The research on viscosity-temperature characteristics of the mixed slag of biomass and bituminous coal
-
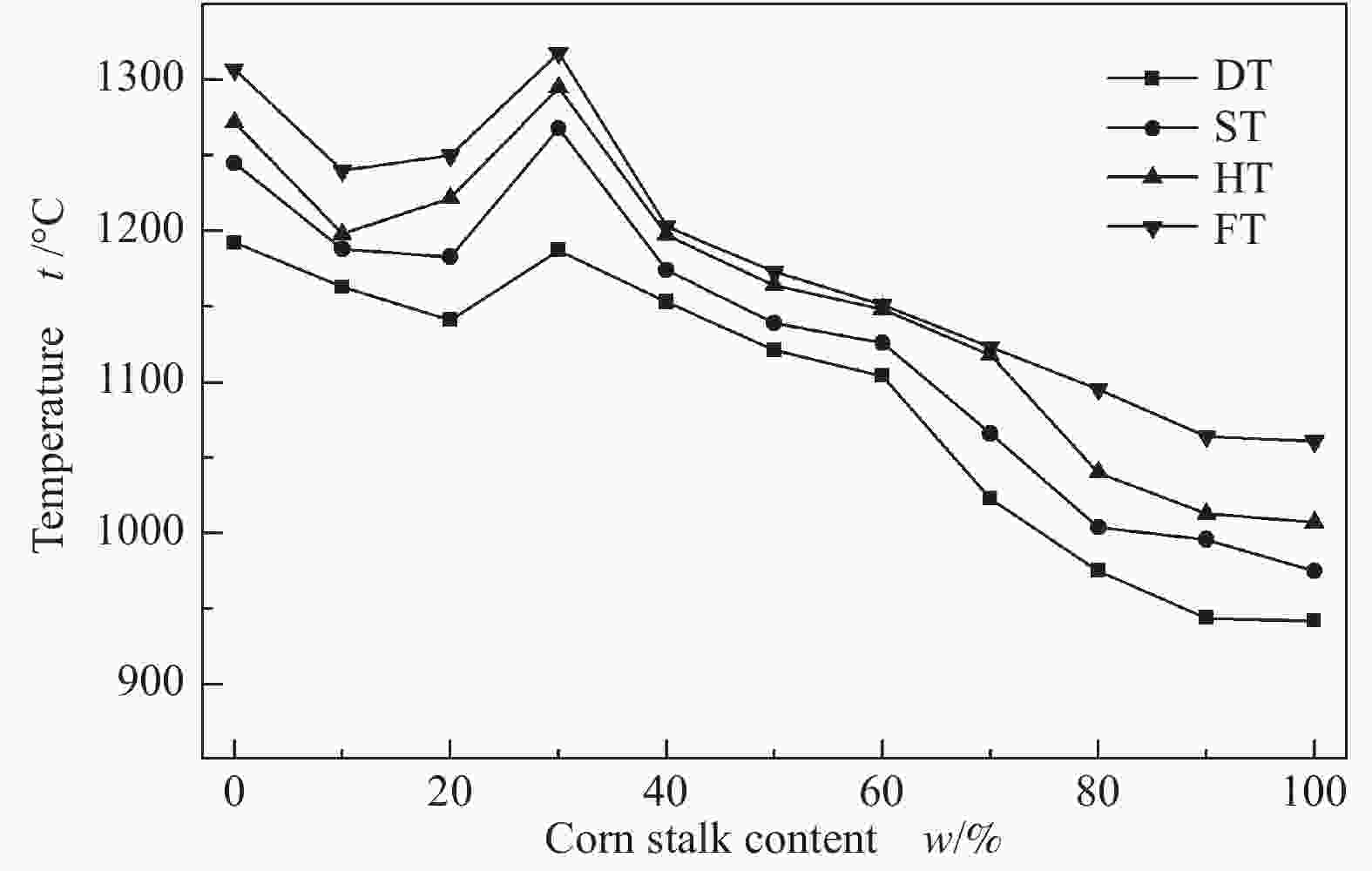
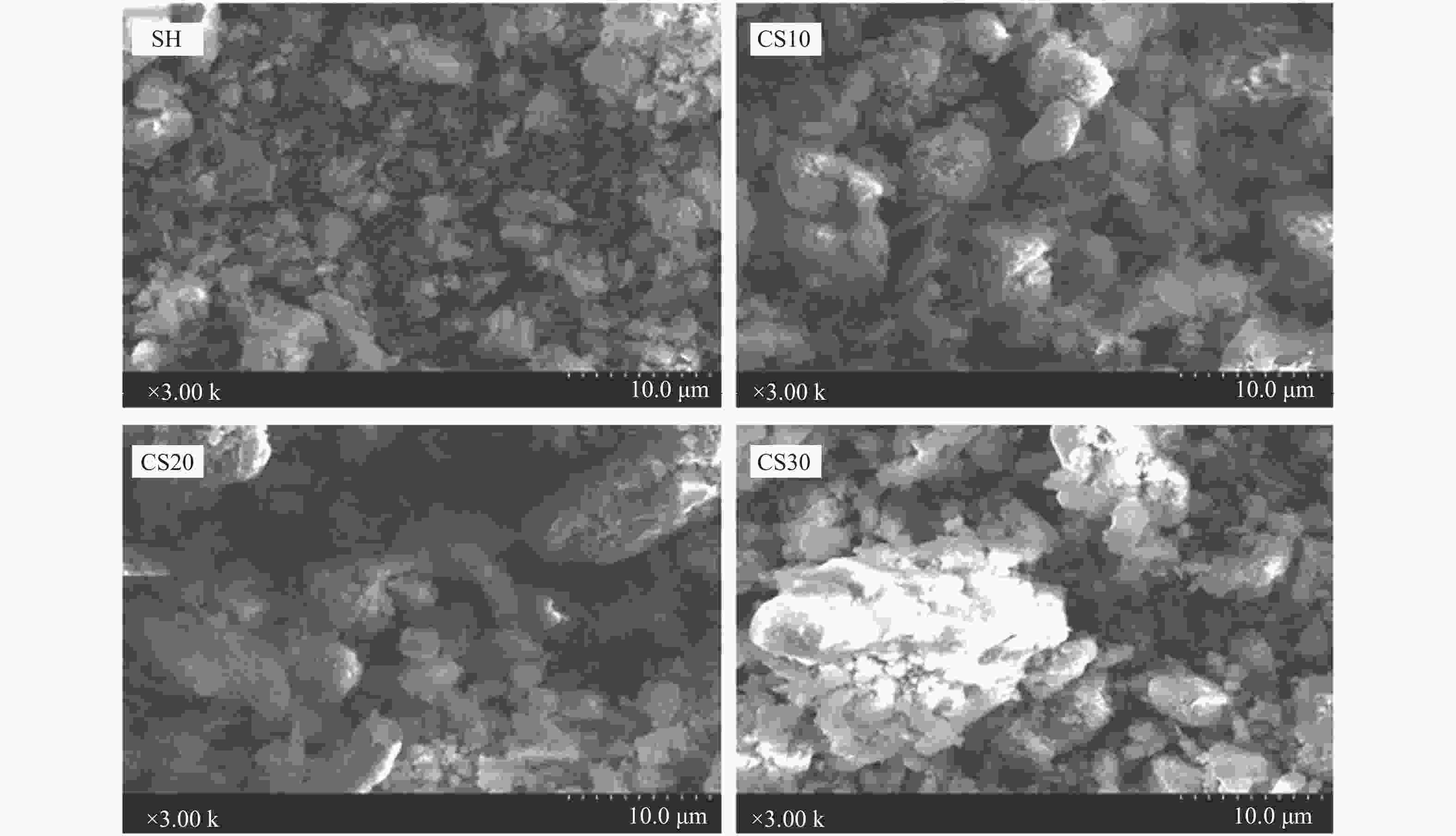
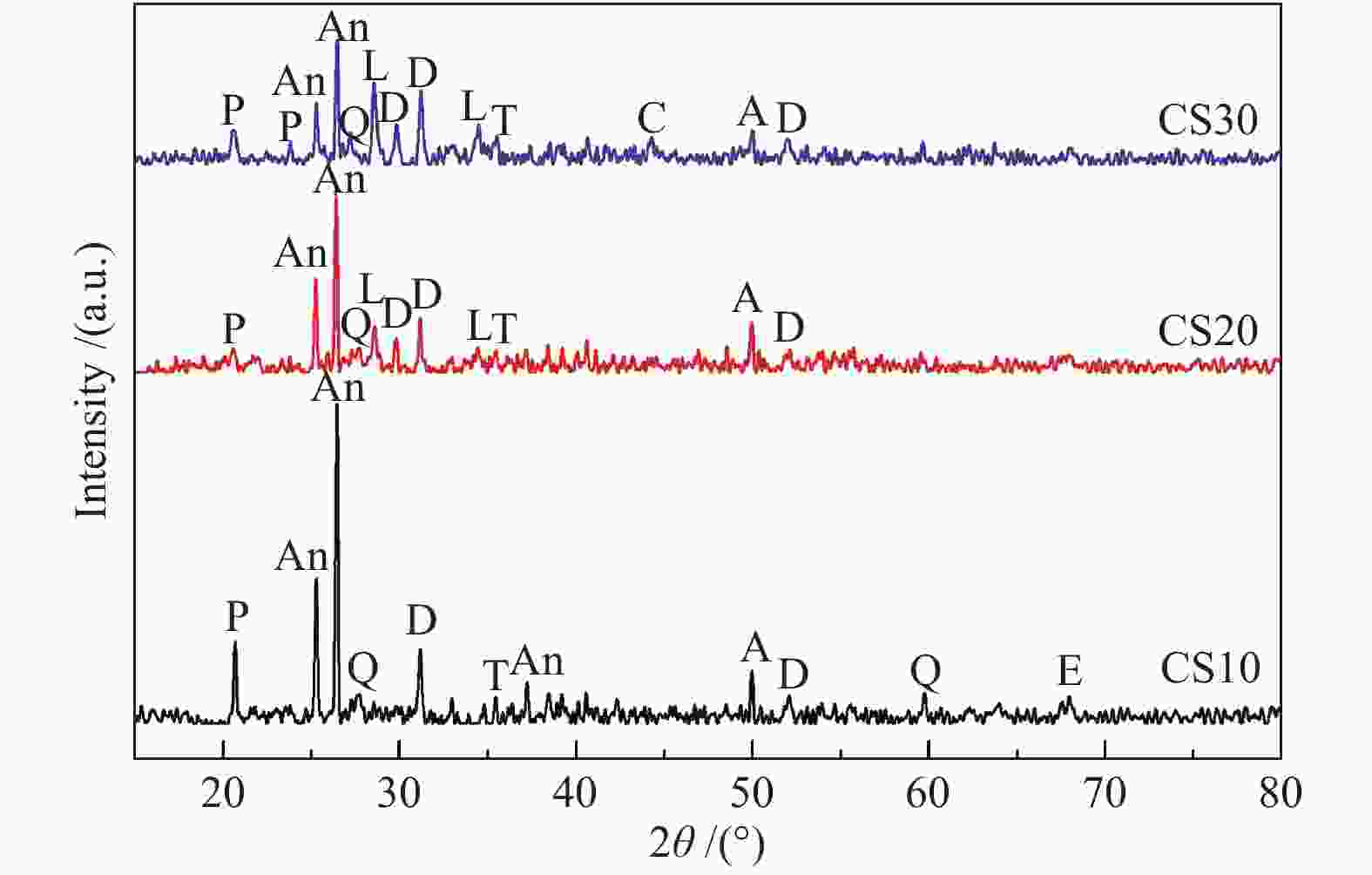
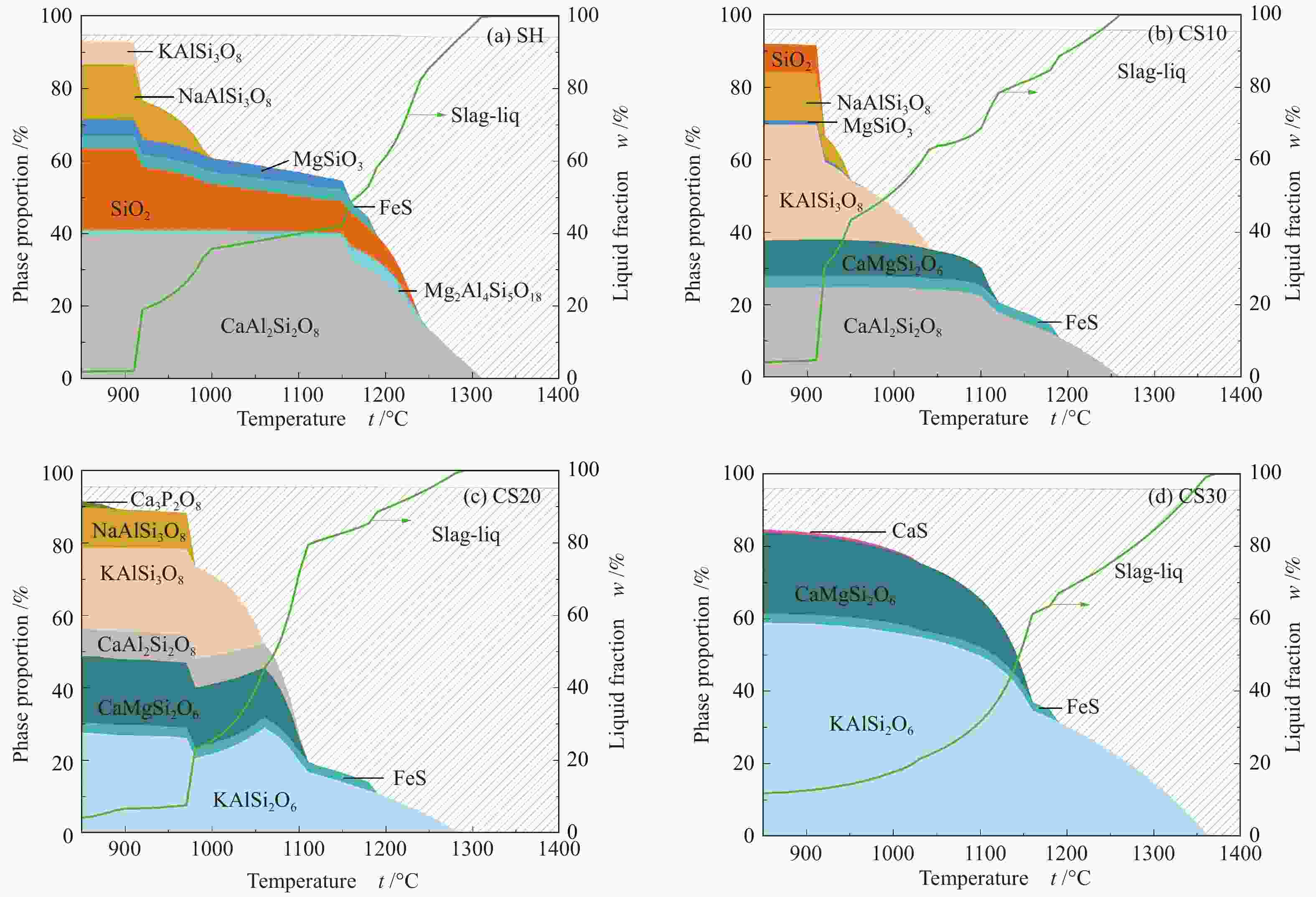
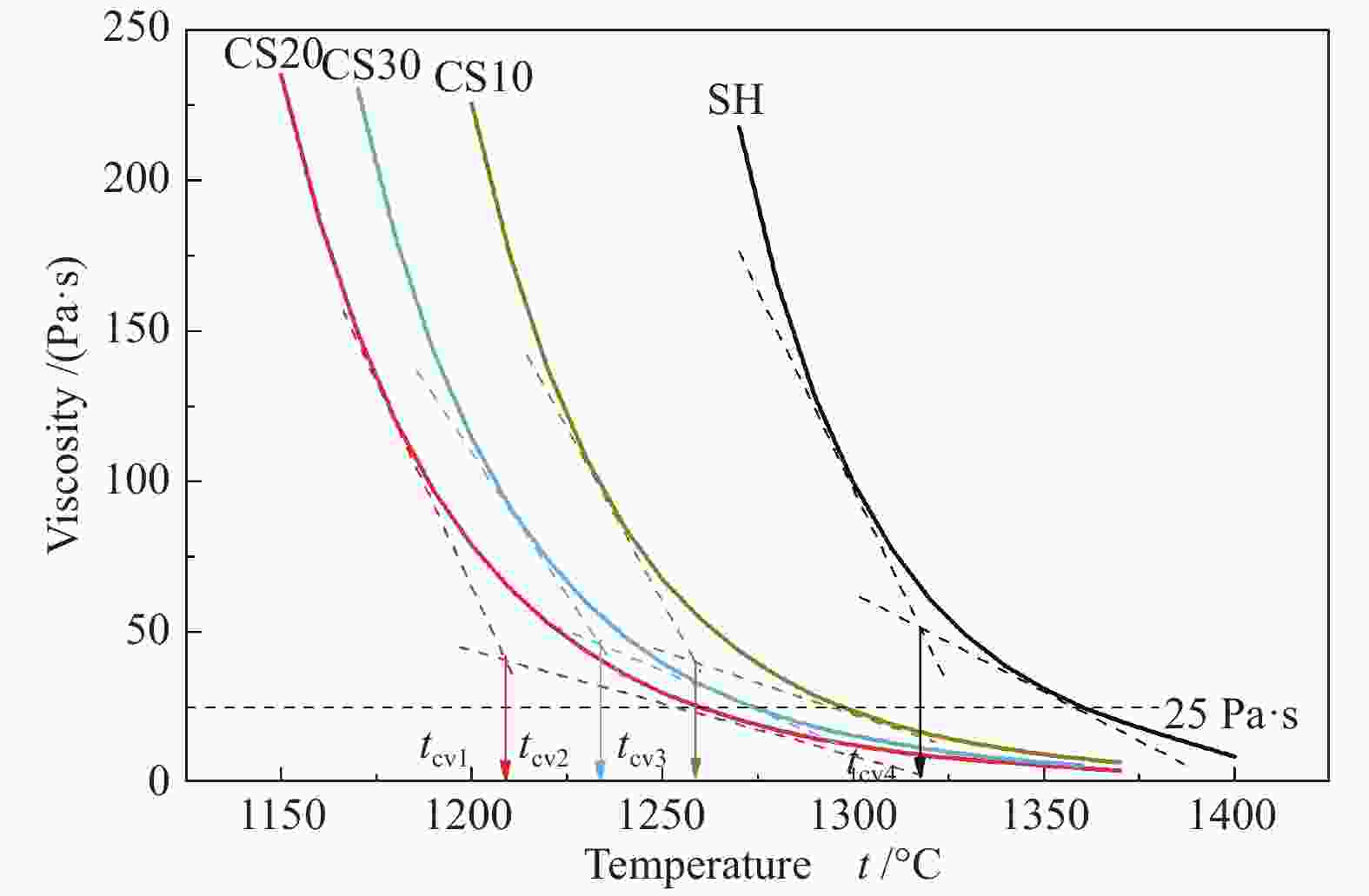
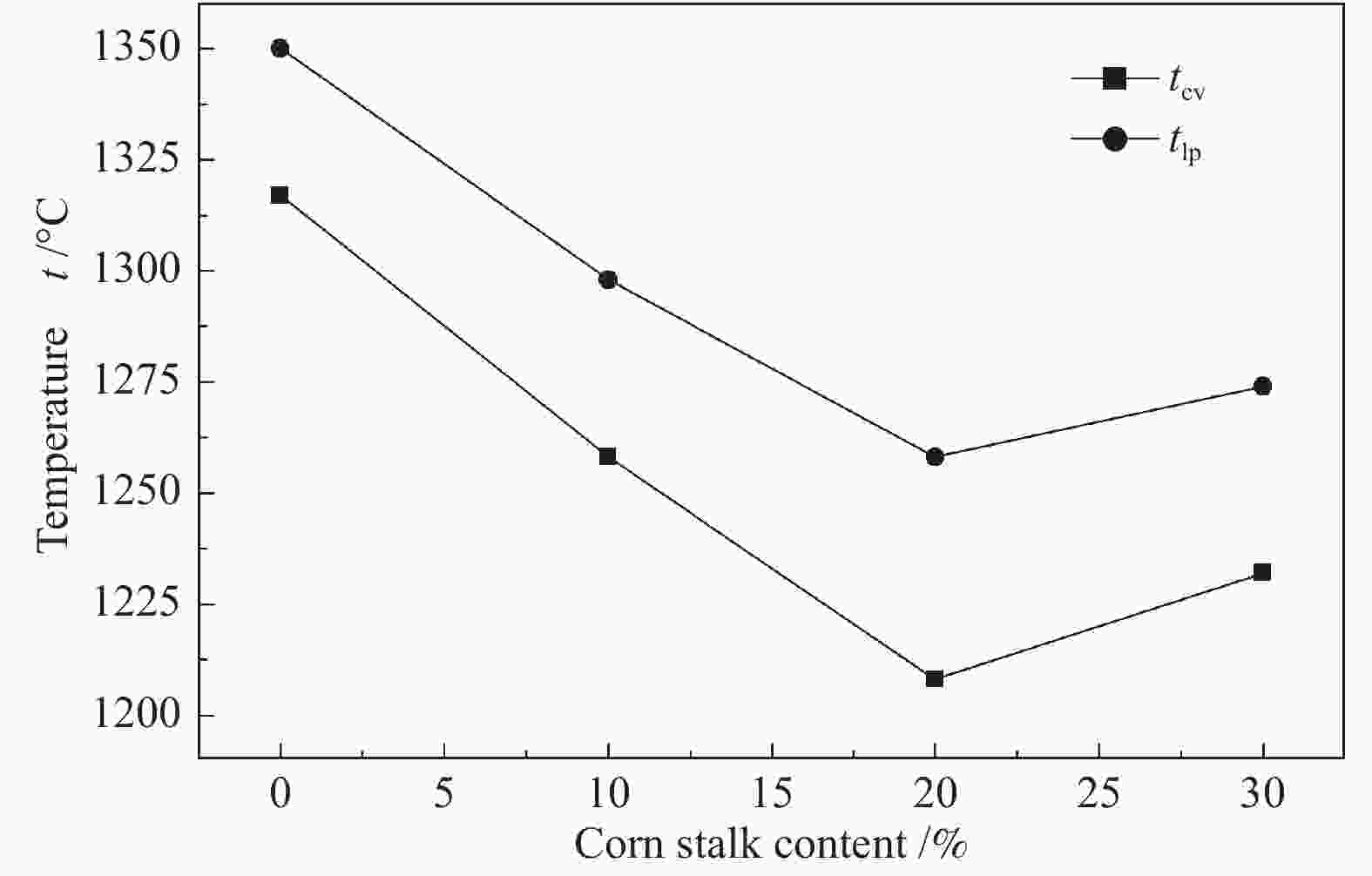
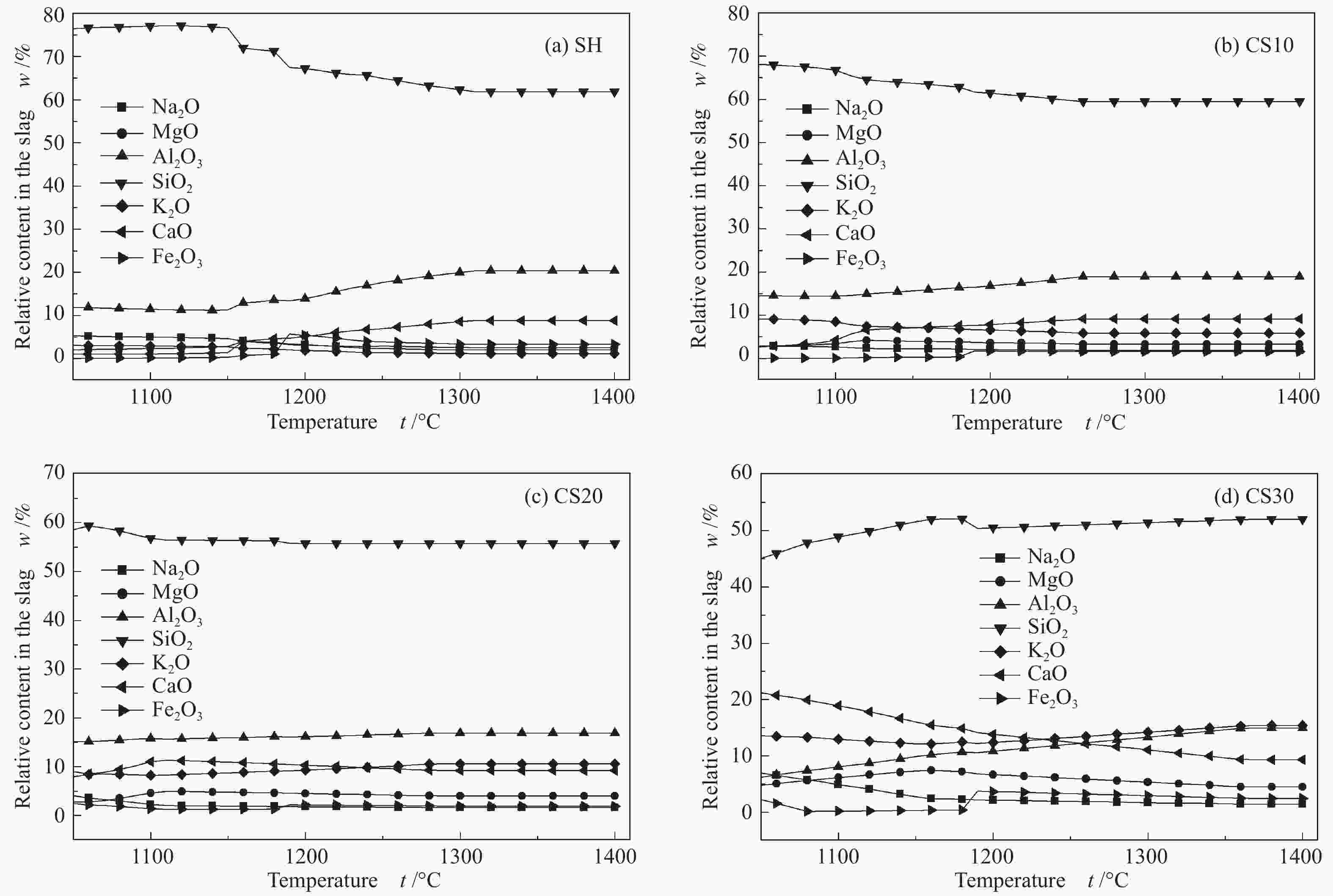
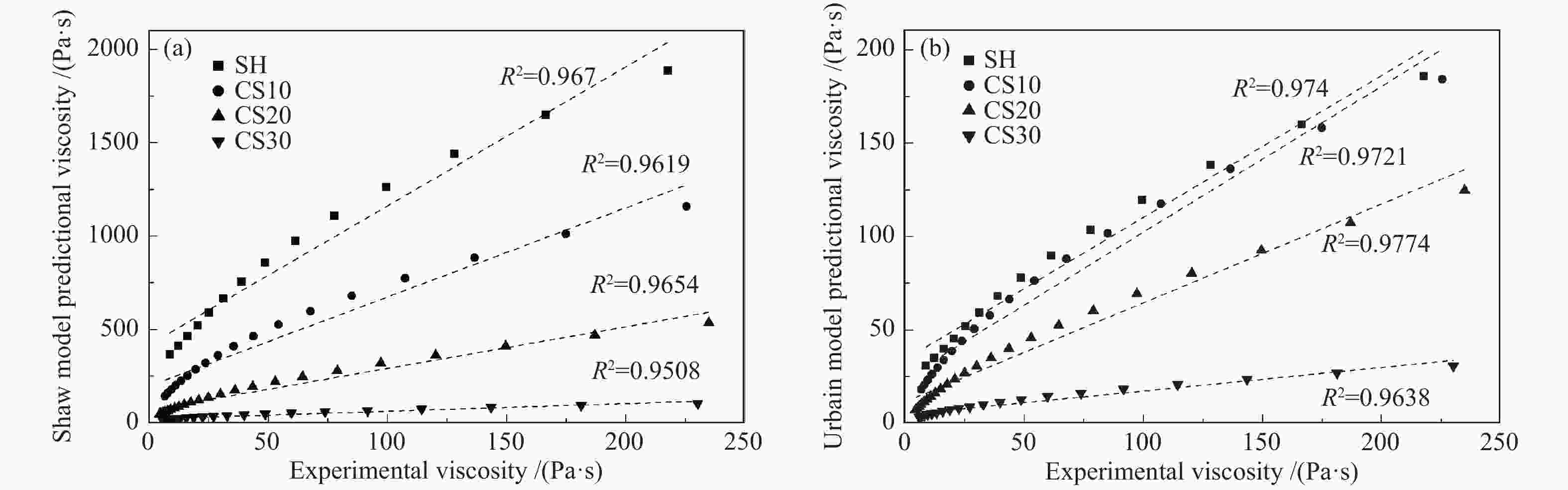
摘要: 以神华烟煤和玉米秸秆为实验原料,研究弱还原性气氛下生物质掺混量对神华烟煤的灰熔融特性和黏温特性的影响。利用XRD和SEM对灰渣的矿物质组成和微观形貌进行检测和表征。并利用热力学软件FactSage对不同温度下灰渣的物相及矿物质转化过程进行模拟计算。结果表明,随着玉米秸秆掺混量的增加,灰渣中高熔点的石英、钙长石和堇青石的含量降低,低熔点的钾长石含量增加,在玉米秸秆掺混量为20%(质量分数)时,灰渣的临界黏度温度(tcv)和最低操作温度(tlp)降到最低,此时灰渣的黏度最低,温度升高至1255 ℃时黏度值小于25 Pa·s,满足气化炉的液态排渣要求。结合Urbain均相模型和Einstein-Roscoe非均相模型,以及FactSage软件计算的不同温度下的液相含量得出适合玉米秸秆和神华烟煤混合灰渣的黏度预测经验公式。
-
关键词:
- 烟煤与生物质混合燃料 /
- 气化 /
- 灰渣 /
- 熔融特性 /
- 黏温特性
Abstract: Shenhua bituminous coal and corn stalks were selected as experimental materials, and the effects of biomass blending ratio on the ash melting characteristics and viscosity-temperature characteristics of Shenhua bituminous ash in a weakly reducing atmosphere were studied. The mineral composition and micro-morphology of ash were detectd by XRD and SEM, and the phase and mineral transformation of ash at different temperatures were simulated by thermodynamic software FactSage. The results indicate that with the increase in corn stalks addition, the contents of high melting point quartz, anorthite and cordierite in ash decrease, and the content of low melting point potassium feldspar increases. Besides, the critical viscosity temperature (tcv), the lowest operation temperature (tlp) of ash and the viscosity of slag reach to the minimum values as the blending ratio of corn stalk to Shenhua coal is 20%. When the temperature arrives at 1255 ℃, the value of viscosity is less than 25 Pa·s, which can meet the liquid slag discharging requirements of the gasifier. Combining the Urbain homogeneous model with the Einstein-Roscoe heterogeneous model and using the FactSage software to calculate the liquid phase content at different temperatures, an empirical formula for the viscosity prediction of the corn stalks and Shenhua bituminous coal mixed gasification slag has been obtained. -
表 1 神华烟煤和玉米秸秆的工业分析
Table 1 Proximate analysis of Shenhua bituminous coal and corn stalk
Sample Proximate analysis wad/% M A V FC SH 10.21 11.83 31.91 46.05 CS 9.21 5.08 66.42 19.29 表 2 烟煤、玉米秸秆及其混合灰的成分分析
Table 2 Chemical compositions of Shenhua bituminous coal ash, corn stalk ash and blending ashes
Sample Content w/% Na2O MgO Al2O3 SiO2 P2O5 SO3 K2O CaO Fe2O3 SH 1.86 2.34 19.12 58.03 0.50 5.66 1.09 8.29 3.11 CS10 1.69 3.02 17.37 54.72 1.11 5.47 5.40 8.38 2.84 CS20 1.51 3.71 15.59 51.35 1.74 5.27 9.78 8.47 2.57 CS30 1.33 4.41 13.79 47.95 2.37 5.08 14.20 8.57 2.29 CS40 1.15 5.13 11.95 44.46 3.02 4.88 18.75 8.66 2.01 CS50 0.97 5.85 10.09 40.94 3.67 4.67 23.33 8.76 1.72 CS60 0.78 6.59 8.19 37.35 4.34 4.46 28.00 8.86 1.43 CS70 0.59 7.34 6.27 33.71 5.01 4.25 32.74 8.96 1.13 CS80 0.40 8.10 4.31 30.01 5.70 4.04 37.55 9.06 0.83 CS90 0.20 8.87 2.32 26.24 6.40 3.82 42.45 9.16 0.52 CS 0 9.66 0.30 22.42 7.11 3.60 47.43 9.27 0.21 表 3 牛顿型流体模型类型和适用范围
Table 3 Newtonian fluid model types and applications
Model type Applicable conditions Reid Slags containing more than 3% MgO and less than 5% CaO, or where the alkalies exceed 2.5% S2 SiO2: 31%−59%; Al2O3: 19%−37%; Fe2O3: 0−38%; CaO: 1%−37%; MgO: 1%−12%; Na2O+K2O: 1%−6%; silica ratio: 45−75; SiO2/Al2O3: 1.2−2.3 Watt SiO2: 30%−60%; Al2O3: 15%−35%; Fe2O3: 3%−30%; CaO: 2%−30%; MgO: 1%−10%; silica ratio: 40−80; SiO2/Al2O3: 1.4−2.4 Shaw any system Lakatos SiO2: 0.61−0.77; Al2O3: 0−0.05; CaO: 0.09−0.14; MgO: 0−0.10; Na2O: 0.10−0.15; K2O: 0−0.06 (ratio is molar) Urbain SiO2-Al2O3-MO and SiO2-Al2O3-M2O mixtures (where MO and M2O represent divalent and monovalent oxides respectively) Riboud SiO2: 27%−56%; Al2O3: 0−12%; CaO: 8%−46%; Na2O: 0−22%; CaF2: 0−18% -
[1] CROMPTON P, WU Y. Energy consumption in China: Past trends and future directions[J]. Energy Econ,2005,27(1):195−208. [2] 乌晓江, 张忠孝, 朴桂林, 小林信介, 森滋勝, 板谷義紀. 高灰熔点煤加压气流床气化特性[J]. 燃烧科学与技术,2009,15(2):182−186. doi: 10.3321/j.issn:1006-8740.2009.02.017WU Xiao-jiang, ZHANG Zhong-xiao, PIAO Gui-lin, KOBAYASHI Nobusuke, MORI Shigekatsu, ITATYA Yoshinori. Gasification characteristics of coal with high ash fusion temperature in lab-scale down-flow gasifier[J]. J Combust Sci Technol,2009,15(2):182−186. doi: 10.3321/j.issn:1006-8740.2009.02.017 [3] 金泽华, 胡瑞生, 龚雪, 胡佳楠, 杜娟, 刘树森, 傅德慧, 李应彤, 李强. 现代煤化工用煤技术条件标准体系分析与思考[J]. 煤化工,2016,44(1):19−22. doi: 10.3969/j.issn.1005-9598.2016.01.006JIN Ze-hua, HU Rui-sheng, GONG Xue, HU Jia-nan, DU Juan, LIU Shu-sen, FU De-hui, LI Ying-tong, LI Qiang. Analysis and consideration of the standardization system of coal utilization technical conditions in the modern coal chemical industry[J]. Coal Chem Ind,2016,44(1):19−22. doi: 10.3969/j.issn.1005-9598.2016.01.006 [4] 韩广怡, 朱治平, 张海霞. 配煤比例及温度对气化反应特性影响[J]. 化学工程,2018,46(4):64−68.HAN Guang-yi, ZHU Zhi-ping, ZHANG Hai-xia. Effect of coal blending ratio and temperature on gasification characteristics[J]. Chem Eng,2018,46(4):64−68. [5] 夏光璧, 朴桂林, 张居兵, 谢浩, 李帅丹. 掺混生物质炭对煤气化反应特性的影响[J]. 动力工程学报,2015,35(8):681−686. doi: 10.3969/j.issn.1674-7607.2015.08.012XIA Guang-bi, PIAO Gui-lin, ZHANG Ju-bing, XIE Hao, LI Shuai. Research on co-gasification characteristics of biomass char and coal[J]. J Chin Soc Power Eng,2015,35(8):681−686. doi: 10.3969/j.issn.1674-7607.2015.08.012 [6] 刘涛, 陈雪莉, 李德侠, 刘霞, 梁钦锋. 生物质与煤混合灰的熔融及黏温特性[J]. 化工学报,2012,63(4):1217−1225. doi: 10.3969/j.issn.0438-1157.2012.04.032LIU Tao, CHEN Xue-li, LI De-xia, LIU Xia, LIANG Qin-feng. Blending ash fusion and viscosity-temperature characteristics of biomass and coal[J]. J Chem Ind Eng,2012,63(4):1217−1225. doi: 10.3969/j.issn.0438-1157.2012.04.032 [7] SEEBOLD S, WU G, MÜLLER M. The influence of crystallization on the flow of coal ash-slags[J]. Fuel,2017,187:376−387. [8] 陈晓东, 孔令学, 白进, 白宗庆, 李文. 高温气化条件下Na2O对煤灰中矿物质演化行为的影响[J]. 燃料化学学报,2016,44(3):263−272. doi: 10.3969/j.issn.0253-2409.2016.03.002CHEN Xiao-dong, KONG Ling-xue, BAI Jin, BAI Zong-qing, LI Wen. Effect of Na2O on mineral transformation of coal ash under high temperature gasification condition[J]. J Fuel Chem Technol,2016,44(3):263−272. doi: 10.3969/j.issn.0253-2409.2016.03.002 [9] 周永刚, 范建勇, 李培, 王炳辉, 赵虹. 高碱金属准东煤灰熔融过程的矿物质衍变[J]. 浙江大学学报(工学版),2015,49(8):1559−1564.ZHOU Yong-gang, FAN Jian-yong, LI Pei, WANG Bing-hui, ZHAO Hong. Mineral transmutation of high alkali zhundong coal in ash melting process[J]. J Zhejiang Univ Eng Sci,2015,49(8):1559−1564. [10] 许洁, 李帅丹, 刘帅君. 典型秸秆类生物质灰的特性研究[J]. 热能动力工程,2019,34(12):137−154.XU Jie, LI Shuai-dan, LIU Shuai-jun. Investigation on the ash properties of straw[J]. J Eng Therm Energy Pow,2019,34(12):137−154. [11] JING N, WANG Q, CHENG L, LUO Z, CEN K, ZHANG D. Effect of temperature and pressure on the mineralogical and fusion characteristics of jincheng coal ash in simulated combustion and gasification environments[J]. Fuel,2013,104:647−655. [12] VORRES K S. Mineral Matter and Ash in Coal[M]. Washington DC: American Chemical Society Symposium Series, 1986: 25-28. [13] WEI B, WANG X, TAN H, ZHANG L, WANG Y, WANG Z. Effect of silicon-aluminum additives on ash fusion and ash mineral conversion of xinjiang high-sodium coal[J]. Fuel,2016,181:1224−1229. [14] ILYUSHECHKIN A Y, HLA S S, CHEN X, ROBERTS D G. Effect of sodium in brown coal ash transformations and slagging behaviour under gasification conditions[J]. Fuel Process Technol,2018,179:86−98. [15] 高娜, 刘胜华, 刘勇晶, 郭延红. 碱性氧化物对煤灰熔融特性的影响及机理研究[J]. 煤炭转化,2014,37(3):42−45. doi: 10.3969/j.issn.1004-4248.2014.03.010GAO Na, LIU Sheng-hua, LIU Yong-jing, GUO Yan-hong. Effect of alkali oxides on ash melting characteristic[J]. Coal Convers,2014,37(3):42−45. doi: 10.3969/j.issn.1004-4248.2014.03.010 [16] 秦育红, 韩晴晴, 任伟平, 赵子兵, 冯杰, 李文英, 高松平. 弱还原气氛下水稻秸秆对晋城无烟煤的助熔机理[J]. 燃料化学学报,2016,44(12):1440−1446. doi: 10.3969/j.issn.0253-2409.2016.12.005QIN Yu-hong, HAN Qing-qing, REN Wei-ping, ZHAO Zi-bing, FENG Jie, LI Wen-ying, GAO Song-ping. Fluxing mechanism of rice straw for jincheng anthracite under weak reducing atmosphere[J]. J Fuel Chem Technol,2016,44(12):1440−1446. doi: 10.3969/j.issn.0253-2409.2016.12.005 [17] XIONG Q, LI J, GUO S, LI G, ZHAO J, FANG Y. Ash fusion characteristics during co-gasification of biomass and petroleum coke[J]. Bioresour Technol,2018,257:1−6. [18] ZHANG S, ZHANG X, LIU W, LV X, BAI C, WANG L. Relationship between structure and viscosity of CaO-SiO2-Al2O3-MgO-TiO2 slag[J]. J Non-Cryst Solids,2014,402:214−222. [19] ROSCOE R. The viscosity of suspensions of rigid spheres[J]. British J App Phys,1952,3(8):267−269. [20] VARGAS S, FRANDSEN F J, DAM-JOHANSEN K. Rheological properties of high-temperature melts of coal ashes and other silicates[J]. Prog Energy Combust,2001,27(3):237−429. -





 下载:
下载: