Preparation of Ni0.6Cu0.4O/NC catalyst and its catalytic performance for hydrogen production from hydrolysis of ammonia borane
-
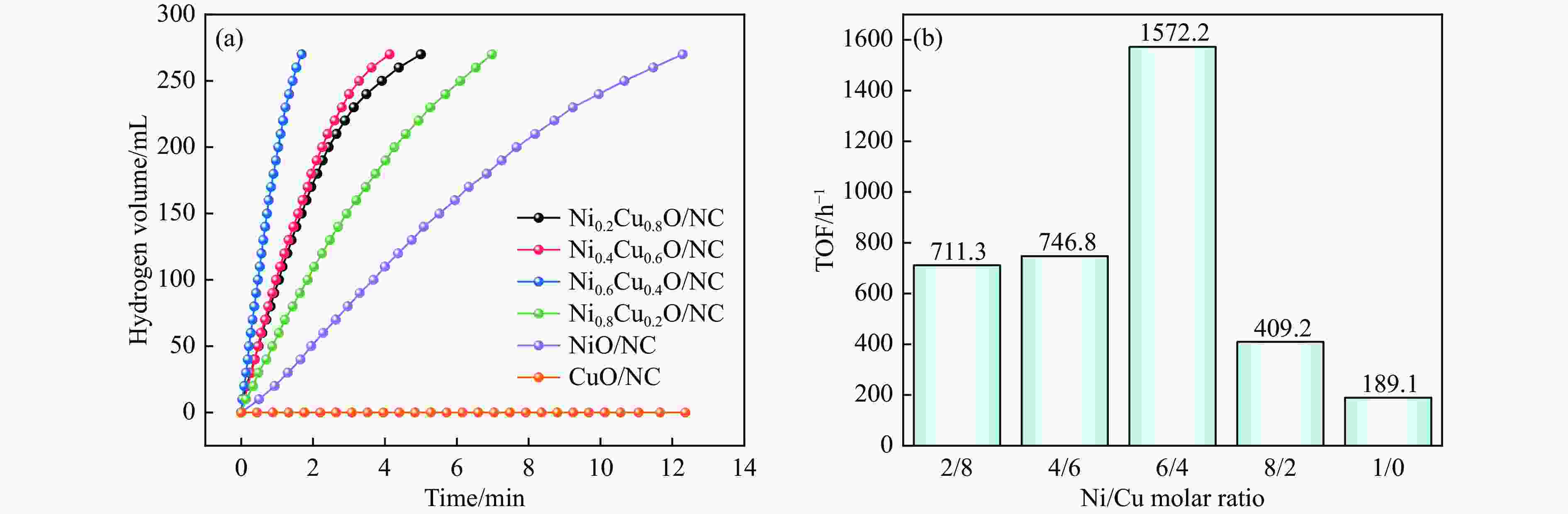
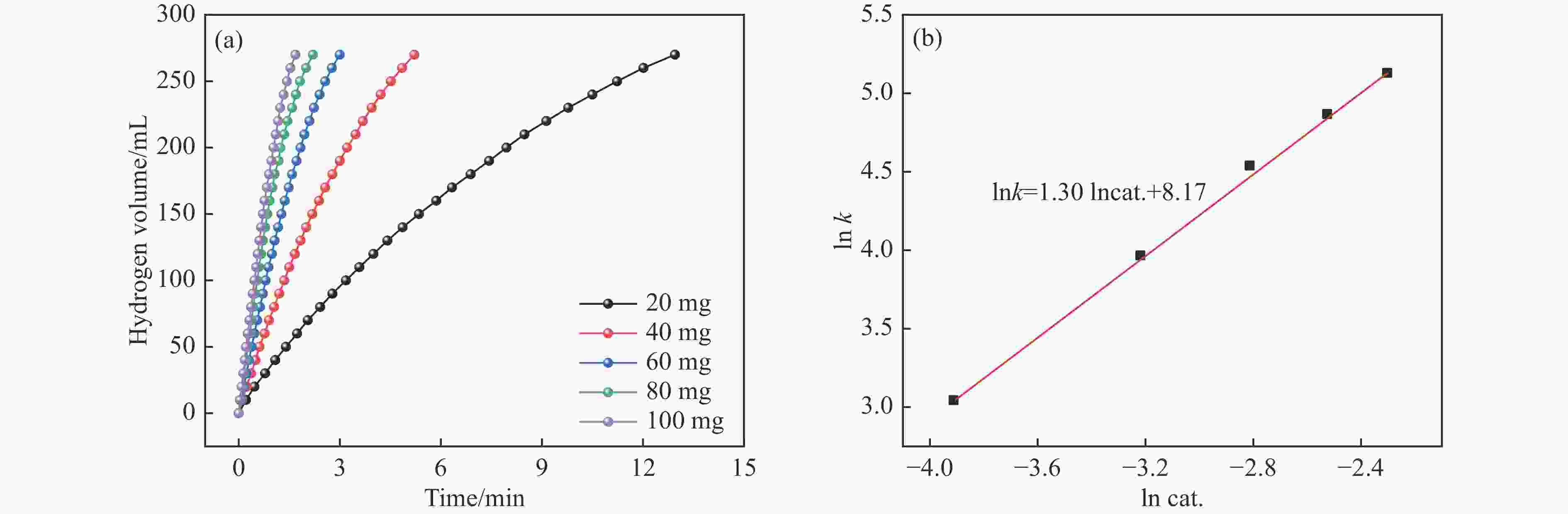
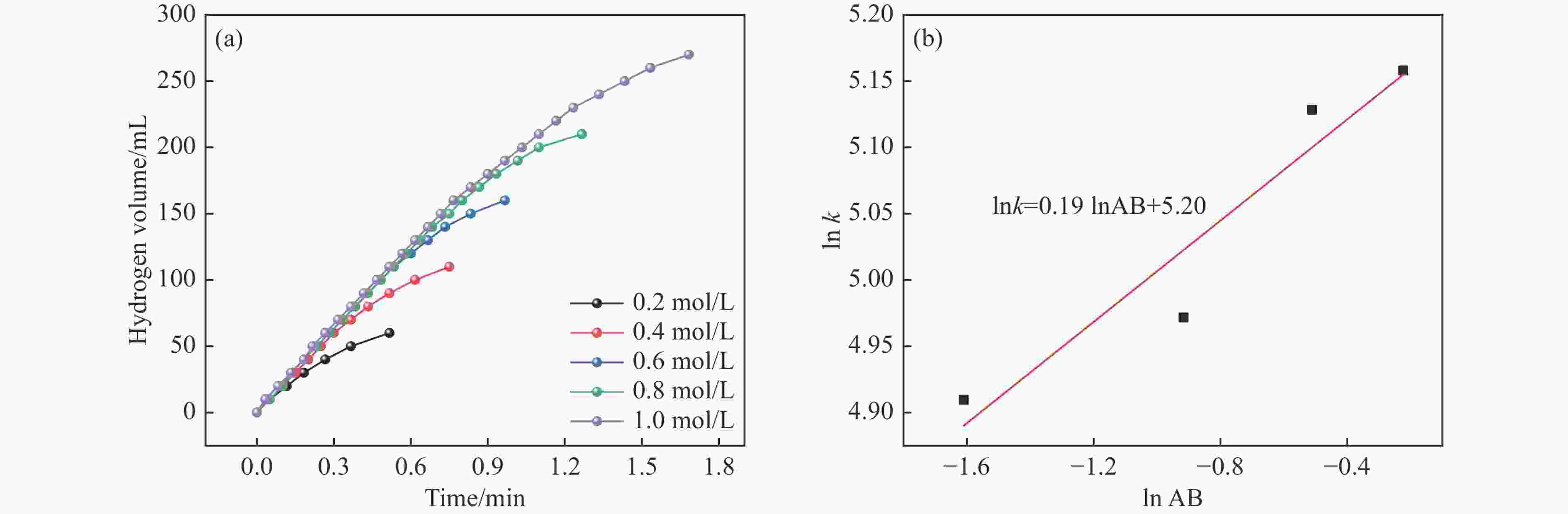
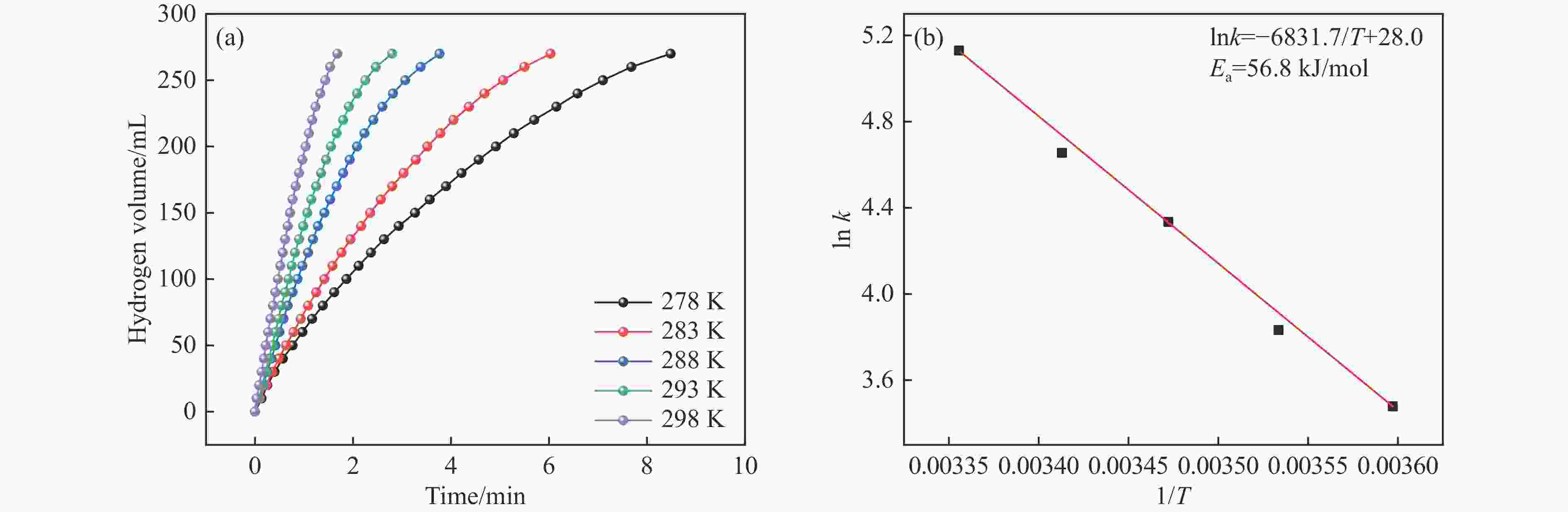
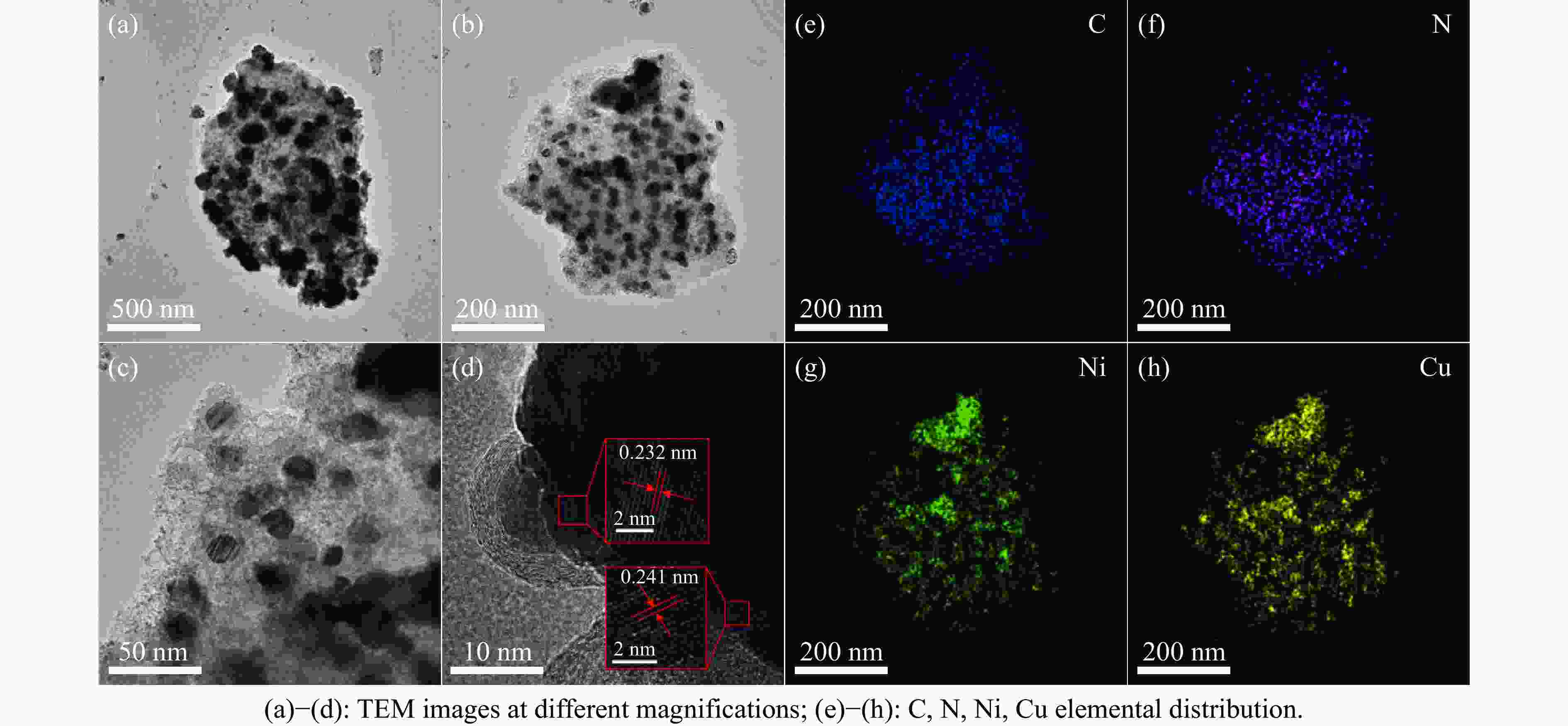
摘要: 氨硼烷(NH3BH3,AB)是一种理想的制氢原料,具有较高的储氢能力。本研究在氮气气氛下高温碳化Ni/Cu-ZIF前驱体制备了一种含氮炭材料(Ni0.6Cu0.4O/NC)催化剂,并采用多种表征方法对所制备催化剂的微观结构以及组成成分进行了研究。此外,通过控制变量法探究了催化剂的催化性能以及变化规律。研究结果表明,Ni0.6Cu0.4O/NC催化AB水解制氢的活化能(Ea)为56.8 kJ/mol,TOF值高达1572.2 h−1。该催化剂催化AB水解制氢速率对于AB自身浓度可近似看作零级反应,而相对于催化剂的用量可近似看作一级反应。催化剂经过10次循环后仍然保持良好的催化活性,表明其具有良好的稳定性。Abstract: Ammonia borane (NH3BH3, AB) is an ideal feedstock for hydrogen production with high hydrogen storage capacity. In this paper, a nitrogen-containing carbon material (Ni0.6Cu0.4O/NC) catalyst was prepared by high-temperature carbonization of Ni/Cu-ZIF precursor under nitrogen atmosphere, and the microstructure as well as the composition of the prepared catalyst were investigated by various characterization methods. In addition, the catalytic performance of the catalyst and the variation rule were investigated by the controlled variable method. The results showed that the activation energy (Ea) of Ni0.6Cu0.4O/NC catalyzed hydrolysis of AB for hydrogen production was 56.8 kJ∙mol−1, and the TOF value was as high as 1572.2 h−1. The rate of hydrogen production from AB hydrolysis catalyzed by this catalyst could be approximated as a zero-order reaction with respect to the concentration of AB itself, and a one-order reaction with respect to the amount of catalyst. The catalyst still maintained good catalytic activity after ten cycles, indicating its good stability.
-
Key words:
- nitrogen-containing carbon material /
- ammonia borane /
- hydrogen hydrolysis
-
表 1 室温下水溶液中AB水解制氢各种金属催化剂的催化活性
Table 1 Catalytic activity of various metal catalysts for hydrogen production by AB hydrolysis in aqueous solution at room temperature
Catalyst TOF/h−1 Ea/(kJ∙mol−1) Ref. Ni0.6Cu0.4O/NC 1572.2 56.8 this work Ni0.3Co0.3Cu0.4 66 58.9 [36] Cu0.64Ni0.36-TiO2 (B) NTs 954 36.14 [37] NiCu/SiO2 1518 34.2 [38] Ni/Cu-c 540 23.7 [39] Ni/Cu-t 282 35 [39] Ni/Cu-o 204 62 [39] Ni/g-C3N4 1122 36 [40] Ni/TiN-TiO2 480 60.7 [41] CoCu-NC-5 487.2 34.25 [42] SCo0.43Cu0.57 340.8 31.06 [43] Cu2Ni1@MIL-101 1254 32.2 [44] Cu0.4Co0.6/BNNFs 505.2 21.8 [45] Ni/BN 72 63.2 [46] Ni/CeO2 504 28.9 [47] -
[1] SUN Z, SUN Z. Hydrogen generation from methanol reforming for fuel cell applications: A review[J]. J Cent South Univ,2020,27(4):1074−1103. doi: 10.1007/s11771-020-4352-8 [2] 吴慧, 郑君宁, 左佑华, 等. NiPd/TiO2催化剂的制备及催化甲酸分解制氢[J]. 精细化工, 2023.WU Hui, ZHENG Junning, ZUO Youhua, et al. Preparation of NiPd/TiO2 catalysts and catalytic hydrogen production from formic acid decomposition[J]. Fine Chem, 2023.) [3] 张唯怡, 张议洁, 王进伟, 等. 电解水制氢技术及大电流析氧反应研究与展望[J]. 工程科学学报,2023,45(7):1057−1070.ZHANG Weiyi, ZHANG Yijie, WANG Jinwei, et al. Research and perspectives on electrocatalytic water splitting and large current density oxygen evolution reaction[J]. Chin J Eng,2023,45(7):1057−1070. [4] LE P A, TRUNG V D, NGUYEN P L, et al. The current status of hydrogen energy: an overview[J]. RSC Adv,2023,13(40):28262−20287. doi: 10.1039/D3RA05158G [5] WEI Y, YANG G, XU X, et al. Ultrafine Ru nanoparticles anchored on core–shell structured zeolite-carbon for efficient catalysis of hydrogen generation[J]. Rare Met,2023,42(7):2324−2334. doi: 10.1007/s12598-022-02246-0 [6] ALPTEKIN F M, CELIKTAS M S. Review on catalytic biomass gasification for hydrogen production as a sustainable energy form and social, technological, economic, environmental, and political analysis of catalysts[J]. ACS Omega,2022,7(29):24918−24941. doi: 10.1021/acsomega.2c01538 [7] 王小燕, 张若凡, 司航, 等. 椰壳炭负载钌催化剂的制备及其催化氨硼烷水解制氢性能[J]. 石油炼制与化工,2023,54(7):64−70. doi: 10.3969/j.issn.1005-2399.2023.07.012WANG Xiaoyan, ZHANG Ruofan, SI Hang, et al. Preparation of coconut shell charcoal-loaded ruthenium catalysts and their catalytic performance for hydrogen production from hydrolysis of ammonia borane[J]. Pet Process Petrochem,2023,54(7):64−70. doi: 10.3969/j.issn.1005-2399.2023.07.012 [8] HUO J, ZHANG K, WEI H, et al. A review on hydrogen production from ammonia borane: Experimental and theoretical studies[J]. Chin Chem Lett,2023,34(12):108280. doi: 10.1016/j.cclet.2023.108280 [9] WANG M, WANG J, ZHAO X, et al. CuCo2O4-NiO heterostructure catalysts for hydrogen production from ammonia borane[J]. Int J Hydrogen Energy,2023,48(51):19543−19553. doi: 10.1016/j.ijhydene.2023.02.068 [10] 曹云钟, 郑君宁, 吴慧, 等. Pt基催化剂催化氨硼烷水解产氢的研究进展[J]. 稀有金属,2023,47(8):1122−1131.CAO Yunzhong, ZHENG Junning, WU Hui, et al. Advances in hydrogen production by ammonia borane hydrolysis over Pt-based catalysts[J]. Rare Met,2023,47(8):1122−1131. [11] ZHANG J, LI J, YANG L, et al. Efficient hydrogen production from ammonia borane hydrolysis catalyzed by TiO2-supported RuCo catalysts[J]. Int J Hydrogen Energy,2021,46(5):3964−3973. doi: 10.1016/j.ijhydene.2020.10.234 [12] WAN C, LI G, WANG J, et al. Modulating electronic metal-support interactions to boost visible-light-driven hydrolysis of ammonia borane: Nickel-Platinum nanoparticles supported on phosphorus-doped titania[J]. Angew Chem Int Ed,2023,62(40):e202305371. doi: 10.1002/anie.202305371 [13] 邱小魁, 张若凡, 王小燕, 等. 竹茹丝炭负载钌催化剂光催化氨硼烷水解产氢研究[J]. 无机盐工业, 2023, 55(10): 153-158.QIU Xiaokui, ZHANG Ruofan, WANG Xiaoyan, et al. Hydrogen production by photocatalytic hydrolysis of ammonia borane over Bamboo Rhizoma Pinelliae silk charcoal loaded ruthenium catalysts[J]. 2023, 55(10): 153-158.) [14] LI J, REN X, LV H, et al. Highly efficient hydrogen production from hydrolysis of ammonia borane over nanostructured Cu@CuCoO x supported on graphene oxide[J]. J Hazard Mater,2020,391:122199. doi: 10.1016/j.jhazmat.2020.122199 [15] 李贵, 梁雨, 郑君宁, 等. Rh/N-GMCs纳米催化剂的制备及其催化氨硼烷水解产氢性能研究[J]. 燃料化学学报,2023,51(4):528−537.LI Gui, LIANG Yu, ZHENG Junning, et al. Preparation of Rh/N-GMCs nanocatalyst and its catalytic performance for the hydrolytic dehydrogenation of ammonia borane[J]. J Fuel Chem Technol,2023,51(4):528−537. [16] WAN C, LIANG Y, ZHOU L, et al. Integration of morphology and electronic structure modulation on cobalt phosphide nanosheets to boost photocatalytic hydrogen evolution from ammonia borane hydrolysis[J]. Green Energy Environ, 2022. [17] FANG M J, LIN Y C, JAN J Y, et al. Au@Cu2O core@shell nanocrystals as sustainable catalysts for efficient hydrogen production from ammonia borane[J]. Appl Catal B Environ,2023,324:122198. doi: 10.1016/j.apcatb.2022.122198 [18] 牛曦若, 张怡菲, 徐靖涵, 等. MOF衍生物(Fe/NC)活化过硫酸盐对土霉素的高效降解[J]. 环境化学, 2023.NIU Ruoxi, ZHANG Yifei, XU Jinghan, et al. Efficient degradation of oxytetracycline by persulfate activated by MOF derivative (Fe/NC)[J]. Environ Chem, 2023.) [19] VILLENOISY T D, ZHENG X, WONG V, et al. Principles of design and synthesis of metal derivatives from MOFs[J]. Adv Mater,2023,35(24):2210166. doi: 10.1002/adma.202210166 [20] SONG G, SHI Y, JIANG S, et al. Recent progress in MOF-derived porous materials as electrodes for high-performance Lithium-ion batteries[J]. Adv Funct Mater,2023,33(42):2303121. doi: 10.1002/adfm.202303121 [21] LI Y-X, HAN Y C, WANG C C. Fabrication strategies and Cr(VI) elimination activities of the MOF-derivatives and their composites[J]. Chem Eng J,2021,405:126648. doi: 10.1016/j.cej.2020.126648 [22] ABDELHAMID H, MEKHEMER I M. A. , GABER A-A M. Metal-organic frameworks (MOFs)-derived ZrOSO4@C for photocatalytic synthesis of benzimidazole derivatives[J]. Mol Catal, 2023, 548 : 113418. [23] XIAO T, LIU D. The most advanced synthesis and a wide range of applications of MOF-74 and its derivatives[J]. Micropor Mesopor Mater,2019,283:88−103. doi: 10.1016/j.micromeso.2019.03.002 [24] SHEN M, MA H. Metal-organic frameworks (MOFs) and their derivative as electrode materials for lithium-ion batteries[J]. Coord Chem Rev,2022,470:214715. doi: 10.1016/j.ccr.2022.214715 [25] KUMAR G, DUTTA A, GOSWAMI M, et al. Synthesis of Zr-MOF/rGO-nanocomposites used for spirooxindole scaffolds derivatives[J]. J Mol Struct,2023,1287:135653. doi: 10.1016/j.molstruc.2023.135653 [26] CAO L, CHEN B, YAN J, et al. Nickel nanoparticle-embedded N-doped carbon catalysts formed by MOF derivatives for the oxygen evolution reaction[J]. New J Chem,2023,47(27):12799−12805. doi: 10.1039/D3NJ01102J [27] PÜSCHEL D, HÉDÉ S, MAISULS I, et al. Enhanced solid-state fluorescence of flavin derivatives by incorporation in the metal-organic frameworks MIL-53(Al) and MOF-5[J]. Molecules,2023,28(6):2877. doi: 10.3390/molecules28062877 [28] FEREJA S L, ZHANG Z, FANG Z. High-entropy oxide derived from metal-organic framework as a bifunctional electrocatalyst for efficient urea oxidation and oxygen evolution reactions[J]. ACS Appl Mater Interfaces,2022,14(34):38727−38738. doi: 10.1021/acsami.2c09161 [29] LI C, LIU X, WANG H, et al. Metal-organic framework derived hexagonal layered cobalt oxides with {112} facets and rich oxygen vacancies: High efficiency catalysts for total oxidation of propane[J]. Adv Powder Technol,2022,33(1):103373. doi: 10.1016/j.apt.2021.11.025 [30] KOTEESWARI P, SAGADEVAN S, FATIMAH I, et al. Green synthesis and characterization of copper oxide nanoparticles and their photocatalytic activity[J]. Inorg Chem Commun,2022,144:109851. doi: 10.1016/j.inoche.2022.109851 [31] 张兴, 刘浩宇, 朱明路. 碳化Ni@MOF808催化剂在甲烷干重整中的研究[J]. 山东化工,2023,52(10):9−11. doi: 10.3969/j.issn.1008-021X.2023.10.003ZHANG Xing, LIU Haoyu, ZHU Minglu. Study on carbonized Ni@MOF808 catalyst in methane dry reforming[J]. Shandong Chem Ind,2023,52(10):9−11. doi: 10.3969/j.issn.1008-021X.2023.10.003 [32] YAN J, ZHOU Y, SHEN J, et al. Facile synthesis of S, N-co-doped carbon dots for bio-imaging, Fe3+ detection and DFT calculation[J]. Spectrochim Acta A Mol Biomol Spectrosc,2023,302:123105. doi: 10.1016/j.saa.2023.123105 [33] Rana L K, Kaur P, Bavandsavadkouhi A, et al. Isostructural coordination polymers of the tethering naphthalene anchored bis (2-methylpyridinecarboxamide) ligand: single crystal, XPS, EDS and theoretical studies[J]. New J Chem,2023,47(11):5477−5487. doi: 10.1039/D3NJ00038A [34] JOHNSON E, KRISHNAN R, CHANDRAN S, et al. Green mediated sol-gel synthesis of copper oxide nanoparticle: An efficient candidate for waste water treatment and antibacterial agent[J]. J Sol-Gel Sci Technol,2023,107:697−710. doi: 10.1007/s10971-023-06172-0 [35] ZHANG L, ZHOU L, YANG K, et al. Pd-Ni nanoparticles supported on MIL-101 as high-performance catalysts for hydrogen generation from ammonia borane[J]. J Alloys Compd,2016,677:87−95. doi: 10.1016/j.jallcom.2016.03.234 [36] YANG X, WANG C, GAO R, et al. Non-noble metallic nanoparticles supported on titania spheres as catalysts for hydrogen generation from hydrolysis of ammonia borane under ultraviolet light irradiation[J]. Int J Hydrogen Energy,2018,43(34):16556−16565. doi: 10.1016/j.ijhydene.2018.07.049 [37] WANG C, SUN D, YU X, et al. Cu/Ni nanoparticles supported on TiO2 (B) nanotubes as hydrogen generation photocatalysts via hydrolysis of ammonia borane[J]. Inorg Chem Front,2018,5(8):2038−2044. doi: 10.1039/C8QI00492G [38] GUO K, DING Y, LUO J, et al. NiCu bimetallic nanoparticles on silica support for catalytic hydrolysis of ammonia borane: composition-dependent activity and support size effect[J]. ACS Appl Energy Mater,2019,2(8):5851−5861. doi: 10.1021/acsaem.9b00997 [39] LI X, ZHANG J, WANG S, et al. Exposed facet tailoring of Ni/Cu catalysts for boosting dehydrogenation of ammonia borane[J]. ACS Appl Nano Mater,2023,6(20):19136−19147. doi: 10.1021/acsanm.3c03621 [40] GAO M, YU Y, YANG W, et al. Ni nanoparticles supported on graphitic carbon nitride as visible light catalysts for hydrolytic dehydrogenation of ammonia borane[J]. Nanoscale,2019,11(8):3506−3513. doi: 10.1039/C8NR09005J [41] LI X, LIU Y, XU P, et al. Enhanced hydrogen production from ammonia borane over Ni nanoparticles supported on TiN-TiO2 composites under sunlight[J]. Energy Fuels,2022,36(5):2901−2909. doi: 10.1021/acs.energyfuels.1c04255 [42] SONG Y, GAO C, LIU J, et al. Fabrication of multiatomic structure of Cu-CoO/Co interface for efficient hydrogen generation from ammonia borane hydrolysis[J]. Int J Hydrogen Energy,2023,48(67):26162−26172. doi: 10.1016/j.ijhydene.2023.03.270 [43] WANG C, LI L, YU X, et al. Regulation of d-band electrons to enhance the activity of Co-based non-noble bimetal catalysts for hydrolysis of ammonia borane[J]. ACS Sustain Chem Eng,2020,8(22):8256−8266. doi: 10.1021/acssuschemeng.0c01475 [44] GAO D, ZHANG Y, ZHOU L, et al. CuNi NPs supported on MIL-101 as highly active catalysts for the hydrolysis of ammonia borane[J]. Appl Surf Sci,2018,427:114−122. doi: 10.1016/j.apsusc.2017.08.167 [45] YANG X, LI Q, LI L, et al. CuCo binary metal nanoparticles supported on boron nitride nanofibers as highly efficient catalysts for hydrogen generation from hydrolysis of ammonia borane[J]. J Power Sources,2019,431:135−143. doi: 10.1016/j.jpowsour.2019.05.038 [46] YANG X, LI L, SANG W, et al. Boron nitride supported Ni nanoparticles as catalysts for hydrogen generation from hydrolysis of ammonia borane[J]. J Alloys Compd,2017,693:642−649. doi: 10.1016/j.jallcom.2016.09.204 [47] YAO Q, LU Z, YANG Y, et al. Facile synthesis of graphene-supported Ni-CeO x nano-composites as highly efficient catalysts for hydrolytic dehydrogenation of ammonia borane[J]. Nano Res,2018,11(8):4412−4422. doi: 10.1007/s12274-018-2031-y [48] 吴慧, 郑君宁, 李蓉, 等. NiPt/CeO2催化剂的制备及其催化水合肼分解制氢性能研究[J]. 中国稀土学报, 2023.WU Hui, ZHENG Junning, LI Rong, et al. Preparation of NiPt/CeO2 catalyst and its catalytic performance for hydrogen production from hydrazine hydrate decomposition[J]. J Chin Soc Rare Earths, 2023.) [49] KARATAS Y, GÜLCAN M, ZAHMAKIRAN M. Silica supported ternary NiRuPt alloy nanoparticles: Highly efficient heterogeneous catalyst for H2 generation via selective decomposition of hydrous hydrazine in alkaline solution[J]. Int J Hydrogen Energy,2020,45(51):27098−27113. doi: 10.1016/j.ijhydene.2020.07.048 -




 下载:
下载: