Promoted stability of Cu/ZnO/Al2O3 catalysts for methanol production from CO2 hydrogenation by La modification
-
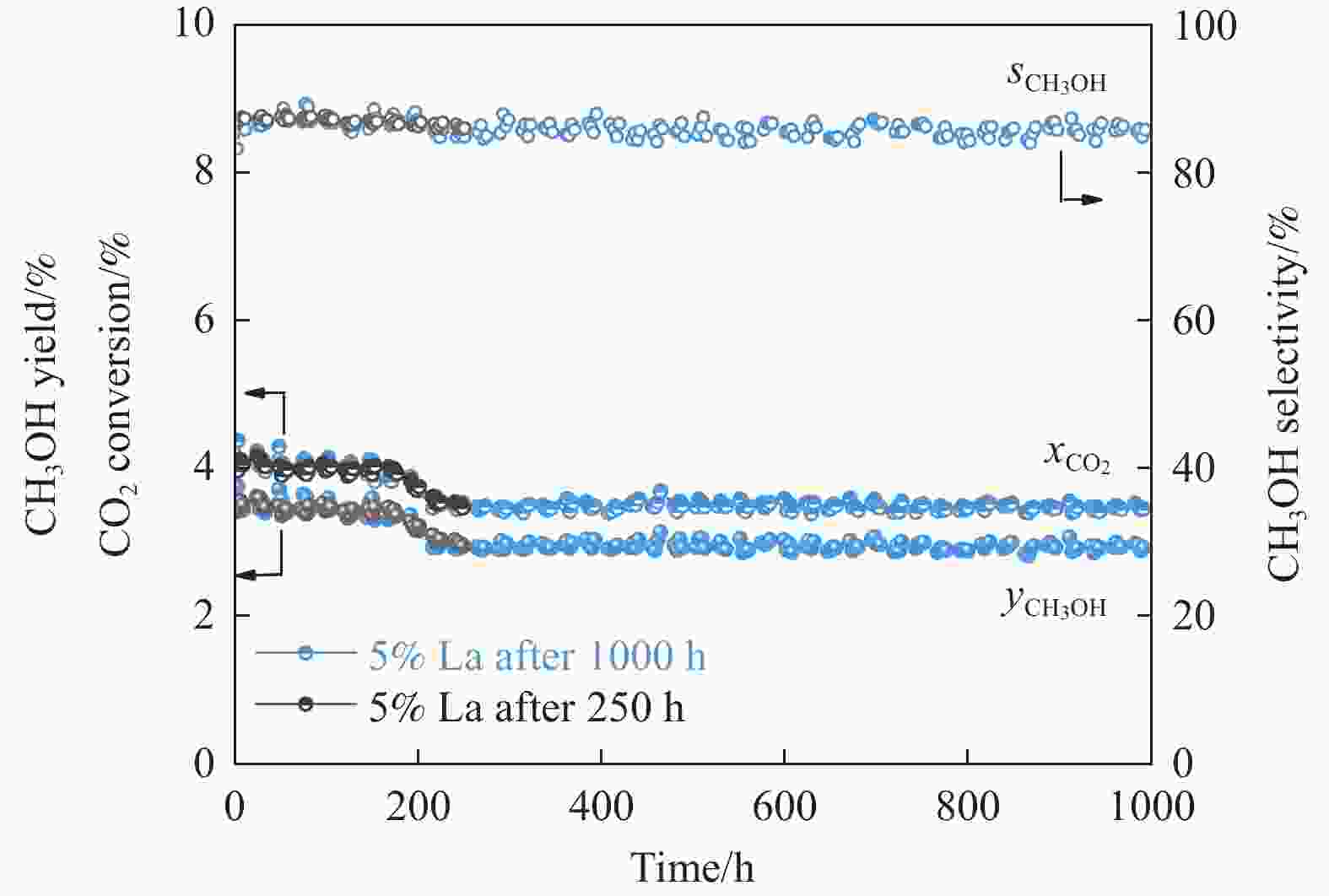
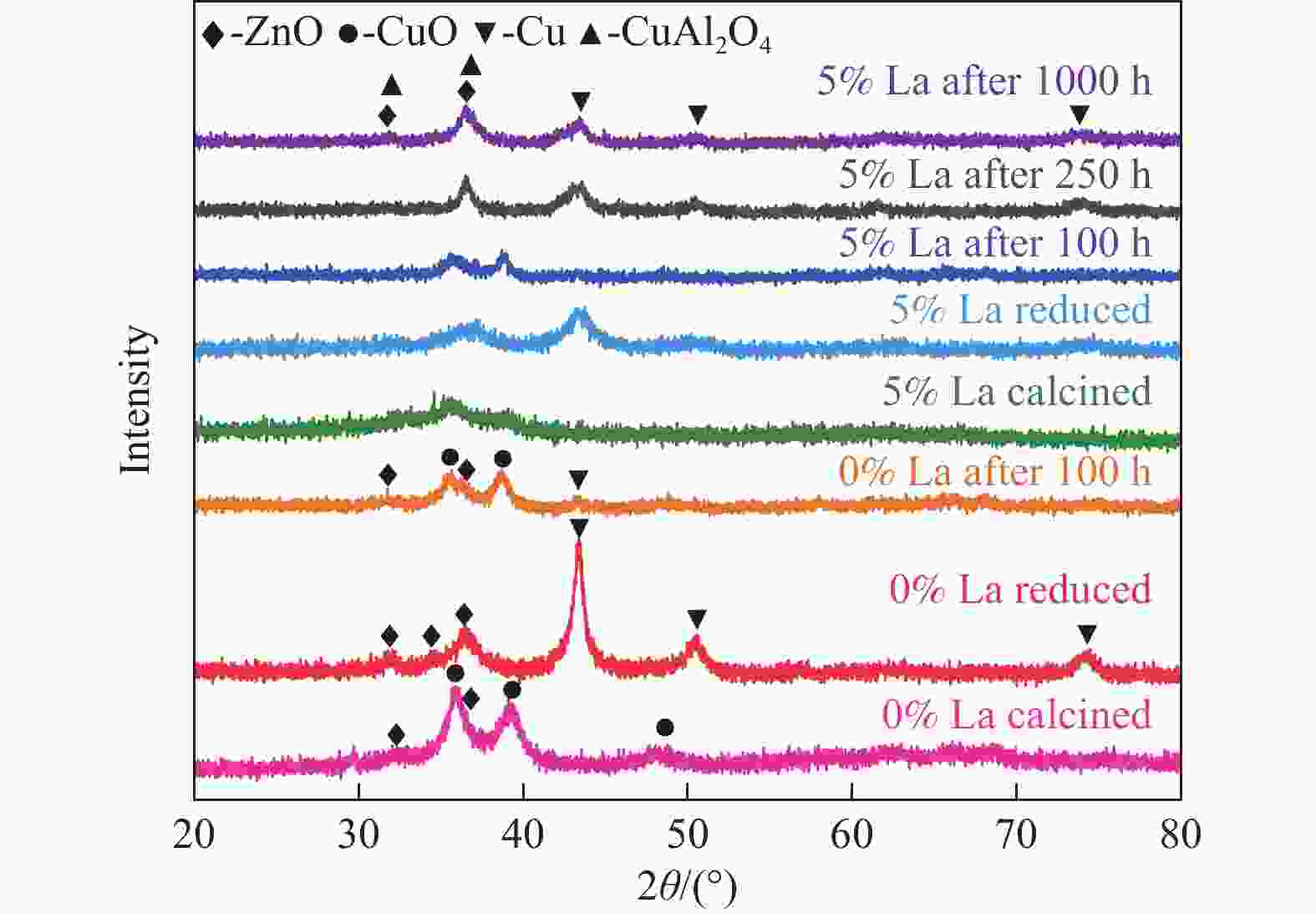
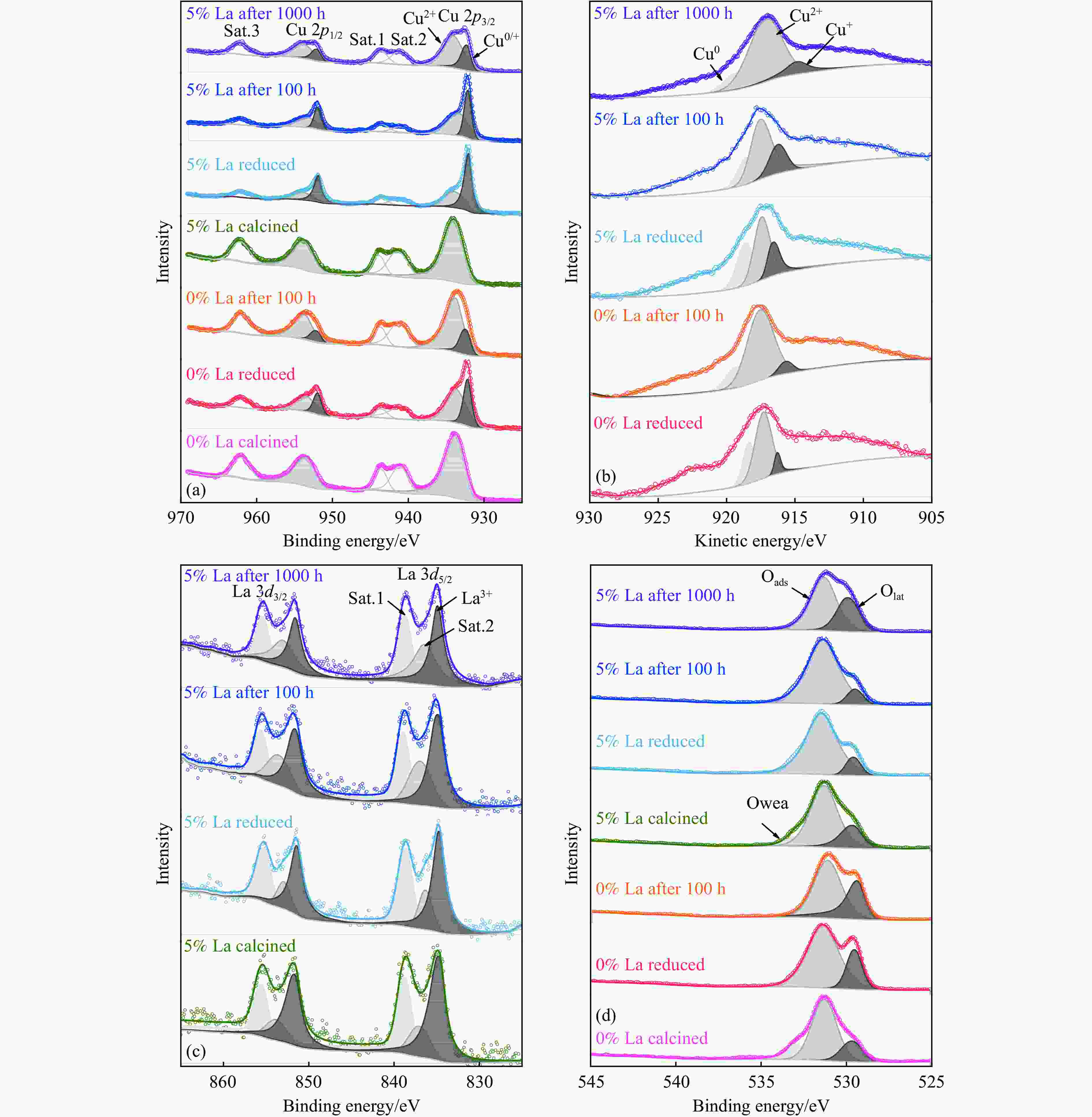
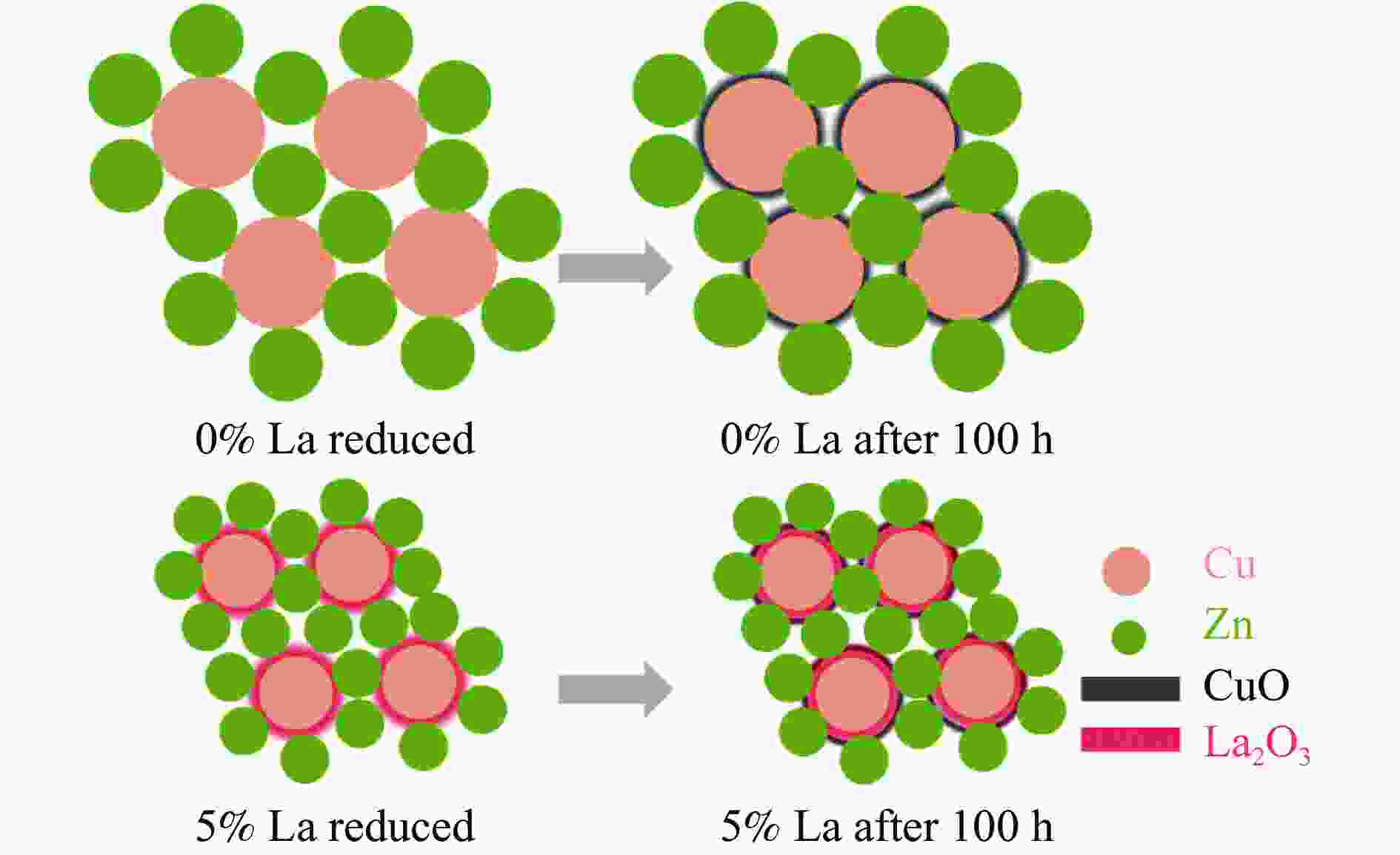
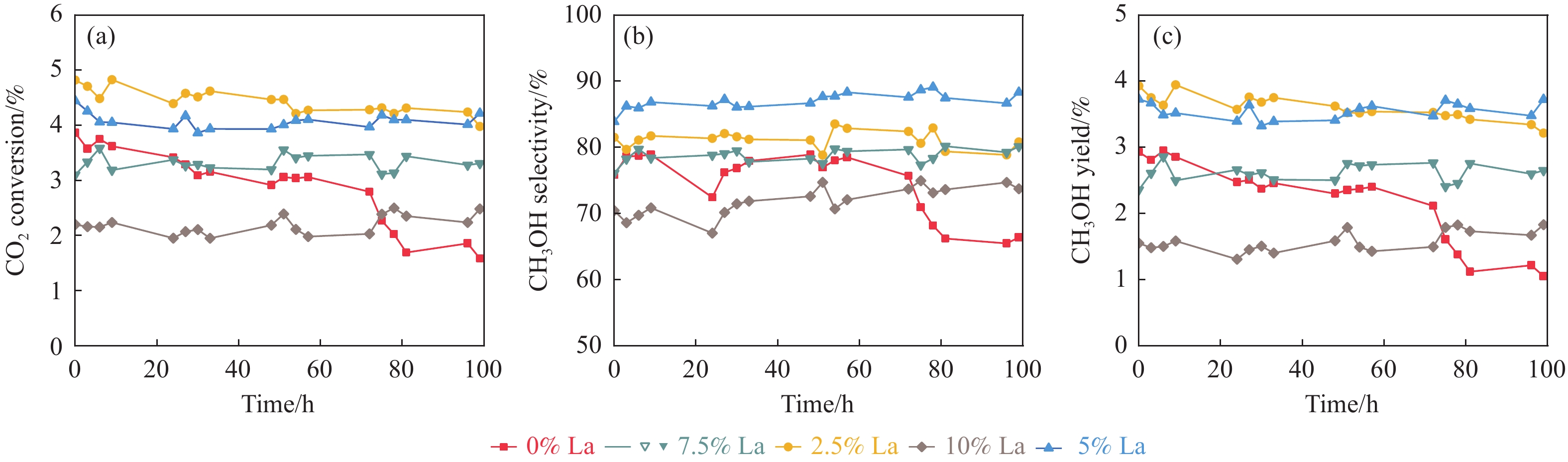
摘要: CO2加氢制甲醇反应中Cu/ZnO/Al2O3催化剂的失活是限制其应用的主要原因之一,实验通过向Cu/ZnO/Al2O3催化剂中添加不同含量的La,合成了一系列La改性的Cu/ZnO/Al2O3催化剂,以提高其对 CO2加氢制甲醇反应的催化稳定性。在温度200 ℃,压力3 MPa,空速12000 mL/(g·h)条件下进行的100 h短期稳定性测试中,未改性的Cu/ZnO/Al2O3催化剂在100 h内活性衰减明显,添加La后催化剂稳定性逐渐得到提高,当La添加量为5% 时活性最佳(CO2转化率4%,甲醇选择性85%),并且该催化剂在1000 h长期稳定性测试中表现出较高的稳定性(在190−220 h失活 17% 后保持稳定)。通过X射线衍射(XRD)、X射线光电子能谱(XPS)表征发现,加入5% La提高了Cu/ZnO/Al2O3催化剂中Cu、ZnO的分散度,抑制了催化剂中Cu的烧结;同时稳定了Cu0/+,延缓了催化剂中Cu的氧化,从而提高了催化剂的稳定性。
-
关键词:
- Cu/ZnO/Al2O3 /
- La 助剂 /
- 稳定性
Abstract: Deactivation of Cu/ZnO/Al2O3 catalysts in CO2 hydrogenation to methanol reaction is one of the main reasons limiting their application. We synthesized a series of La modified Cu/ZnO/Al2O3 catalysts by adding different contents of La to improve the stability. In the 100 h short-term stability test at 200 ℃ under 3 MPa with a GHSV of 12000 mL/(g·h), the unmodified Cu/ZnO/Al2O3 catalysts degraded obviously over 100 h. In sharp contrast, the stability was significantly promoted by the addition of La. The best activity was achieved with 5% La added samples (4% CO2 conversion and 85% methanol selectivity). Which also showed impressive stability over 1000 h except about17% deactivation during the initial 190−220 h. The X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) results revealed that the addition of 5% La improved the dispersion of Cu and Zn , inhibited the sintering of Cu, stabilized the Cu0/+ species and retarded oxidation of Cu in catalysts, which attributed to the high stability of the catalysts.-
Key words:
- Cu/ZnO/Al2O3 /
- La promoter /
- stability
-
表 1 0%、5% La Cu/ZnO/Al2O3催化剂还原后及稳定性测试后的不同价态Cu含量和煅烧后、还原后及稳定性测试后的不同O物种含量
Table 1 0%, 5% La Cu/ZnO/Al2O3 catalysts with different valence Cu contents after calcination, reduction and stability tests and different O species contents after calcination, reduction and stability tests
Samples Cu/% O/% Cu0 Cu+ Cu2+ Olat Oads Owea 0% La calcined − − 100.0 18.8 63.0 18.2 0% La reduced 27.6 7.9 64.5 23.2 76.8 − 0% La after 100 h 13.1 7.0 79.9 36.5 63.5 − 5% La calcined − − 100.0 18.5 70.0 11.5 5% La reduced 32.8 16.8 50.4 11.6 88.4 − 5% La after 100 h 19.8 20.0 60.2 12.4 87.6 − 5% La after 250 h 13.4 15.2 71.4 32.6 67.4 − 5% La after 1000 h 12.8 14.3 72.9 34.0 66.0 − -
[1] NEETZOW P. The effects of power system flexibility on the efficient transition to renewable generation[J]. Appl Energy,2021,283:116278 doi: 10.1016/j.apenergy.2020.116278 [2] ANDERSSON J and GRONKVIST S. Large-scale storage of hydrogen[J]. Int J Hydrogen Energy,2019,44:11901−11919 doi: 10.1016/j.ijhydene.2019.03.063 [3] ALVAREZ A, BANSODE A, URAKAWA A, et al. Challenges in the greener production of formates formic acid, methanol, and DME by heterogeneously catalyzed CO2 hydrogenation processes[J]. Chem Rev,2017,117:9804−9838 doi: 10.1021/acs.chemrev.6b00816 [4] BOWKER M. Methanol synthesis from CO2 hydrogenation[J]. ChemCatChem,2019,11:4238−4246 doi: 10.1002/cctc.201900401 [5] ONISHI N, HIMEDA Y. Homogeneous catalysts for CO2 hydrogenation to methanol and methanol dehydrogenation to hydrogen generation[J]. Coordin Chem Rev,2022,472:214767 doi: 10.1016/j.ccr.2022.214767 [6] RUI N, WANG Z, SUN K, et al. CO2 hydrogenation to methanol over Pd/In2O3: effects of Pd and oxygen vacancy[J]. Appl Catal B:Environ,2017,218:488−497 doi: 10.1016/j.apcatb.2017.06.069 [7] TIAN G, WU Y, WU S, et al. Solid-state synthesis of Pd/In2O3 catalysts for CO2 hydrogenation to methanol[J]. Catal Lett,2023,153:903−910 doi: 10.1007/s10562-022-04030-2 [8] CUI G, ZHANG Q, ZHOU L, et al. Hybrid MOF template-directed construction of hollow-structured In2O3@ZrO2 heterostructure for enhancing hydrogenation of CO2 to methanol[J]. Small,2023,19:2204914 doi: 10.1002/smll.202204914 [9] YANG C, PEI C, LUO R, et al. Strong electronic oxide-support interaction over In2O3/ZrO2 for highly selective CO2 hydrogenation to methanol[J]. J Am Chem Soc,2020,142:19523−19531 doi: 10.1021/jacs.0c07195 [10] HAN Z, TANG C, SHA F, et al. CO2 hydrogenation to methanol on ZnO-ZrO2 solid solution catalysts with ordered mesoporous structure[J]. J Catal,2021,396:242−250 doi: 10.1016/j.jcat.2021.02.024 [11] WANG J, LI G, LI Z, et al. A highly selective and stable ZnO-ZrO2 solid solution catalyst for CO2 hydrogenation to methanol[J]. Sci Adv,2017,3:e1701290 doi: 10.1126/sciadv.1701290 [12] NIU J, LIU H, JIN Y, et al. Comprehensive review of Cu-based CO2 hydrogenation to CH3OH: Insights from experimental work and theoretical analysis[J]. Int J Hydrogen Energy,2022,47:9183−9200 doi: 10.1016/j.ijhydene.2022.01.021 [13] TWIGG V and SPENCER S. Deactivation of supported copper metal catalysts for hydrogenation reactions[J]. Appl Catal A:Genl,2001,212:161−174 doi: 10.1016/S0926-860X(00)00854-1 [14] DANG S, YANG H, GAO P, et al. A review of research progress on heterogeneous catalysts for methanol synthesis from carbon dioxide hydrogenation[J]. Catal Today,2019,330:61−75 doi: 10.1016/j.cattod.2018.04.021 [15] NATESAKHAWAT S, OHODNICKI R, HOWARD B H, et al. Adsorption and deactivation characteristics of Cu/ZnO-based catalysts for methanol synthesis from carbon dioxide[J]. Top Catal,2013,56:1752−1763 doi: 10.1007/s11244-013-0111-5 [16] GONG J, YUE H, ZHAO Y, et al. Synthesis of ethanol via syngas on Cu/SiO2 catalysts with balanced Cu0-Cu+ sites[J]. J Am Chem Soc,2012,134:13922−13925 doi: 10.1021/ja3034153 [17] LIANG B, MA J, SU X, et al. Investigation on deactivation of Cu/ZnO/Al2O3 catalyst for CO2 hydrogenation to methanol[J]. Ind Eng Chem Res,2019,58:9030−9037 doi: 10.1021/acs.iecr.9b01546 [18] LI H, WANG L and XIAO S. Silica-modulated Cu-ZnO-Al2O3 catalyst for efficient hydrogenation of CO2 to methanol[J]. Catal Today,2023,418:114051 doi: 10.1016/j.cattod.2023.114051 [19] AN B, ZHANG J, CHENG K, et al. Confinement of ultrasmall Cu/ZnO x nanoparticles in metal-organic frameworks for selective methanol synthesis from catalytic hydrogenation of CO2[J]. J Am Chem Soc,2017,139:3834−3840 doi: 10.1021/jacs.7b00058 [20] WANG P, ZHANG H, WANG S, et al. Controlling H2 adsorption of Cu/ZnO/Al2O3/MgO with enhancing the performance of CO2 hydrogenation to methanol at low temperature[J]. J Alloy Compd,2023,966:171577 doi: 10.1016/j.jallcom.2023.171577 [21] GUO X, MAO D, LU G, et al. The influence of La doping on the catalytic behavior of Cu/ZrO2 for methanol synthesis from CO2 hydrogenation[J]. J Mol Catal A:Chem,2011,345:60−68 doi: 10.1016/j.molcata.2011.05.019 [22] JI Y, LIN S, XU G, et al. Enhancing CO2 hydrogenation to methanol via constructing Cu–ZnO–La2O3 interfaces[J]. Catal Lett,2023,23:10562 [23] CHEN K, DUAN X, FANG H, et al. Selective hydrogenation of CO2 to methanol catalyzed by Cu supported on rod-like La2O2CO3[J]. Catal Sci & Technol,2018,84:1062−1069 [24] ALI S, KUMAR D, KHADER M, et al. Synthesis and evaluation of lanthana modified Cu-based catalysts for CO2 hydrogenation to value added products[J]. Mol Catal,2023,543:113164 [25] KOURTELESIS M, KOUSI K and KONDARIDES I. CO2 hydrogenation to methanol over La2O3 promoted CuO/ZnO/Al2O3 catalysts: A kinetic and mechanistic study[J]. Catalysts,2020,10:183 doi: 10.3390/catal10020183 [26] ZHONG J, YANG X, WU Z, et al. State of the art and perspectives in heterogeneous catalysis of CO2 hydrogenation to methanol[J]. Chem Soc Rev,2020,49:1385−1413 doi: 10.1039/C9CS00614A [27] CHEN K, YU J, LIU B, et al. Simple strategy synthesizing stable CuZnO/SiO2 methanol synthesis catalyst[J]. J Catal,2019,372:163−173 doi: 10.1016/j.jcat.2019.02.035 [28] HU W, DONAT F, SCOTT A, et al. The interaction between CuO and Al2O3 and the reactivity of copper aluminates below 1000 ℃ and the implication on the use of the Cu–Al–O system for oxygen storage and production[J]. RSC Adv,2016,6:113016−113024 doi: 10.1039/C6RA22712K [29] RAJESH C and RAON A Solid-state reaction based study synthesis and characterization for CuAl2O4 nanocrystalline powder[J]. Optik, 2023, 294 : 171472 [30] TETSUJI Y , MAKOTO E, SHUICHI S, et al. Anomalous chemical shifts of Cu 2p and Cu LMM Auger spectra of silicate glasses[J]. J Electron Spectrosc, 2003, 131 : 133–144 [31] CHEN K, FANG H, WU S et al. CO2 hydrogenation to methanol over Cu catalysts supported on La-modified SBA-15: The crucial role of Cu–LaO x interfaces[J]. Appl Catal B Environ,2019,251:119−129 doi: 10.1016/j.apcatb.2019.03.059 [32] JI Y, LIN S, XU G, et al. Enhancing CO2 hydrogenation to methanol via constructing Cu–ZnO–La2O3 interfaces[J]. Catal Lett,2023,25:119−129 [33] SUN X, JIN Y, CHENG Z, et al. Dual active sites over Cu-ZnO-ZrO2 catalysts for carbon dioxide hydrogenation to methanol[J]. J Environ Sci (China),2023,131:162−172 doi: 10.1016/j.jes.2022.10.002 [34] ALVARO A, CARLOS A, MARTHA E, et al. XPS fitting model proposed to the study of Ni and La in deactivated FCC catalysts[J]. J Electron Spectrosc,2019,233:5−10 doi: 10.1016/j.elspec.2019.03.007 [35] PAPARAZZO E. XPS, AES and EELS studies of Al surfaces[J]. Vacuum,2001,62:47−60 doi: 10.1016/S0042-207X(01)00123-3 [36] ZHANG C, WANG L, ETIMU J, et al. Oxygen vacancies in Cu/TiO2 boost strong metal-support interaction and CO2 hydrogenation to methanol[J]. J Catal,2022,413:284−296 doi: 10.1016/j.jcat.2022.06.026 -




 下载:
下载: