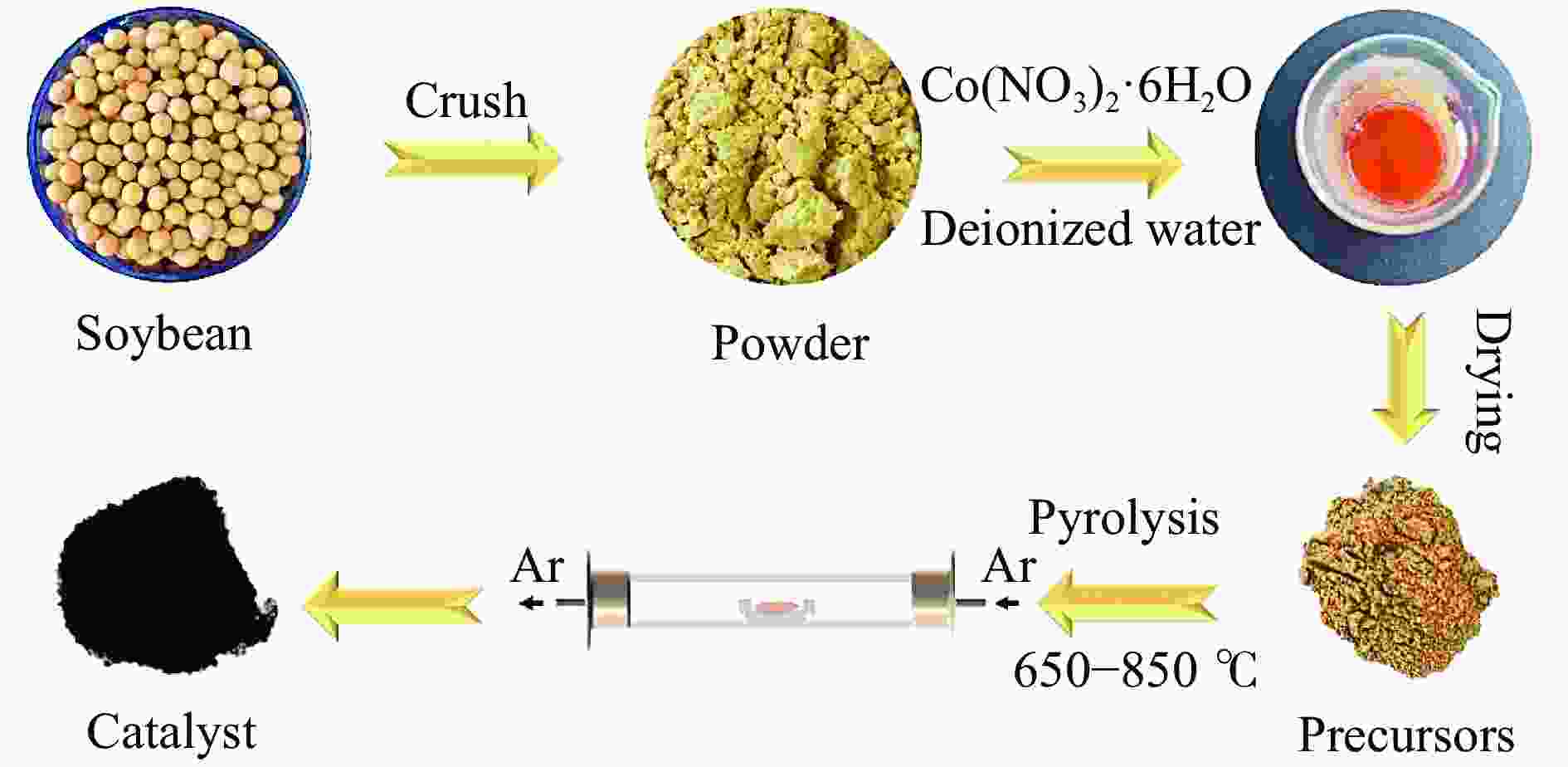
摘要: 本研究报道了一种通过大豆和钴盐热解制备的甲酸脱氢用Co2P催化剂,制备过程简单且环境友好。催化过程中,催化剂上的含K固体碱可作为路易斯酸性位点促进HCOO−中间体的吸附,而自掺杂的N可作为碱性位点促进H+的吸附。大豆生物质中的P可与钴盐结合并热解成Co2P,该位点可裂解HCOO−的H−C键。催化剂制备过程中当Co(NO3)2·6H2O/大豆的质量比为1∶15时,所得Co2P催化剂对甲酸脱氢反应的产气率可达237.47 mL/(g·h),并展现出良好的稳定性。本研究结果可为甲酸选择性产氢用非贵金属非均相催化剂的开发提供一定的借鉴基础。Abstract: Formic acid (FA) is a sustainable liquid organic hydrogen carrier and the catalyst for hydrogen production from FA has received significant attention. However, the development of efficient non-noble metal catalysts still remains challenges. In this work, we provide a technologically rather simple and environmental-friendly strategy to synthesize Co2P catalyst for dehydrogenation of FA by pyrolyzing soybean powder and cobalt salt. The K-containing solid bases in catalyst could act as Lewis acid sites for the HCOO− intermediate adsorption while the self-doped N could act as Lewis base sites to enhance the H+ adsorption. The P contained in soybean could combine with Co to form Co2P for H−C bond cleavage of HCOO−. At a Co(NO3)2·6H2O/soybean mass ratio of 1∶15, the as prepared Co2P catalyst demonstrated a gas production rate of 237.47 mL/(g·h) and a good stability. This study provides a novel strategy to develop non-noble metal heterogeneous catalysts for FA dehydrogenation.
-
Key words:
- formic acid /
- heterogeneous catalyst /
- biomass /
- dehydrogenation
-
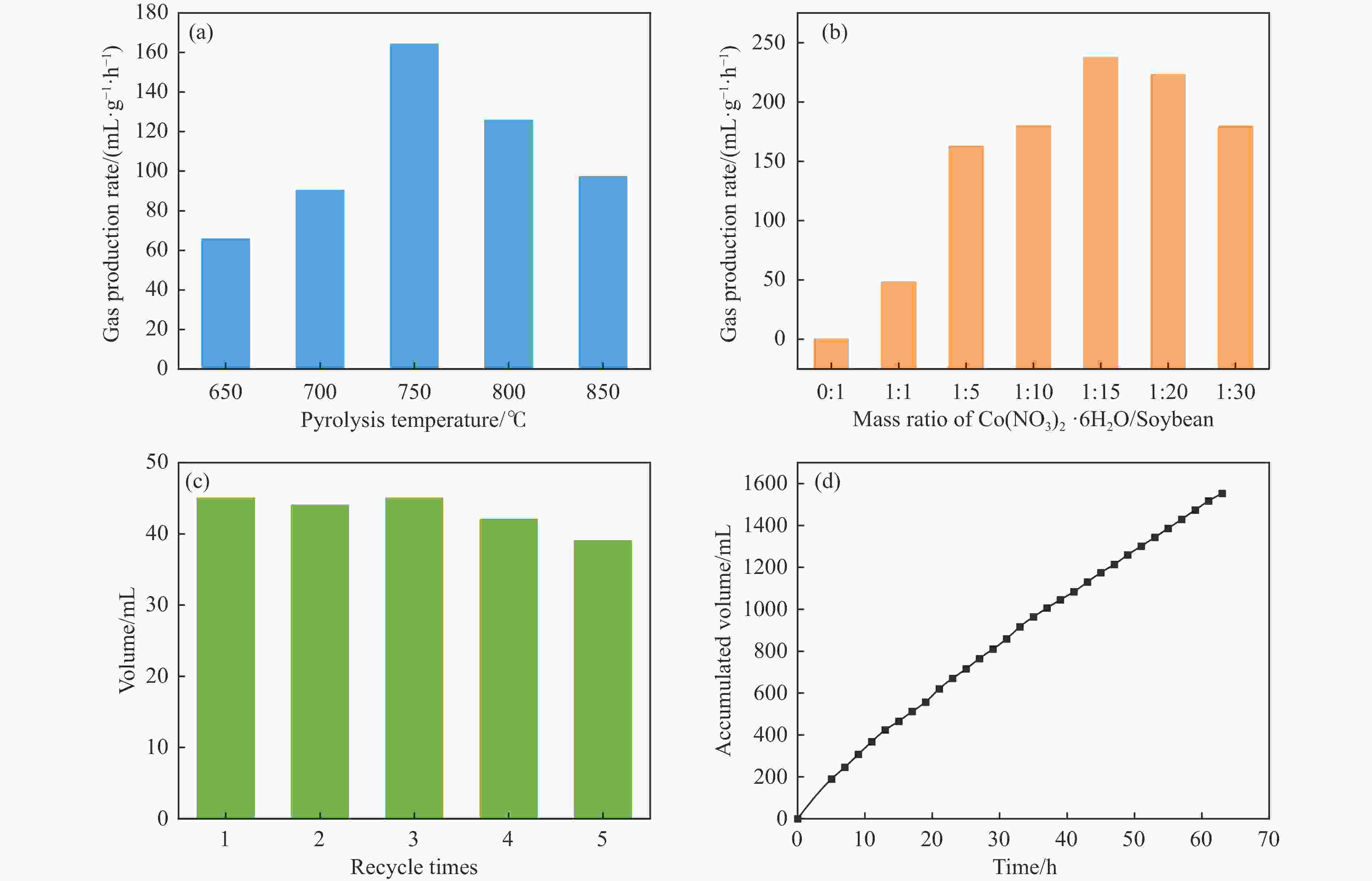
Figure 6 Gas production rates of (a) (Co2P)1-SB10 synthesized at different pyrolysis temperatures and (b) (Co2P)x-SBy pyrolyzed at 750 °C, (c) volume of gas produced during five cycles and (d) accumulated gas volume in a 63 h-continuous test over (Co2P)1-SB15
(Reaction conditions: 20 mL of 10.39 mol/L FA, 98 °C)
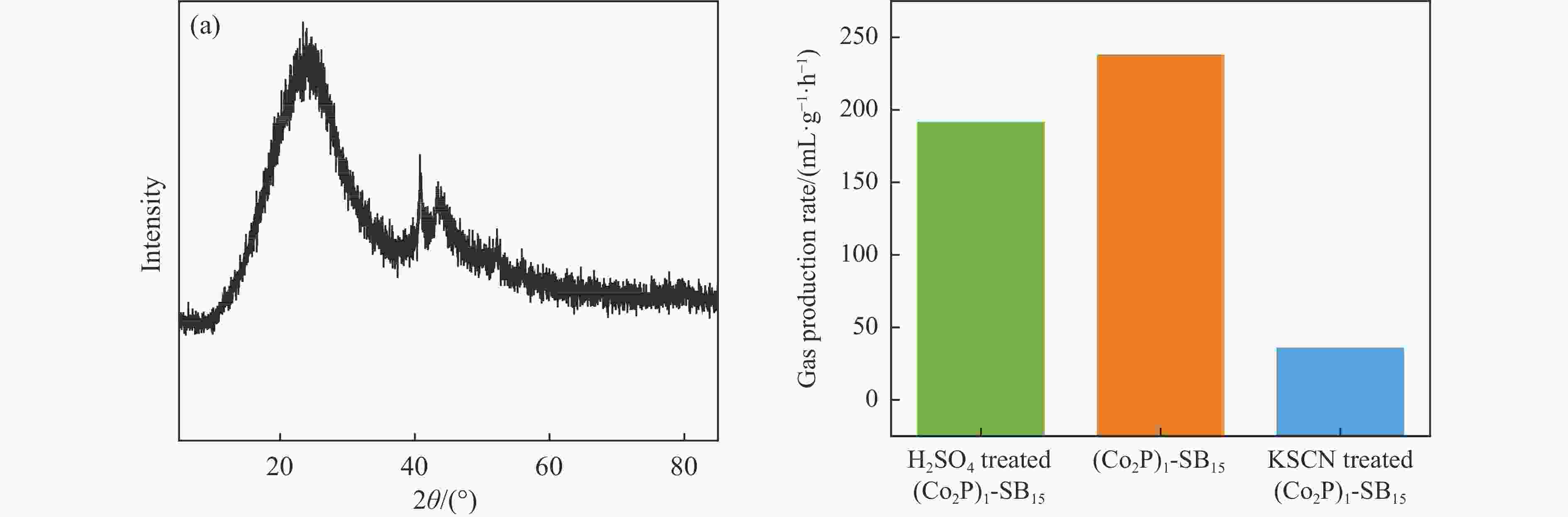
Table 1 Co and P contents of catalyst and corresponding FA solution in 10.39 mol/L FA, 98 °C
Sample Co P Unit (Co2P)1-SB15 1.04 0.77 % (Co2P)1-SB15-1 h 0.86 0.28 % (Co2P)1-SB15-63 h 0.81 0.27 % FA solution-63 h 95.3 139.3 mg/L Table 2 K contents of (Co2P)1-SB15 collected after 1 h and 63 h reaction in 10.39 M FA, 98 °C
Sample K Unit (Co2P)1-SB15-1 h 0.59 wt.% (Co2P)1-SB15-63 h 0.13 wt.% -
[1] GRASEMANN M, LAURENCZY G. Formic acid as a hydrogen source - recent developments and future trends[J]. Energy Environ Sci,2012,5(8):8171−8181. doi: 10.1039/c2ee21928j [2] PRETI D, RESTA C, SQUARCIALUPI S, et al. Carbon dioxide hydrogenation to formic acid by using a heterogeneous gold catalyst[J]. Angew Chemi,2011,123(52):12759−12762. doi: 10.1002/ange.201105481 [3] CHEN X, LIU Y, WU J. Sustainable production of formic acid from biomass and carbon dioxide[J]. Mol Catal,2020,483:110716. doi: 10.1016/j.mcat.2019.110716 [4] PREUSTER P, PAPP C, WASSERSCHEID P. Liquid organic hydrogen carriers (LOHCs): toward a hydrogen-free hydrogen economy[J]. Acc Chem Res,2017,50(1):74−85. doi: 10.1021/acs.accounts.6b00474 [5] MARS P, SCHOLTEN J J F, ZWIETERING P. The catalytic decomposition of formic acid[J]. Adv Catal,1963,14:35−113. [6] WANG Z L, YAN J M, WANG H L, et al. Q. Pd/C synthesized with citric acid: An efficient catalyst for hydrogen generation from formic acid/sodium formate[J]. Sci Rep,2012,2:1−6. [7] ZHANG Z, LUO Y, LIU S, et al. A PdAg-CeO2 Nanocomposite anchored on mesoporous carbon: A highly efficient catalyst for hydrogen production from formic acid at room temperature[J]. J Mater Chem A: Mater,2019,7(37):21438−21446. doi: 10.1039/C9TA06987A [8] LUO Y, YANG Q, NIE W, et al. Anchoring IrPdAu nanoparticles on NH2-SBA-15 for fast hydrogen production from formic acid at room temperature[J]. ACS Appl Mater Interfaces,2020,12(7):8082−8090. doi: 10.1021/acsami.9b16981 [9] BULLOCK R M, CHE J G, GAGLIARDI L, et al. Using nature’s blueprint to expand catalysis with earth-abundant metals[J]. Science,2020,369(6505):eabc3183. doi: 10.1126/science.abc3183 [10] DUAN J, XIANG Z, ZHANG H, et al. Pd-Co2P nanoparticles supported on N-doped biomass-based carbon microsheet with excellent catalytic performance for hydrogen evolution from formic acid[J]. Appl Surf Sci,2020,530:147191. doi: 10.1016/j.apsusc.2020.147191 [11] CAO S, CHEN Y, WANG H, et al. Ultrasmall CoP nanoparticles as efficient cocatalysts for photocatalytic formic acid dehydrogenation[J]. Joule,2018,2(3):549−557. doi: 10.1016/j.joule.2018.01.007 [12] KOÓS á, SOLYMOSI F. Production of CO-free H2 by formic acid decomposition over Mo2C/carbon catalysts[J]. Catal Lett,2010,138(1/2):23−27. doi: 10.1007/s10562-010-0375-3 [13] WANG B, YANG S, YU Z, et al. Performance modulation strategies of heterogeneous catalysts for formic acid dehydrogenation: A review[J]. Mater Today Commun,2022,31:103617. doi: 10.1016/j.mtcomm.2022.103617 [14] JIA L, BULUSHEV D A, PODYACHEVA O Y, et al. Pt nanoclusters stabilized by N-doped carbon nanofibers for hydrogen production from formic acid[J]. J Catal,2013,307:94−102. doi: 10.1016/j.jcat.2013.07.008 [15] YU Z, AN X, KURNIA I, et al. Full spectrum decomposition of formic acid over γ-Mo2N-based catalysts: from dehydration to dehydrogenation[J]. ACS Catal,2020,10(9):5353−5361. doi: 10.1021/acscatal.0c00752 [16] YU Z, YANG Y, YANG S, et al. Selective dehydrogenation of aqueous formic acid over multifunctional γ-Mo2N catalysts at a temperature lower than 100 ℃[J]. Appl Catal B: Environ,2022,313:121445. doi: 10.1016/j.apcatb.2022.121445 [17] BULUSHEV D A, JIA L, BELOSHAPKIN S, et al. Improved hydrogen production from formic acid on a Pd/C catalyst doped by potassium[J]. Chem Commun (Camb),2012,48(35):4184−4186. doi: 10.1039/c2cc31027a [18] JIA L, BULUSHEV D A, BELOSHAPKIN S, et al. Hydrogen production from formic acid vapour over a Pd/C catalyst promoted by potassium salts: Evidence for participation of buffer-like solution in the pores of the catalyst[J]. Appl Catal B: Environ,2014,160−161:35−43. [19] WANG G, PENG H, QIAO X, et al. Biomass-derived porous heteroatom-doped carbon spheres as a high-performance catalyst for the oxygen reduction reaction[J]. Int J Hydrogen Energy,2016,41(32):14101−14110. doi: 10.1016/j.ijhydene.2016.06.023 [20] JI T, CHEN L, MU L, et al. Green processing of plant biomass into mesoporous carbon as catalyst support[J]. Chem Eng J,2016,295:301−308. doi: 10.1016/j.cej.2016.03.033 [21] MATSAGAR B M, YANG R X, DUTTA S, et al. Recent progress in the development of biomass-derived nitrogen-doped porous carbon[J]. J Mater Chem A: Mater,2021,9(7):3703−3728. doi: 10.1039/D0TA09706C [22] LIU F, PENG H, QIAO X, et al. High-performance doped carbon electrocatalyst derived from soybean biomass and promoted by zinc chloride[J]. Int J Hydrogen Energy,2014,39(19):10128−10134. doi: 10.1016/j.ijhydene.2014.04.176 [23] LIU X, ZHOU Y, ZHOU W, et al. Biomass-derived nitrogen self-doped porous carbon as effective metal-free catalysts for oxygen reduction reaction[J]. Nanoscale,2015,7(14):6136−6142. doi: 10.1039/C5NR00013K [24] BEDA A, LE MEINS J M, TABERNA P L, et al. Impact of biomass inorganic impurities on hard carbon properties and performance in Na-Ion batteries[J]. Sustainable Mater Technol,2020,26:e00227. doi: 10.1016/j.susmat.2020.e00227 [25] ZHU Y, XU G, ZHANG X, et al. Hierarchical porous carbon derived from soybean hulls as a cathode matrix for lithium-sulfur batteries[J]. J Alloys Compd,2017,695:2246−2252. doi: 10.1016/j.jallcom.2016.11.075 [26] PU Z, ZHANG C, AMIINU I S, et al. General strategy for the synthesis of transition-metal phosphide/N-doped carbon frameworks for hydrogen and oxygen evolution[J]. ACS Appl Mater Interfaces,2017,9(19):16187−16193. doi: 10.1021/acsami.7b02069 [27] XIE H, ZENG D, DU B, et al. Co2P encapsulated in N, O co-doped carbons as bifunctional electrocatalysts for oxygen evolution and reduction reactions[J]. Int J Hydrogen Energy,2023,48(25):9273−9284. doi: 10.1016/j.ijhydene.2022.11.336 [28] YANG J, GUO D, ZHAO S, et al. Cobalt phosphides nanocrystals encapsulated by P-doped carbon and married with P-doped graphene for overall water splitting[J]. Small,2019,15(10):1804546. doi: 10.1002/smll.201804546 [29] XU Y, MO Y, TIAN J, et al. The synergistic effect of graphitic N and pyrrolic N for the enhanced photocatalytic performance of nitrogen-doped graphene/TiO2 nanocomposites[J]. Appl Catal B: Environ,2016,181:810−817. doi: 10.1016/j.apcatb.2015.08.049 [30] HU L, HU Y, LIU R, et al. Co-based MOF-derived Co/CoN/Co2P ternary composite embedded in N- and P-doped carbon as bifunctional nanocatalysts for efficient overall water splitting[J]. Int J Hydrogen Energy,2019,44(23):11402−11410. doi: 10.1016/j.ijhydene.2019.03.157 [31] JIANG S, WU M, XIAO T, et al. Tailoring the activity of electrocatalytic methanol oxidation on cobalt hydroxide by the incorporation of catalytically inactive zinc ions[J]. ACS Appl Mater Interfaces,2023,15(48):55870−55876. doi: 10.1021/acsami.3c13624 [32] HUANG T, XU G, DING H, et al. Ultrafine cobalt molybdenum phosphide nanoparticles embedded in crosslinked nitrogen-doped carbon nanofiber as efficient bifunctional catalyst for overall water splitting[J]. J Colloid Interface Sci,2022,625:956−964. doi: 10.1016/j.jcis.2022.06.093 [33] LONI E, SIADATI M H, SHOKUHFAR A. Mesoporous cobalt-cobalt phosphide electrocatalyst for water splitting[J]. Mater Today Energy,2020,16:100398. doi: 10.1016/j.mtener.2020.100398 [34] MIYAKOSHI A, UENO A, ICHIKAWA M. XPS and TPD characterization of manganese-substituted iron-potassium oxide catalysts which are selective for dehydrogenation of ethylbenzene into styrene[J]. Appl Catal A: Gen,2001,219(1/2):249−258. doi: 10.1016/S0926-860X(01)00697-4 [35] ZHAO S, LI C, HUANG H, et al. Phosphate functionalized activated carbon as an efficient metal-free electrocatalyst for the oxygen reduction reaction[J]. New J Chem,2015,39(11):8881−8886. doi: 10.1039/C5NJ01270H [36] YANG N, ZHAO Y, ZHANG H, et al. Sintering activated atomic palladium catalysts with high-temperature tolerance of ~1, 000°C[J]. Cell Rep Phys Sci,2021,2(1):100287. doi: 10.1016/j.xcrp.2020.100287 [37] LIU, Z, YANG S, YANG Y, et al. Efficient hydrogen production from high-concentration aqueous formic acid over bio-based γ-Mo2N catalysts[J]. Carbon Res Convers, 2023: 100209. [38] LIU Q, YANG X, HUANG Y, et al. A schiff base modified gold catalyst for green and efficient H2 production from formic acid[J]. Energy Environ Sci,2015,8(11):3204−3207. doi: 10.1039/C5EE02506K [39] LI M, CHEN S, JIANG Q, et al. Origin of the activity of Co-N-C catalysts for chemoselective hydrogenation of nitroarenes[J]. ACS Catal,2021,11(5):3026−3039. doi: 10.1021/acscatal.0c05479 [40] XU D, ZHAO H, DONG Z, et al. Catalytically active Co-Nx species stabilized on nitrogen-doped porous carbon for efficient hydrogenation and dehydrogenation of N-heteroarenes[J]. ChemCatChem,2020,12(17):4406−4415. doi: 10.1002/cctc.202000826 [41] TANG C, SURKUS A E, CHEN F, et al. A stable nanocobalt catalyst with highly dispersed CoNx active sites for the selective dehydrogenation of formic acid[J]. Angew Chem Int Ed Eng,2017,56(52):16616−16620. [42] LIU M, XU Y, MENG Y, et al. Heterogeneous catalysis for carbon dioxide mediated hydrogen storage technology based on formic acid[J]. Adv Energy Mater,2022,12(31):2200817. [43] JIA L, BULUSHEV D A, ROSS J R H. Formic acid decomposition over palladium based catalysts doped by potassium carbonate[J]. Catal Today,2016,259:453−459. doi: 10.1016/j.cattod.2015.04.008 [44] BULUSHEV D A, ZACHARSKA M, GUO, Y, et al. CO-Free hydrogen production from decomposition of formic acid over Au/Al2O3 catalysts doped with potassium ions[J]. Catal Commun,2017,92:86−89. doi: 10.1016/j.catcom.2017.01.011 [45] ZHAO H B, XU W T, SONG Q, et al. Effect of steam and SiO2 on the release and transformation of K2CO3 and KCl during biomass thermal conversion[J]. Energy Fuels,2018,32(9):9633−9639. doi: 10.1021/acs.energyfuels.8b02269 -




 下载:
下载: