Effects of preparation methods on the performance of InZr/SAPO-34 composite catalysts for CO2 hydrogenation to light olefins
-
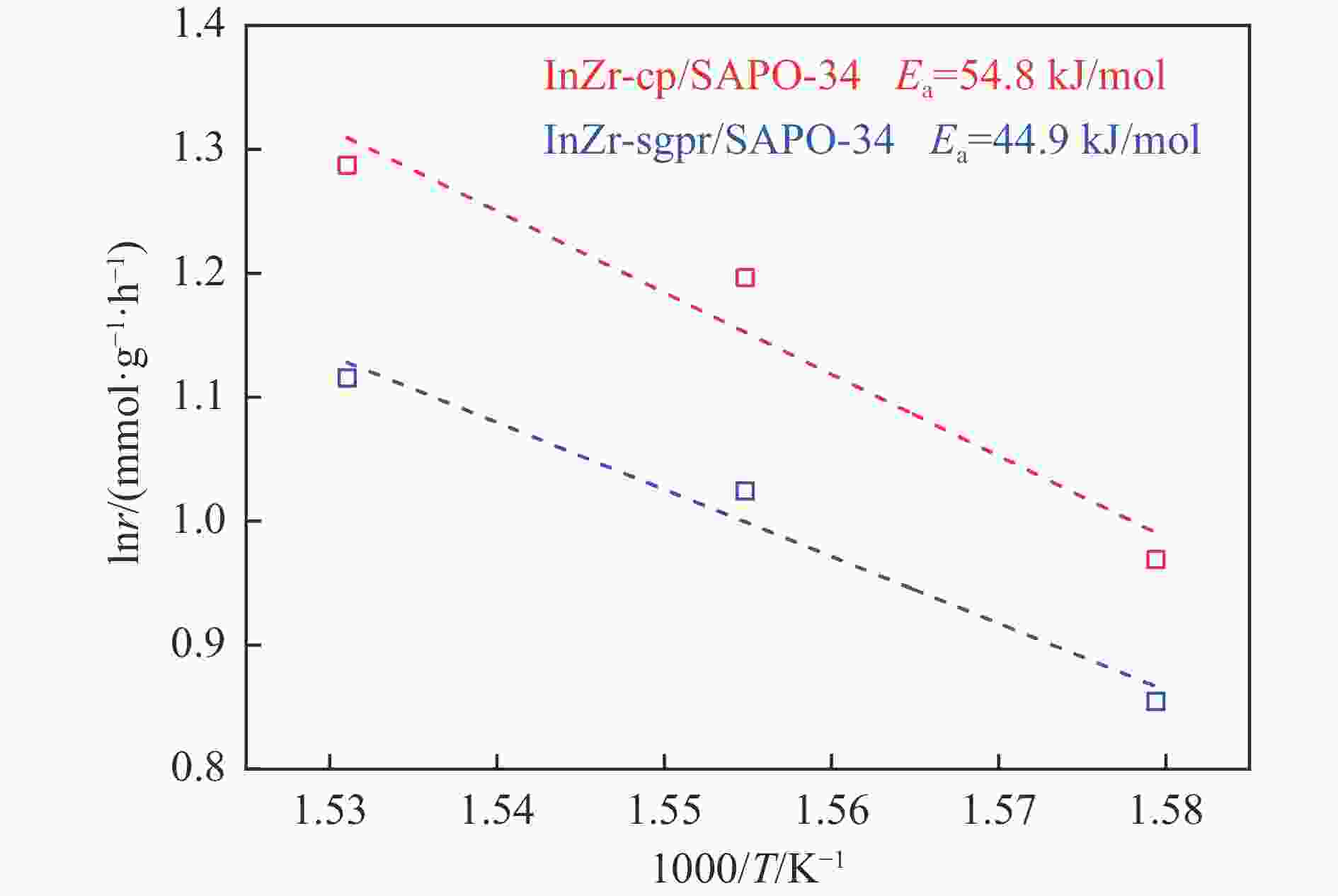
摘要: 低碳烯烃是重要的化工原料,乙烯更是作为衡量一个国家石油化工发展水平的重要标志,使用CO2催化加氢制备低碳烯烃是实现CO2高值化利用的重要途径。在众多催化剂中,InZr复合氧化物/SAPO-34催化剂因其高低碳烯烃选择性和高稳定性展现出潜在的研究和应用前景。本工作研究了不同制备方法对InZr复合氧化物/SAPO-34催化剂CO2加氢制备低碳烯烃的影响,结果表明,共沉淀法制备的催化剂具有最高的催化活性,溶胶凝胶-沉积法制备的催化剂具有最高的低碳烯烃选择性,并结合多种表征手段揭示了InZr复合氧化物/SAPO-34催化剂内在构效关系。Abstract: Light olefins are of great importance as chemical raw materials, and ethylene is a crucial symbol to evaluate the development level of petrochemical industry. Catalytic hydrogenation of CO2 to light olefins is one of the most vital approaches to utilize CO2 with high added valued. InZr/SAPO-34 composite catalyst shows prominent potential in research and application because of their high light olefins selectivity and high stability in CO2 hydrogenation. In this study, the effects of different preparation methods of InZr/SAPO-34 composite catalyst for CO2 hydrogenation to light olefins were studied in depth. The catalyst prepared by co-precipitation method showed the highest catalytic activity, and the catalyst prepared by sol-gel-precipitation method showed the highest light olefins selectivity. The structure-activity relationship of InZr/SAPO-34 catalysts were revealed by various characterization methods.
-
Key words:
- CO2 hydrogenation /
- light olefins /
- InZr composite oxide /
- SAPO-34 /
- structure-activity relationship
-
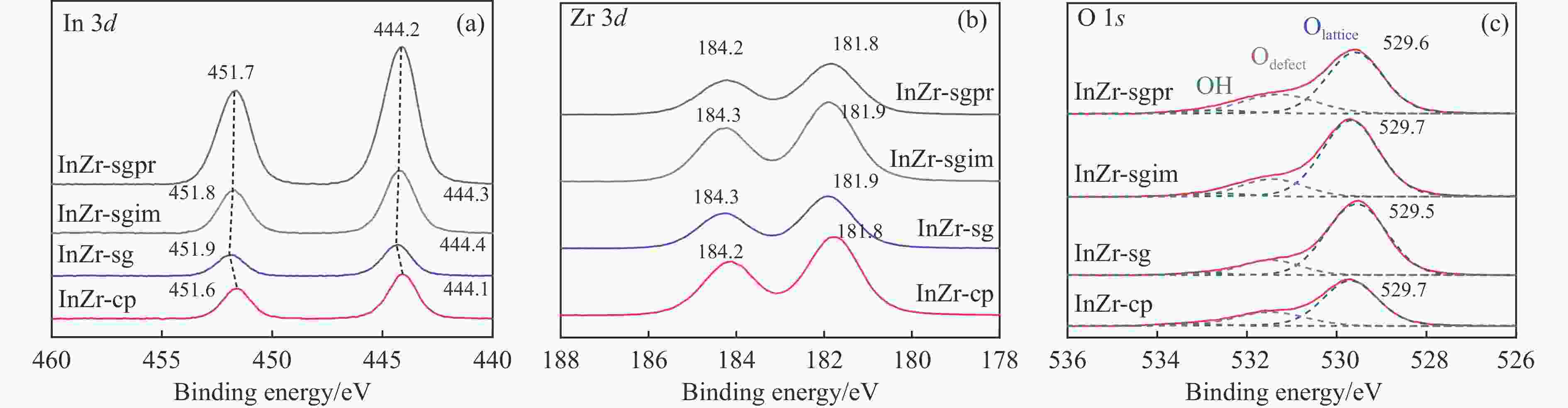
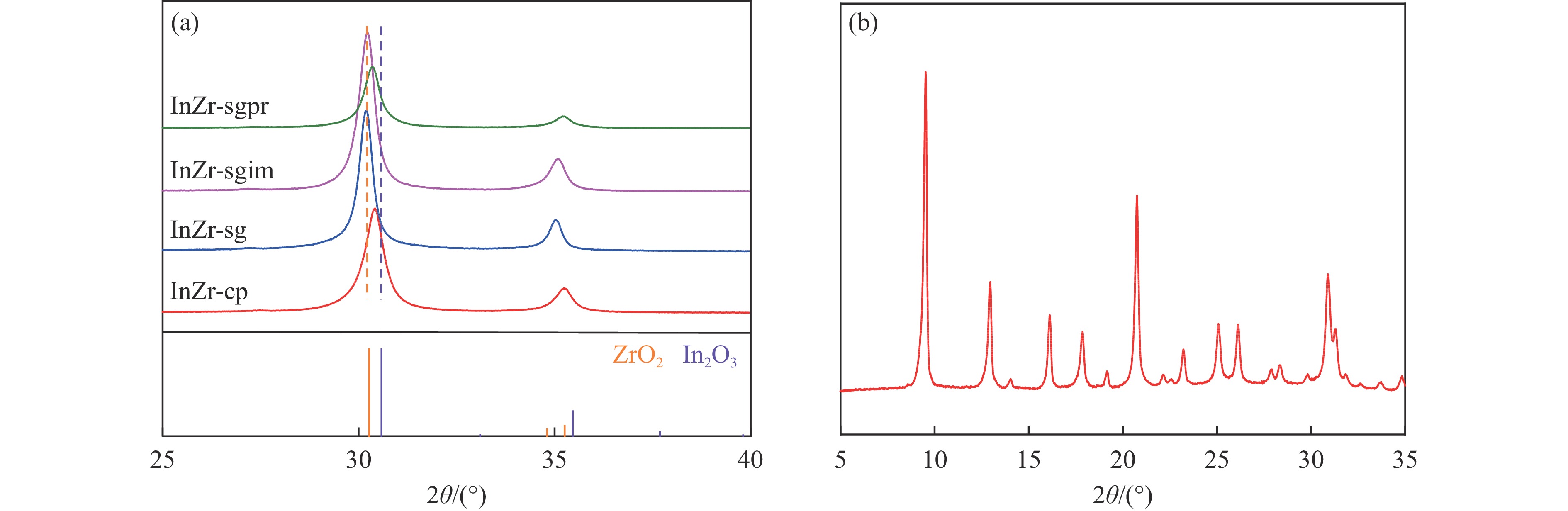
表 1 系列InZr复合氧化物表面金属元素和氧物种含量
Table 1 Surface content of metal elements and oxygen species on InZr composite oxides
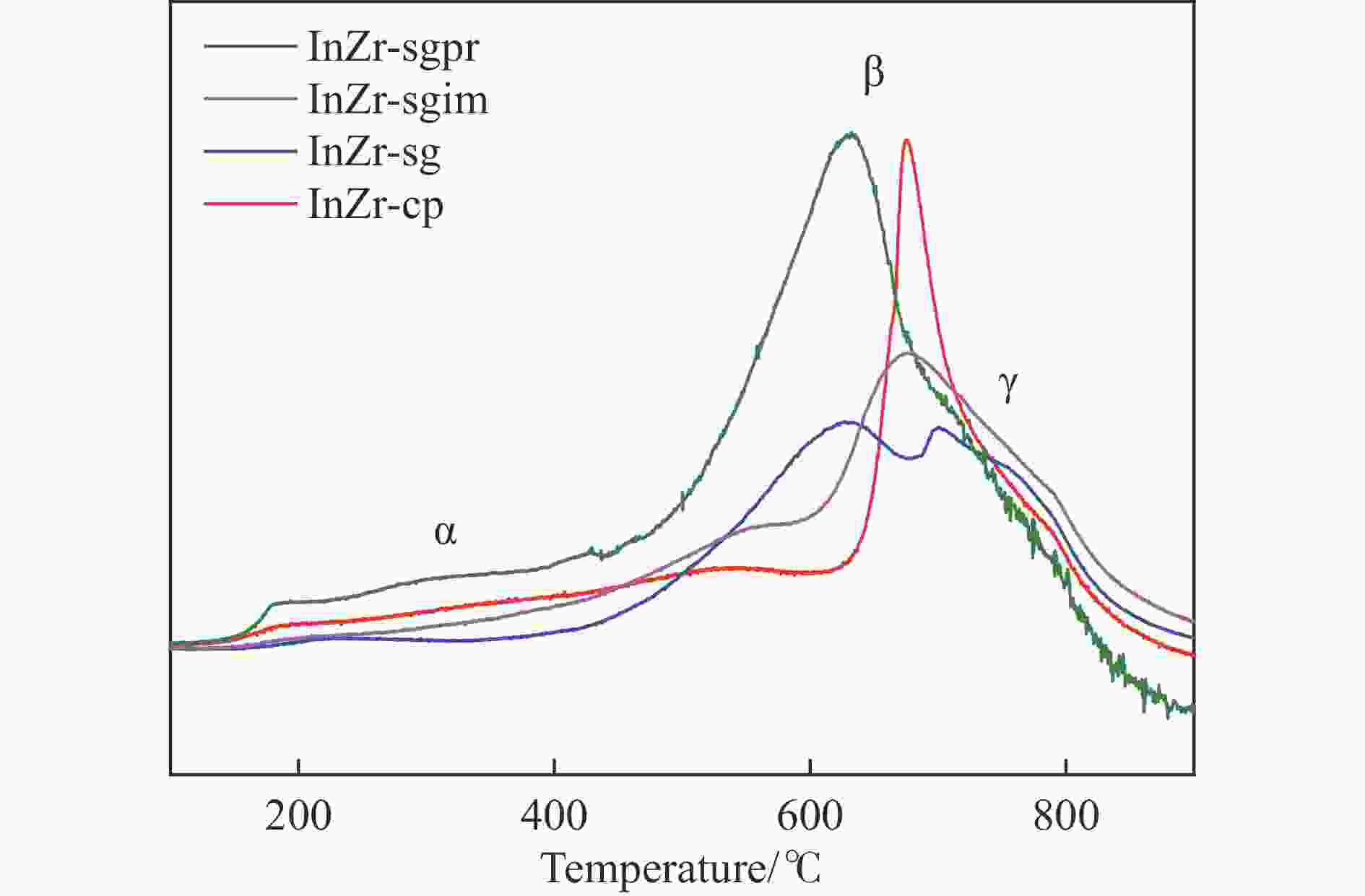
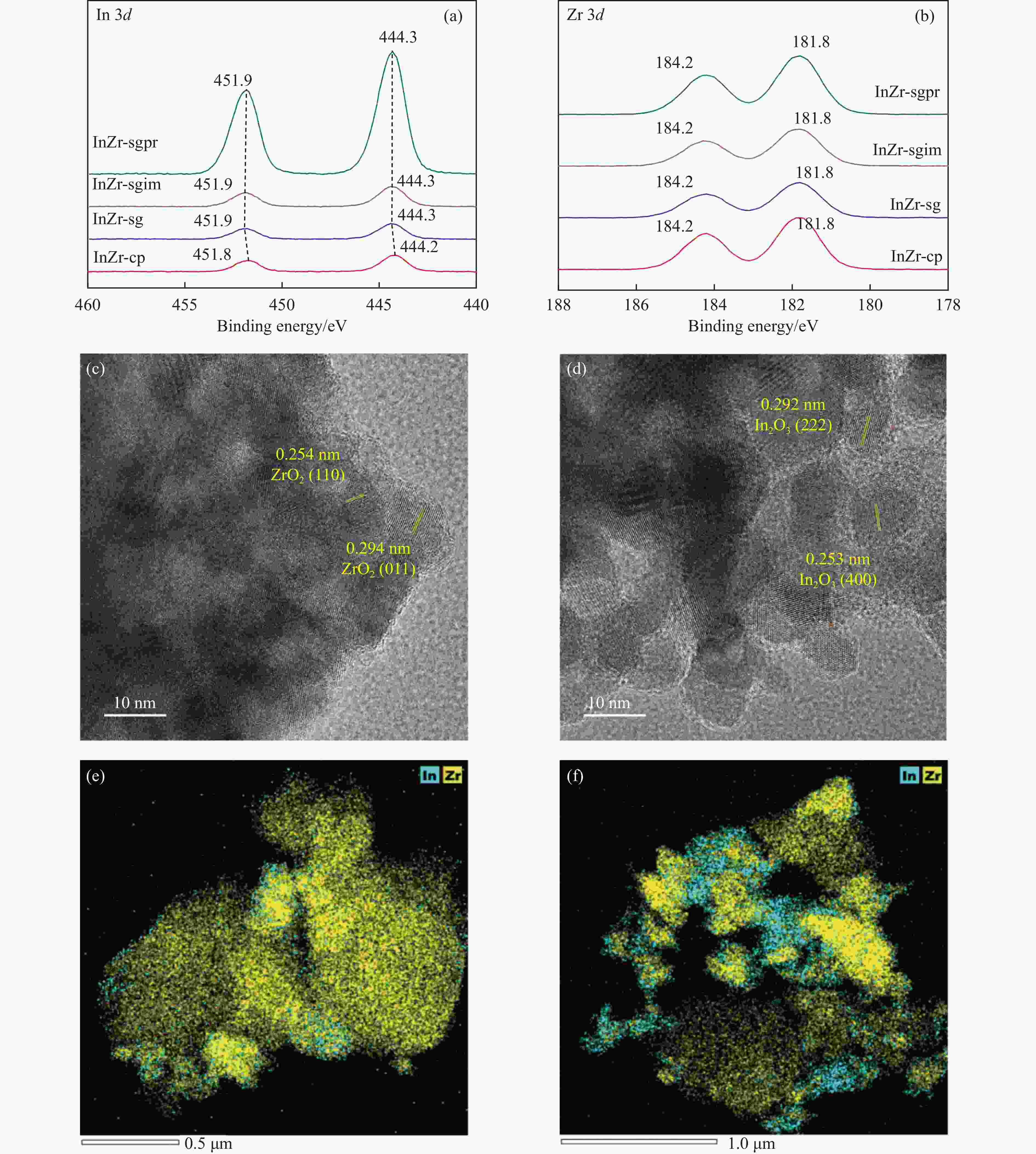
Catalyst Surface element content/% In/Zr ratio O species content/% In Zr Olattice Odefect OH InZr-cp/SAPO-34 3.83 22.25 0.17 70.7 26.2 3.1 InZr-sg/SAPO-34 3.38 17.94 0.19 81.5 16.7 1.8 InZr-sgim/SAPO-34 5.3 21.13 0.25 77.2 19.1 3.7 InZr-sgpr/SAPO-34 12.52 13.93 0.90 68.1 27.5 4.4 表 2 InZr复合氧化物/SAPO-34催化剂CO2加氢制备低碳烯烃性能
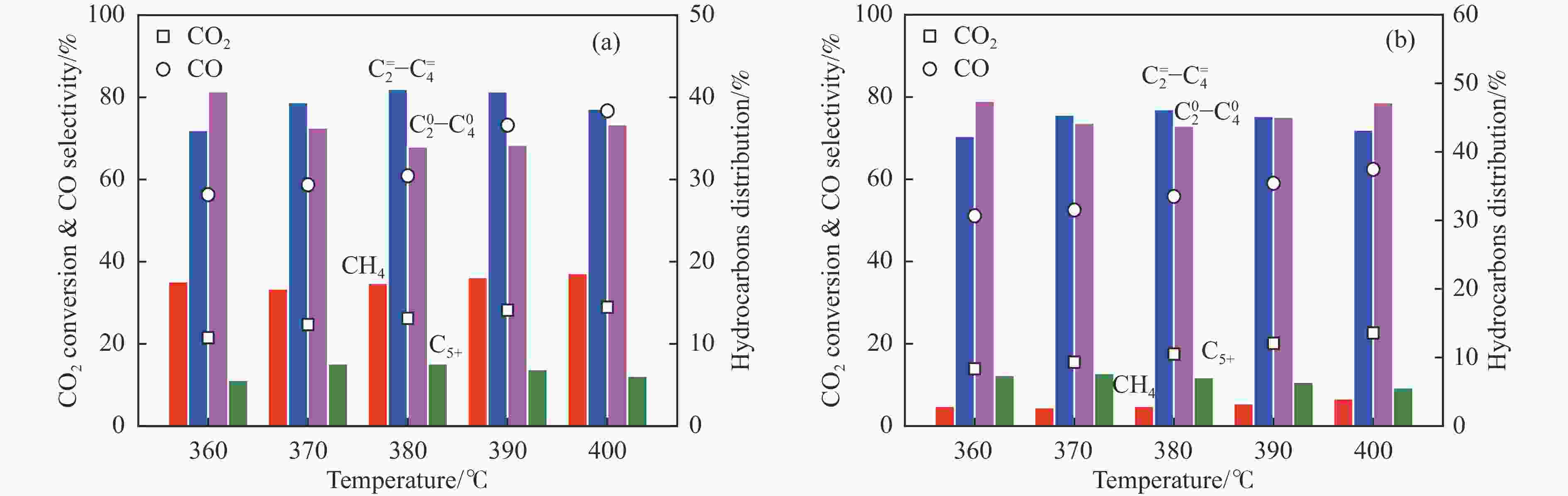
Table 2 Catalytic performance of InZr/SAPO-34 catalysts for CO2 hydrogenation to light olefins
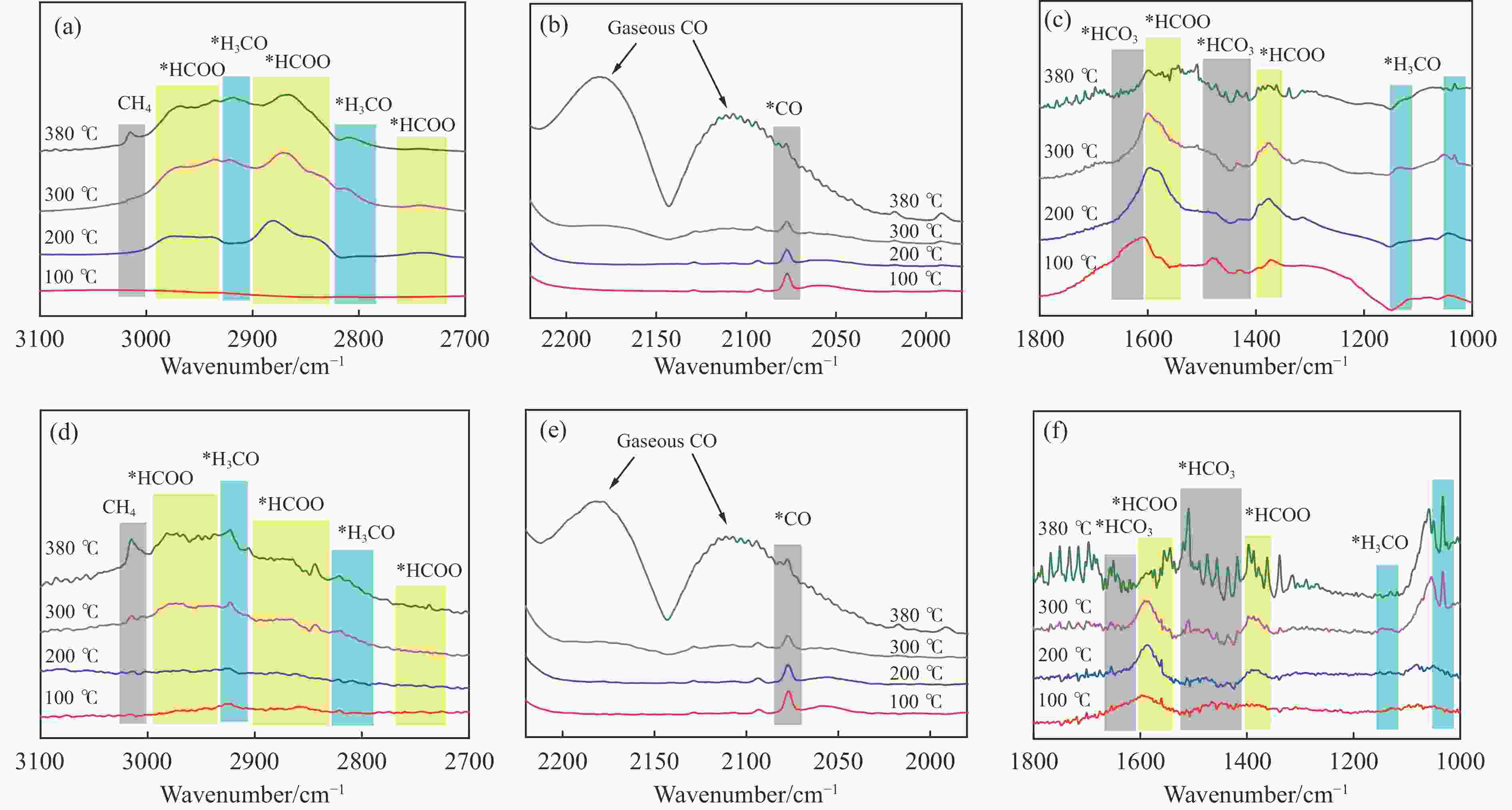
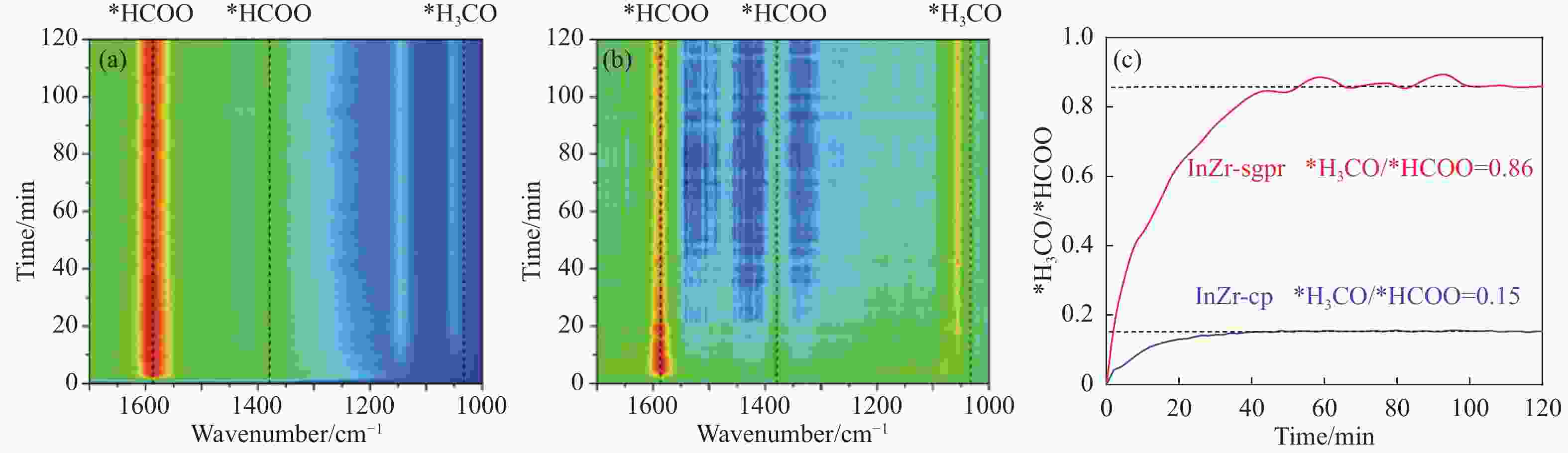
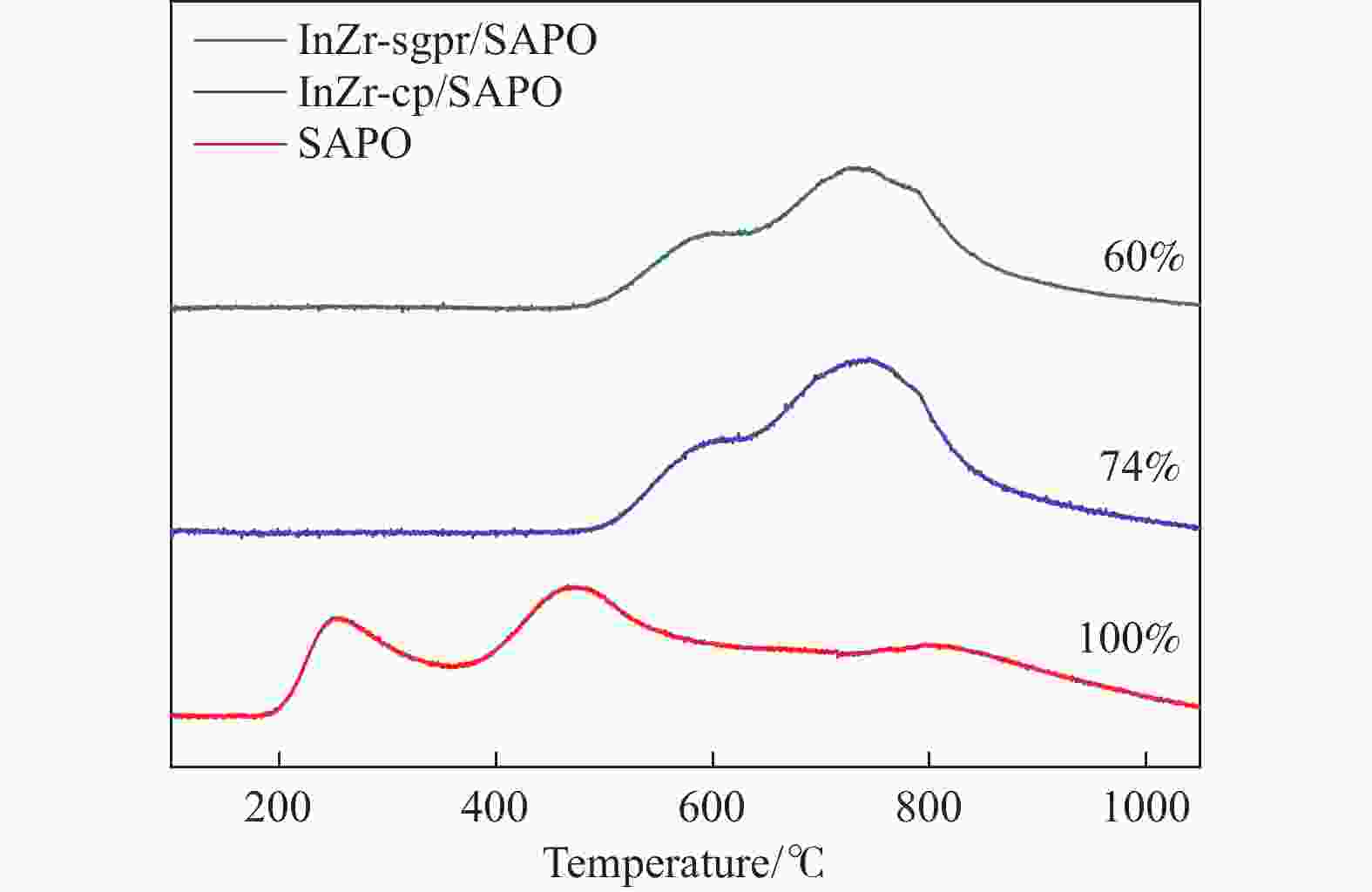
Catalyst CO2 conversion/% CO selectivity/% Hydrocarbons selectivity/% O/P ratio C2 =−C4 = yield/% CH4 C2 =−C4 = C2 0−C4 0 C5+ InZr-cp/SAPO-34 26.2 60.9 17.4 41.0 34.0 7.6 1.12 4.20 InZr-sg/SAPO-34 16.8 69.0 3.6 55.9 33.8 6.7 1.49 2.91 InZr-sgim/SAPO-34 15.9 100 N.D. N.D. N.D. N.D. − − InZr-sgpr/SAPO-34 17.5 55.8 2.9 46.2 43.8 7.1 0.98 3.57 Reaction conditions: H2/CO2=3, 380 ℃, 3 MPa, 6000 mL/(g·h). -
[1] ÁLVAREZ A, BANSODE A, URAKAWA A, et al. Challenges in the greener production of formates/formic acid, methanol, and DME by heterogeneously catalyzed CO2 hydrogenation processes[J]. Chem Rev,2017,117(14):9804−9838. doi: 10.1021/acs.chemrev.6b00816 [2] KONDRATENKO E V, MUL G, BALTRUSAITIS J, et al. Status and perspectives of CO 2 conversion into fuels and chemicals by catalytic, photocatalyticand electrocatalytic processes[J]. Energy Environ Sci, 2013, 6(11): 3112−3135. [3] 王林祥, 常敏. 全球乙烯供需分析及预测[J]. 世界石油工业,2021,28(5):54−61.WANG Linxiang, CHANG Min. Analysis and forecast of global ethylene supply and demand[J]. World Petro Ind,2021,28(5):54−61. [4] 马龙. 全球丙烯供需分析与预测[J]. 世界石油工业,2021,28(5):47−53.MA L. Global propylene supply and demand analysis and forecast[J]. World Petro Ind,2021,28(5):47−53. [5] ZHOU W, CHENG K, KANG J, et al. New horizon in C1 chemistry: Breaking the selectivity limitation in transformation of syngas and hydrogenation of CO2 into hydrocarbon chemicals and fuels[J]. Chem Soc Rev,2019,48(12):3193−3228. doi: 10.1039/C8CS00502H [6] WANG D, XIE Z, POROSOFF M D, et al. Recent advances in carbon dioxide hydrogenation to produce olefins and aromatics[J]. Chem,2021,7(9):2277−2311. doi: 10.1016/j.chempr.2021.02.024 [7] XU Y, ZHAI P, DENG Y, et al. Highly selective olefin production from CO2 hydrogenation on iron catalysts: a subtle synergy between manganese and sodium additives[J]. Angew Chem Int Ed,2020,132(48):21920−21928. doi: 10.1002/ange.202009620 [8] LI Z, WANG J, QU Y, et al. Highly selective conversion of carbon dioxide to lower olefins[J]. ACS Catal,2017,7(12):8544−8548. doi: 10.1021/acscatal.7b03251 [9] GAO P, DANG S, LI S, et al. Direct production of lower olefins from CO2 conversion via bifunctional catalysis[J]. ACS Catal,2018,8(1):571−578. doi: 10.1021/acscatal.7b02649 [10] WANG S, ZHANG L, WANG P, et al. Highly effective conversion of CO2 into light olefins abundant in ethene[J]. Chem,2022,8(5):1376−1394. doi: 10.1016/j.chempr.2022.01.004 [11] SUN K, FAN Z, YE J, et al. Hydrogenation of CO2 to methanol over In2O3 catalyst[J]. J CO2 Util,2015,12:1−6. doi: 10.1016/j.jcou.2015.09.002 [12] MARTIN O, MARTÍN A J, MONDELLI C, et al. Indium oxide as a superior catalyst for methanol synthesis by CO2 hydrogenation[J]. Angew Chem Int Ed,2016,128(21):6369−6373. doi: 10.1002/ange.201600943 [13] DANG S, QIN B, YANG Y, et al. Rationally designed indium oxide catalysts for CO2 hydrogenation to methanol with high activity and selectivity[J]. Sci Adv,2020,6(25):eaaz2060. doi: 10.1126/sciadv.aaz2060 [14] LI K, CHEN J G. CO2 hydrogenation to methanol over ZrO2-containing catalysts: insights into ZrO2 induced synergy[J]. ACS Catal,2019,9(9):7840−7861. doi: 10.1021/acscatal.9b01943 [15] FREI M S, MONDELLI C, CESARINI A, et al. Role of zirconia in indium oxide-catalyzed CO2 hydrogenation to methanol[J]. ACS Catal,2019,10(2):1133−1145. [16] YANG C, PEI C, LUO R, et al. Strong electronic oxide-support interaction over In2O3/ZrO2 for highly selective CO2 hydrogenation to methanol[J]. J Am Chem Soc,2020,142(46):19523−19531. doi: 10.1021/jacs.0c07195 [17] YE J, LIU C, MEI D, et al. Active oxygen vacancy site for methanol synthesis from CO2 hydrogenation on In2O3 (110): A DFT study[J]. ACS Catal,2013,3(6):1296−1306. doi: 10.1021/cs400132a [18] FREI M S, CAPDEVILA-CORTADA M, GARCÍA-MUELAS R, et al. Mechanism and microkinetics of methanol synthesis via CO2 hydrogenation on indium oxide[J]. J Catal,2018,361:313−321. doi: 10.1016/j.jcat.2018.03.014 [19] CAO A, WANG Z, LI H, et al. Relations between surface oxygen vacancies and activity of methanol formation from CO2 hydrogenation over In2O3 surfaces[J]. ACS Catal,2021,11(3):1780−1786. doi: 10.1021/acscatal.0c05046 [20] GHOSH S, SEBASTIAN J, OLSSON L, et al. Experimental and kinetic modeling studies of methanol synthesis from CO2 hydrogenation using In2O3 catalyst[J]. Chem Eng J,2021,416:129120. doi: 10.1016/j.cej.2021.129120 [21] WANG Y, WANG G, VAN DER WAL L I, et al. Visualizing element migration over bifunctional metal-zeolite catalysts and its impact on catalysis[J]. Angew Chem Int Ed,2021,133(32):17876−17884. doi: 10.1002/ange.202107264 [22] RUI N, WANG Z, SUN K, et al. CO2 hydrogenation to methanol over Pd/In2O3: Effects of Pd and oxygen vacancy[J]. Appl Catal B: Environ,2017,218:488−497. [23] SHEN C, SUN K, ZHANG Z, et al. Highly active Ir/In2O3 catalysts for selective hydrogenation of CO2 to methanol: experimental and theoretical studies[J]. ACS Catal,2021,11(7):4036−4046. doi: 10.1021/acscatal.0c05628 [24] ZHU J, CANNIZZARO F, LIU L, et al. Ni-In synergy in CO2 hydrogenation to methanol[J]. ACS Catal,2021,11(18):11371−11384. doi: 10.1021/acscatal.1c03170 [25] LIU T, HONG X, LIU G. In Situ generation of the Cu@ 3D-ZrO x framework catalyst for selective methanol synthesis from CO2/H2[J]. ACS Catal,2019,10(1):93−102. [26] LIU T, XU D, SONG M, et al. K-ZrO2 interfaces boost CO2 hydrogenation to higher alcohols[J]. ACS Catal,2023,13(7):4667−4674. doi: 10.1021/acscatal.3c00074 [27] WEI W, WEI Z, LI R, et al. Subsurface oxygen defects electronically interacting with active sites on In2O3 for enhanced photothermocatalytic CO2 reduction[J]. Nat Commun,2022,13:3199. doi: 10.1038/s41467-022-30958-5 [28] FREI M S, MONDELLI C, GARCÍA-MUELAS R, et al. Nanostructure of nickel-promoted indium oxide catalysts drives selectivity in CO2 hydrogenation[J]. Nat Commun,2021,12(1):1960. doi: 10.1038/s41467-021-22224-x [29] CHRISTOPHER N C, BRADY J C, HSIANG W C, et al. Aerogel synthesis of yttria-stabilized zirconia by a non-alkoxide sol-gel route[J]. Chem Mater,2005,17:3345−3351. doi: 10.1021/cm0503679 [30] XU D, HONG X, LIU G. Highly dispersed metal doping to ZnZr oxide catalyst for CO2 hydrogenation to methanol: Insight into hydrogen spillover[J]. J Catal,2021,393:207−214. doi: 10.1016/j.jcat.2020.11.039 [31] FENG Z, TANG C, ZHANG P, et al. Asymmetric sites on the ZnZrO x catalyst for promoting formate formation and transformation in CO2 hydrogenation[J]. J Am Chem Soc,2023,145:12663−12672. [32] LI Z, MARTÍNEZ-TRIGUERO J, CONCEPCIÓN P, et al. Methanol to olefins: activity and stability of nanosized SAPO-34 molecular sieves and control of selectivity by silicon distribution[J]. Phys Chem Chem Phys,2013,15(35):14670−14680. doi: 10.1039/c3cp52247d [33] SUN Q, WANG N, GUO G, et al. Ultrafast synthesis of nano-sized zeolite SAPO-34 with excellent MTO catalytic performance[J]. Chem Commun,2015,51(91):16397−16400. doi: 10.1039/C5CC07343J -




 下载:
下载: