Performance of Ce-modified CuZnAl catalyst in the dehydrogenation of sec-butanol to methyl ethyl ketone
-
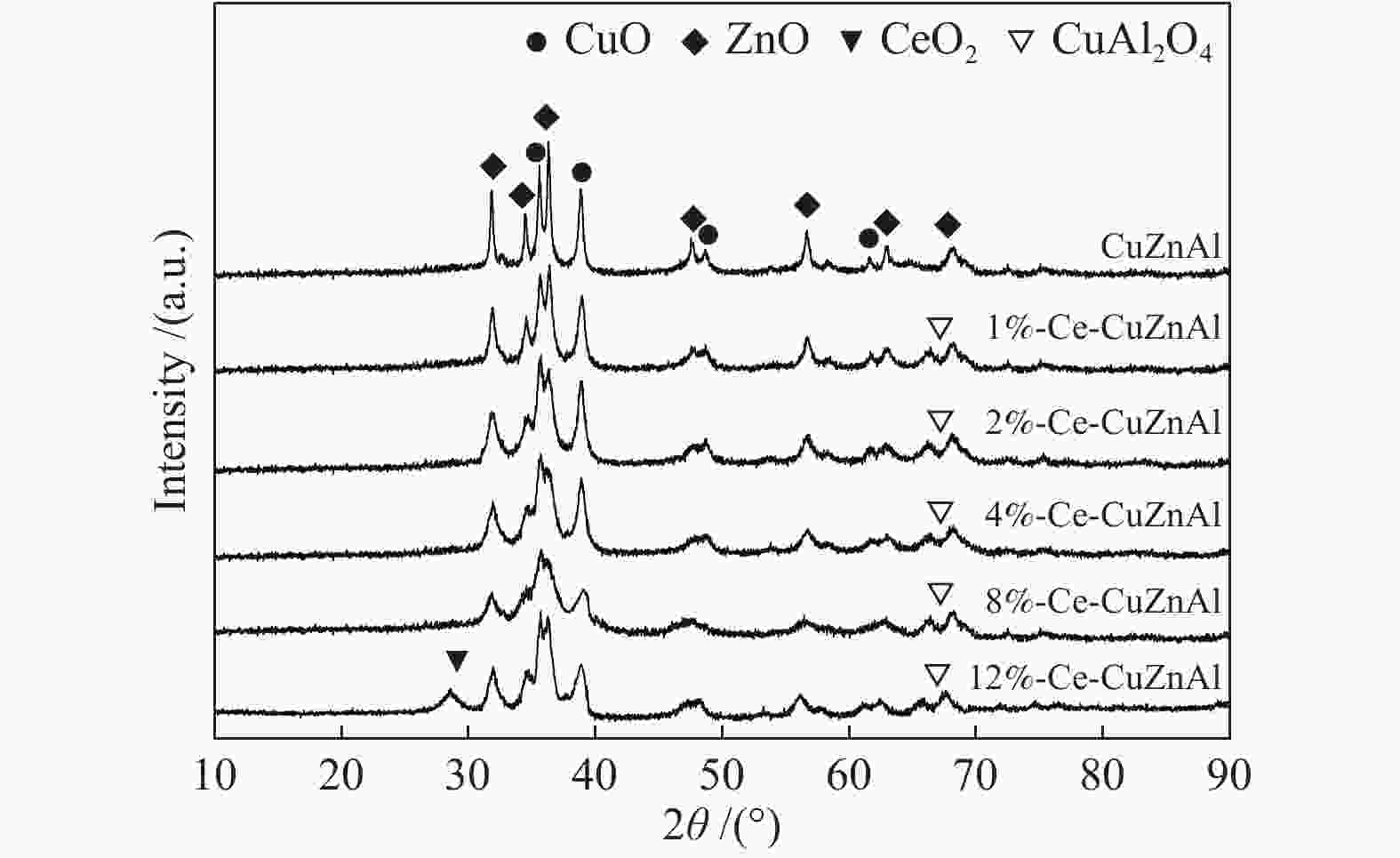
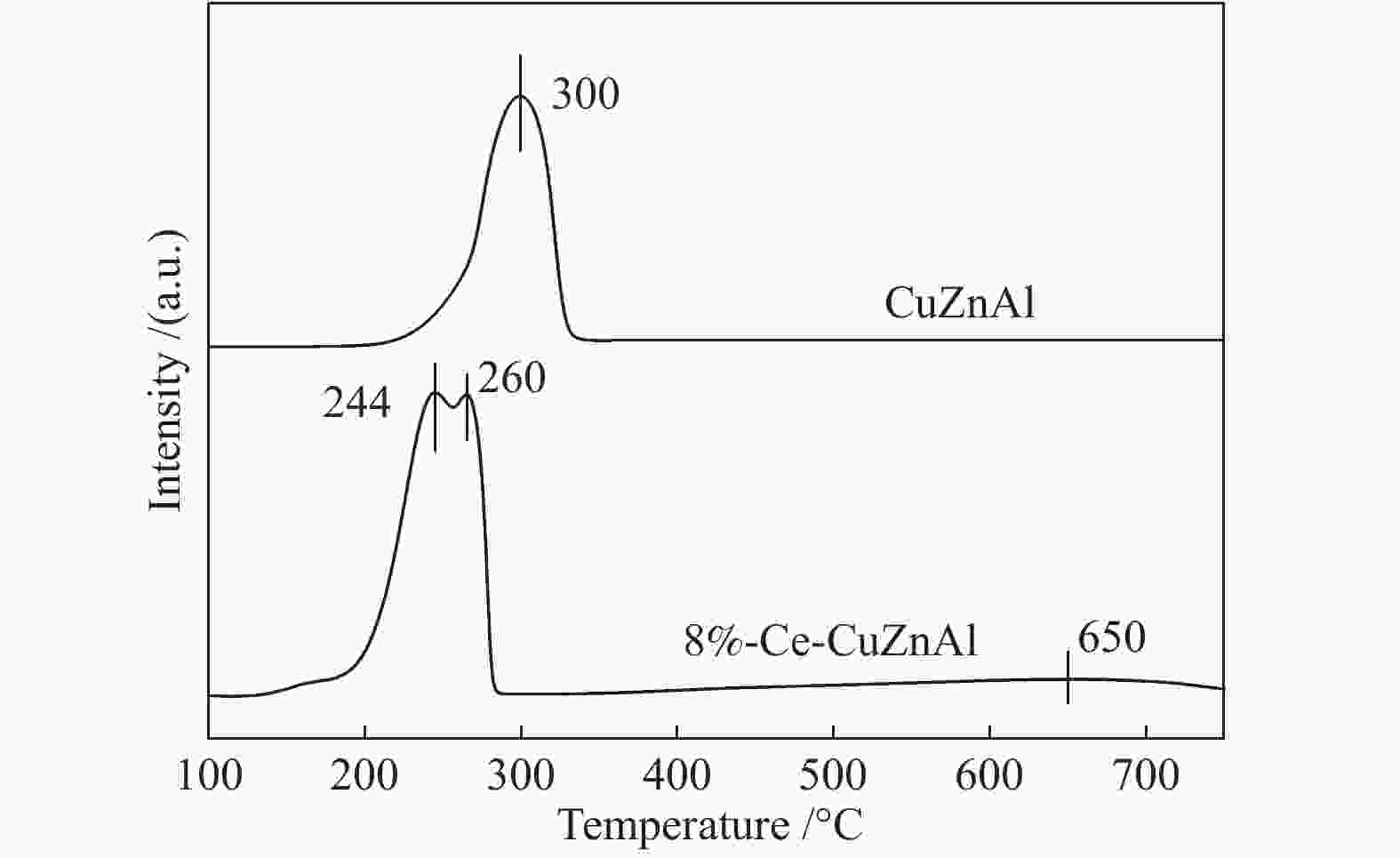
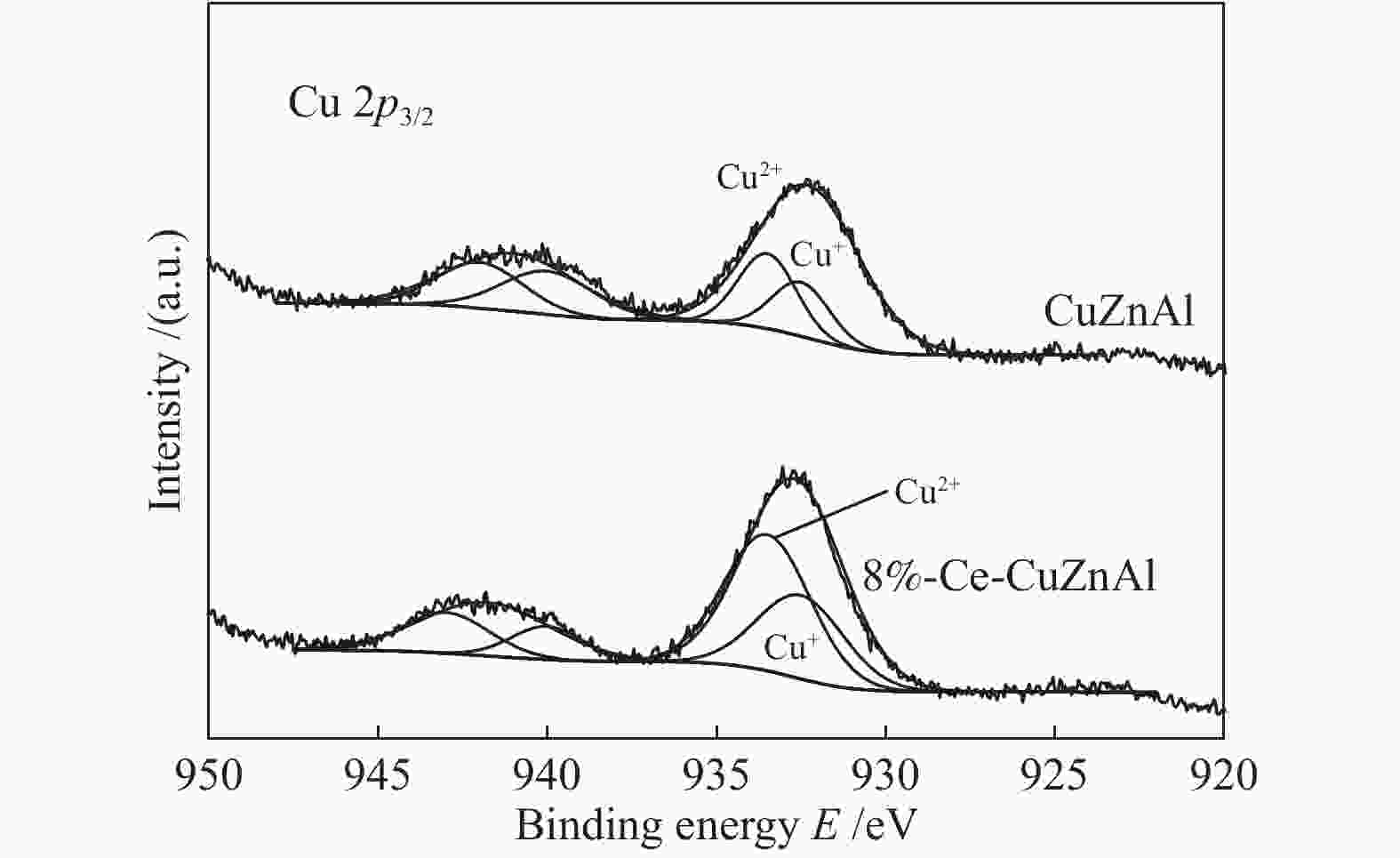
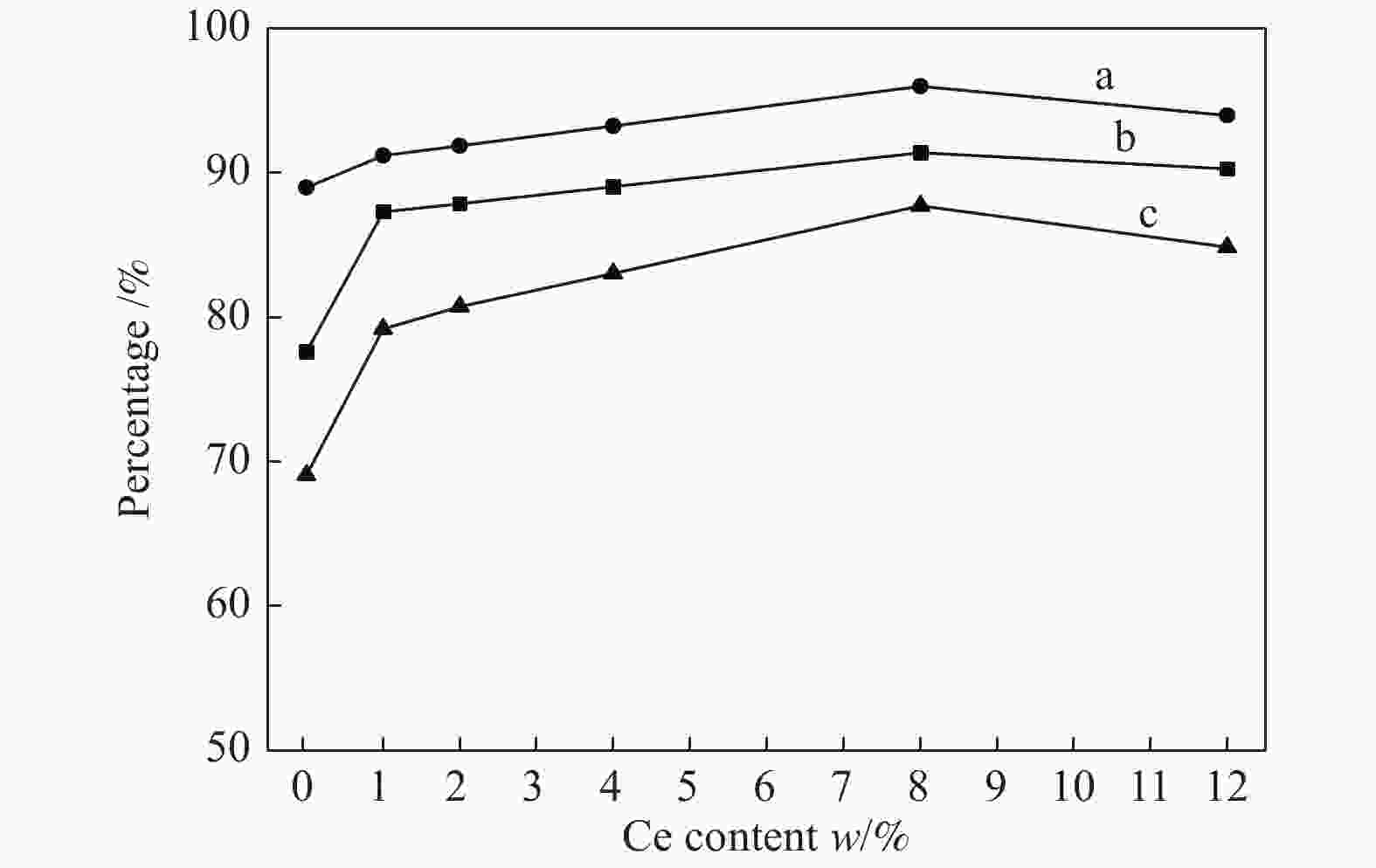
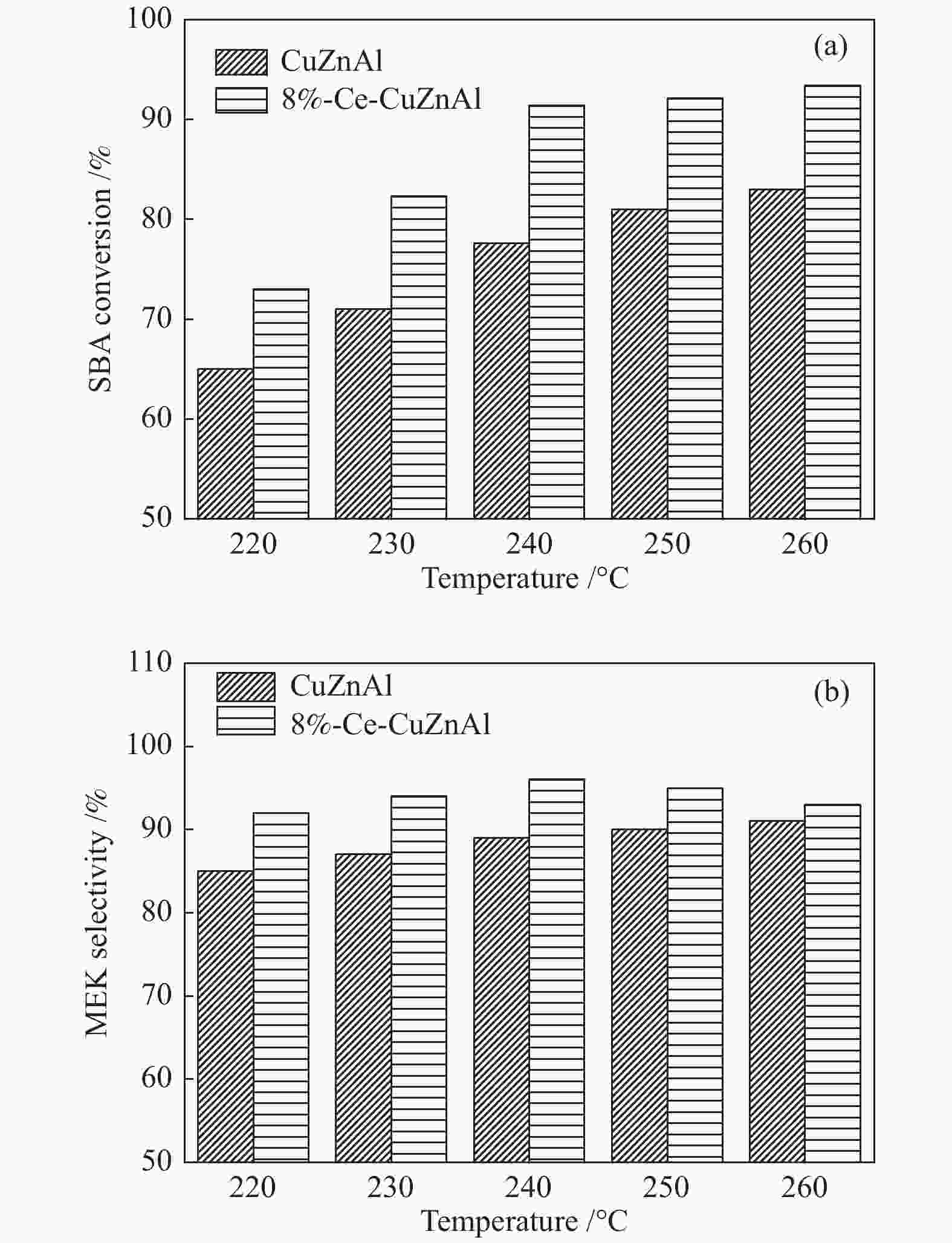
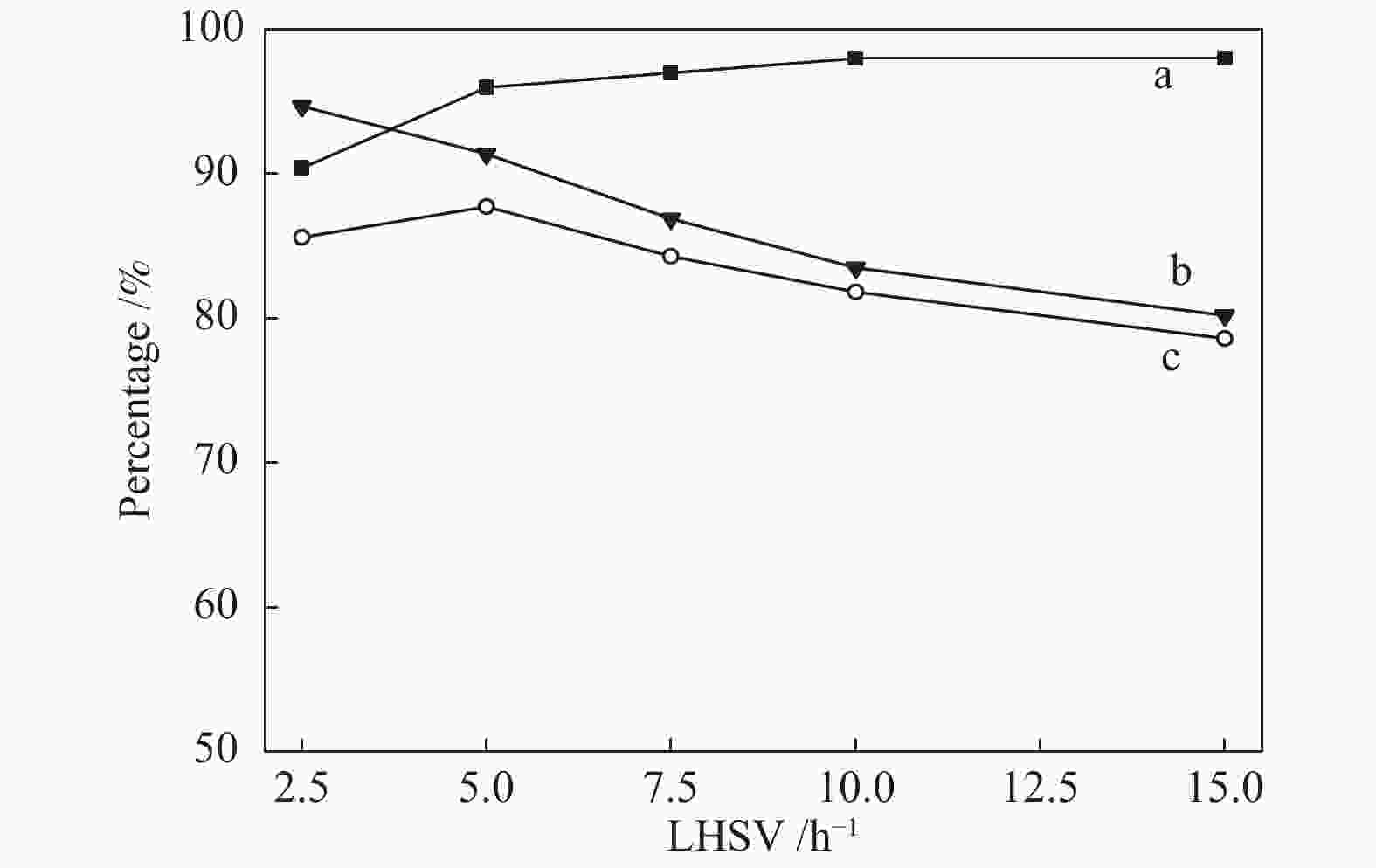
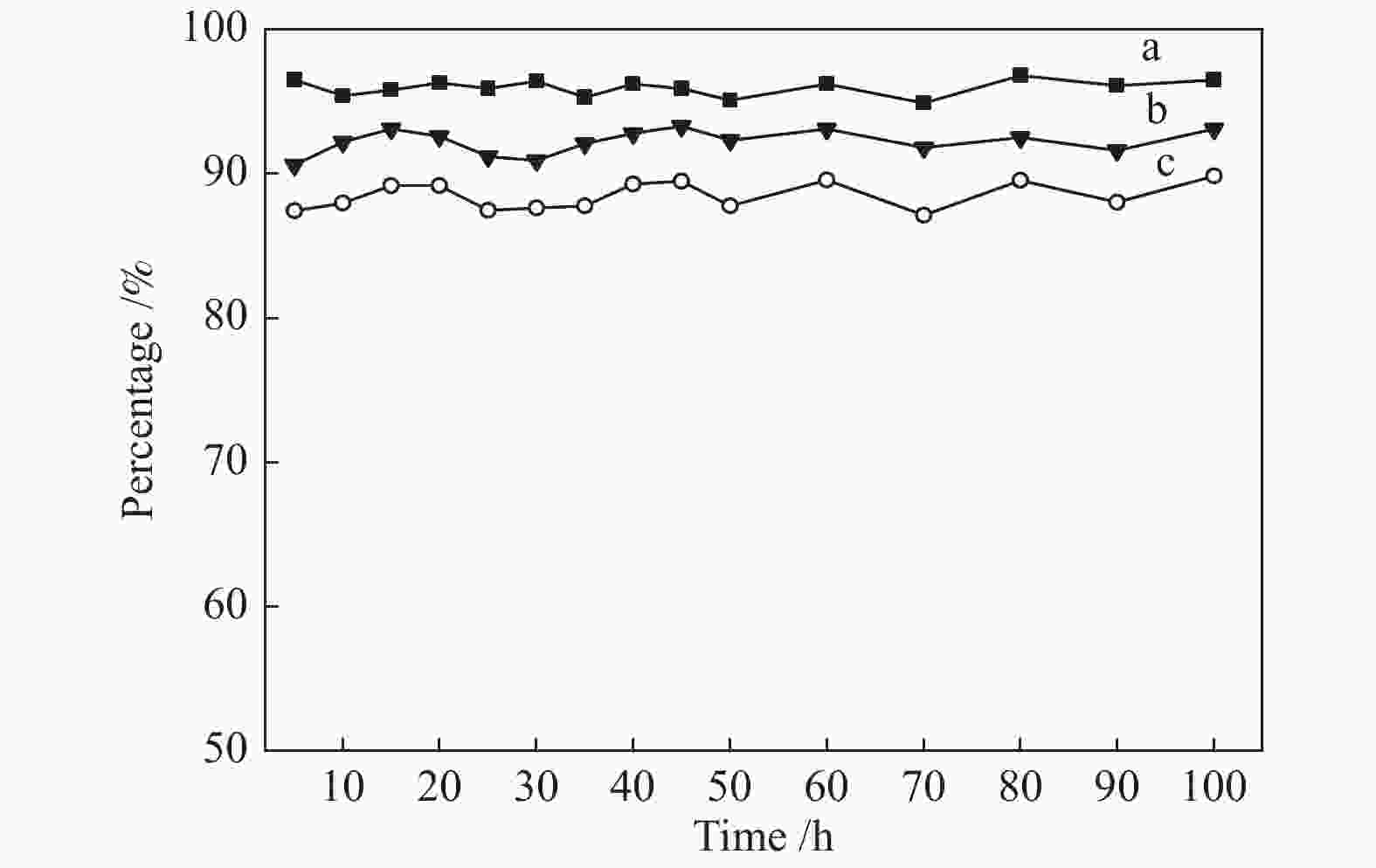
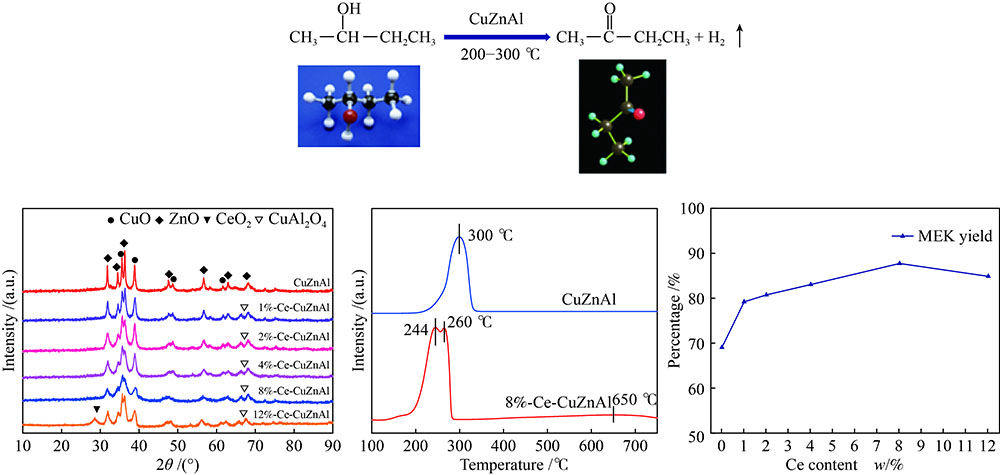
摘要: 采用共沉淀法制备了CuZnAl催化剂,并通过浸渍法将不同含量Ce引入到CuZnAl催化剂中,将其用于仲丁醇(SBA)脱氢制甲乙酮(MEK),研究了Ce改性对其催化性能的影响。结果表明,Ce的引入可以促使CuZnAl催化剂中Zn物种和Al物种发生反应,生成尖晶石(CuAl2O4)结构,有利于提高其催化稳定性;同时引入Ce能降低催化剂晶粒尺寸、提高CuO和ZnO的分散度,并使催化剂中Cu2+含量提高、还原温度降低、还原后催化剂中活性组分Cu0含量增加。Ce改性后的8%-Ce-CuZnAl催化剂对仲丁醇脱氢具有良好的活性,在240 °C、质量空速为5 h−1的条件下,SBA转化率达91.4%,MEK收率为87.74%;而且在100 h内催化活性稳定,SBA转化率保持在92%左右、MEK收率保持在88%左右。Abstract: The CuZnAl catalyst prepared by co-precipitation was further modified with different contents of Ce through impregnation and used in the dehydrogenation of sec-butanol (SBA) to methyl ethyl ketone (MEK); the effect of Ce modification on the performance of CuZnAl catalyst was investigated. The results illustrate that the introduction of Ce in CuZnAl can promote the formation of CuAl2O4 spinel and thus improve the stability of Ce-modified CuZnAl catalyst; meanwhile, Ce is also conducive to reducing the grain size, enhancing the dispersion of CuO and ZnO, lowering the reduction temperature, and increasing the content of Cu2+ and consequently the content of active Cu0 species upon reduction. Over the Ce-modified 8%-Ce-CuZnAl catalyst, the conversion of SBA reaches 91.4% under 240 °C and a mass space velocity of 5 h−1, with a selectivity of 96% to MEK; during the 100 h reaction test, the SBA conversion keeps at about 92%, with the selectivity to MEK at about 96%, demonstrating excellent stability of the Ce-modified CuZnAl catalyst.
-
Key words:
- CuZnAl catalyst /
- dehydrogenation /
- sec-butanol /
- methyl ethyl ketone /
- Ce modification
-
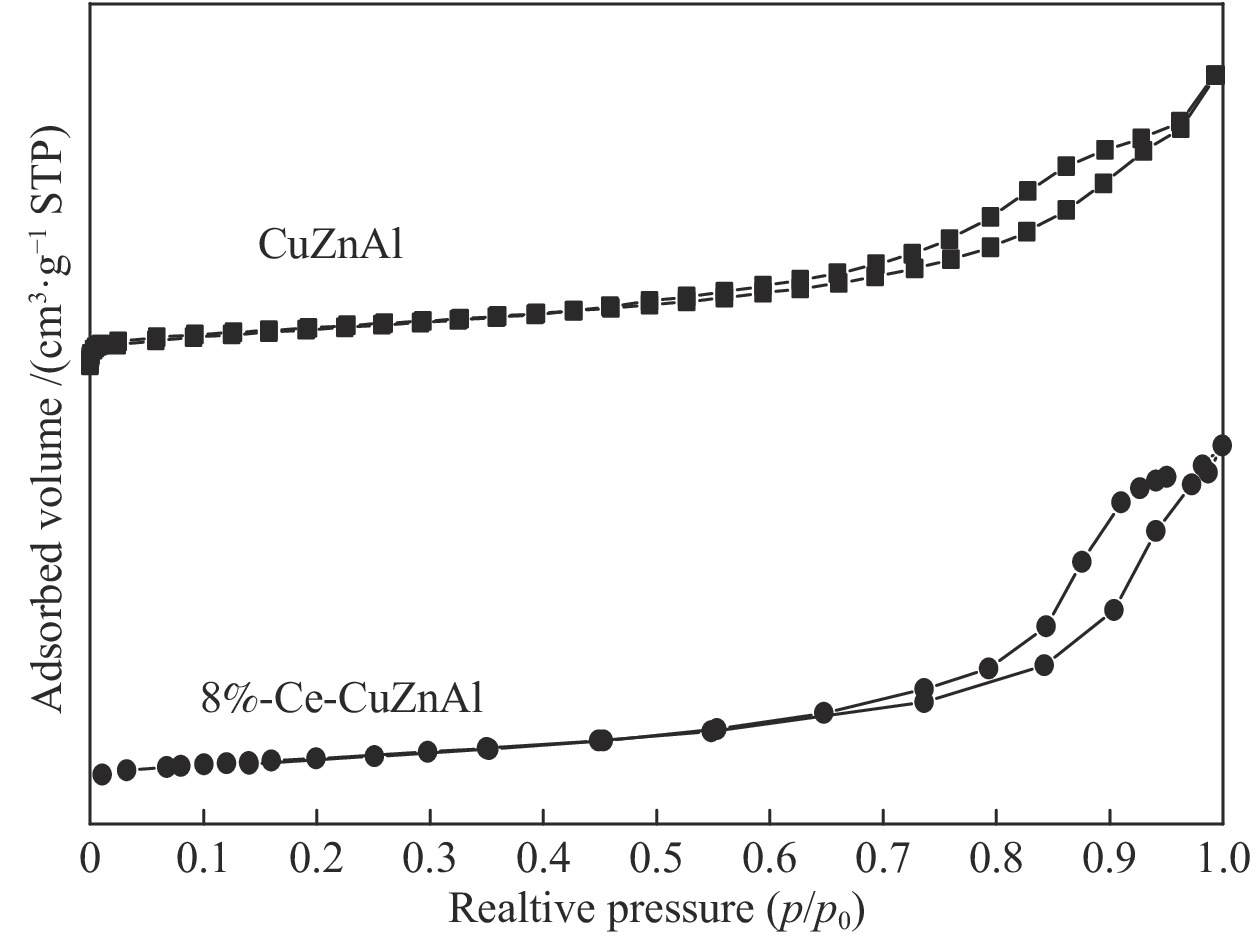
表 1 CuZnAl and 8%-Ce-CuZnAl催化剂的结构参数
Table 1 Structural parameters of CuZnAl and 8%-Ce-CuZnAl catalysts
Sample Pore diameter
d/nmPore volume
v/(cm3·g−1)ABET/
(m2·g−1)CuZnAl 7 0.200 66 8%-Ce-CuZnAl 12 0.241 79 表 2 CuZnAl和Ce-CuZnAl催化剂的表面元素组成
Table 2 Surface element composition of the CuZnAl and Ce-CuZnAl catalysts
Catalyst Element composition w/% Cu Zn Al CuZnAl 7.65 6.32 23.11 8%-Ce-CuZnAl 7.12 5.86 22.46 -
[1] FANG D R, REN W Z, LIU Z G, XU X F, XU L, LU H Y, LIAO W P, ZHANG H M. Synthesis and applications of mesoporous Cu-Zn-Al2O3 catalyst for dehydrogenation of 2-butanol[J]. J Nat Gas Chem,2009,18(2):179−182. doi: 10.1016/S1003-9953(08)60099-7 [2] VEERESHGOUDAV S, PANDA P K. Electrospinning of cellulose acetate nanofiber membrane using methyl ethyl ketone and N, N-Dimethylacetamide as solvents[J]. Mater Chem Phys,2020,240(15):122147−122155. [3] GERAVAND E, SHARIATINIA Z, YARIPOUR F, SAHEBDELFAR S. Copper-based nanocatalysts for 2-butanol dehydrogenation: screening and optimization of preparation parameters by response surface methodology[J]. Korean J Chem Eng,2015,32(12):2418−2428. doi: 10.1007/s11814-015-0087-x [4] SONG D, YOON Y G, LEE C J. Conceptual design for the recovery of 1,3-butadiene and methyl ethyl ketone via a 2,3-Butanediol-dehydration process[J]. Chem Eng Res Des,2017,123:268−276. doi: 10.1016/j.cherd.2017.05.019 [5] 李玉芳, 伍小明. 甲乙酮生产技术及国内外市场分析[J]. 上海化工,2012,37(4):32−37. doi: 10.3969/j.issn.1004-017X.2012.04.013LI Yu-fang, WU Xiao-ming. Methyl ethyl ketone production technology and analysis of domestic and foreign markets[J]. Shanghai Chem Ind,2012,37(4):32−37. doi: 10.3969/j.issn.1004-017X.2012.04.013 [6] ODYAKOV V F, ZHIZHINA E G. Kinetics and mechanism of the homogeneous oxidation of n-butenes to methyl ethyl ketone in a solution of Mo-V-phosphoric heteropoly acid in the presence of palladium pyridine-2,6-dicarboxylate[J]. Kinet Catal,2011,52(6):828−834. doi: 10.1134/S0023158411060164 [7] LIU Z H, HUO W Z, MA H, QIAO K. Development and commercial application of MEK production technology[J]. Chin J Chem Eng,2006,14(5):676−684. doi: 10.1016/S1004-9541(06)60134-1 [8] 付朋, 李永刚, 宁春利. Cu/ZnO/Al2O3催化剂用于醋酸仲丁酯加氢制备仲丁醇联产乙醇[J]. 工业催化,2017,25(4):68−73. doi: 10.3969/j.issn.1008-1143.2017.04.012FU Peng, LI Yong-gang, NING Chun-li. Cu/ZnO/Al2O3 catalyst for hydrogenation of sec-butyl acetate to sec-butanol for ethanol production[J]. Ind Catal,2017,25(4):68−73. doi: 10.3969/j.issn.1008-1143.2017.04.012 [9] FANG D, LIU Z M, MENG S H, WANG L G, XU L, WANG H. Influence of aging time on the properties of precursors of CuO/ZnO catalysts for methanol synthesis[J]. J Nat Gas Chem,2005,14:107−114. [10] LI J L, INUI T. Characterization of precursors of methanol synthesis catalysts, copper/zinc/aluminium oxides, precipitated at different pHs and temperatures[J]. Appl Catal A: Gen,1996,137:105−117. doi: 10.1016/0926-860X(95)00284-7 [11] ZHU W C, WANG L X, LIU S Y, WANG Z L. Characterization and catalytic behavior of silica-supported copper catalysts prepared byimpregnation and ion-exchange methods[J]. React Kinet Catal Lett,2008,93(1):93−99. doi: 10.1007/s11144-008-5178-9 [12] KEULER J N, LORENZEN L, MIACHON S. The dehydrogenation of 2-butanol over copper-based catalysts: Optimising catalyst composition and determining kinetic parameters[J]. Appl Catal A: Gen,2001,218(1):171−180. [13] MARCHIAJ, FIERRO J L G, SANTAMARIA J, MONZON A. Dehydrogenation of isopropylic alcohol on a Cu/SiO2 catalyst: A study of the activity evolution and reactivation of the catalyst[J]. Appl Catal A: Gen,1996,142:375−386. doi: 10.1016/0926-860X(96)00087-7 [14] CRIVELLO M, PEREZ C, FERNANDEZ J, EIMER G, HERRERO E, CASUSCELLI S, ENRIQUE R C. Synthesis and characterization of Cr/Cu/Mg mixed oxides obtained from hydrotalcite-type compounds and their application in the dehydrogenation of isoamylic alcohol[J]. Appl Catal A: Gen,2007,317:11−19. doi: 10.1016/j.apcata.2006.08.035 [15] 姜广申, 胡云峰, 蔡俊, 许鹏, 从亮, 方菲. 仲丁醇脱氢制甲乙酮的Cu-ZnO催化剂[J]. 化工进展,2013,32(2):352−358.JIANG Guang-shen, HU Yun-feng, CAI Jun, XU Peng, CHONG Liang, FANG Fei. Cu-ZnO catalyst for the dehydrogenation of sec-butanol to methyl ethyl ketone[J]. Chem Ind Eng Prog,2013,32(2):352−358. [16] SUN D, MISU T, TAMADA Y, SATO S. Advantages of using Cu/SiO2 catalyst for vapor-phase dehydrogenation of 1-decanol into decanal[J]. Appl Catal A: Gen,2019,582(25):117109. [17] 马依文, 包桂蓉, 王青青, 李法社. La改性Cu/Zn/Al催化剂的制备及其催化纤维素液化性能[J]. 化工进展,2016,35(1):179−187.MA Yi-wen, BAO Gui-rong, WANG Qing-qing, LI Fa-she. Preparation of La modified Cu/Zn/Al catalyst and its catalytic performance for cellulose liquefaction[J]. Chem Ind Eng Prog,2016,35(1):179−187. [18] TOYIR J, FIERRO J L G, HOMS N, PISCINA P R D L. Catalytic performance for CO2 conversion to methanol of gallium-promoted copper-based catalysts: Influence of metallic precursors[J]. Appl Catal B: Environ,2001,34(4):255−266. doi: 10.1016/S0926-3373(01)00203-X [19] LI B X, HAO Y G, ZHANG B S, SHAO S K, HU L Y. A multifunctional noble-metal-free catalyst of CuO/TiO2 hybrid nanofibers[J]. Appl Catal A: Gen,2017,531(3):1−12. [20] 王爱丽, 贾星原, 卢志鹏, 殷恒波, 邵守言, 朱桂生. 稀土元素(La, Ce, Nd)改性Cu/SiO2催化甲醇脱氢制备甲酸甲酯[J]. 精细石油化工,2019,36(1):20−25. doi: 10.3969/j.issn.1003-9384.2019.01.005WANG Ai-li, JIA Xing-yuan, LU Zhi-peng, YIN Heng-bo, SHAO Shou-yan, ZHU Gui-sheng. Rare earth elements (La, Ce, Nd) modified Cu/SiO2 catalyzed dehydrogenation of methanol to methyl formate[J]. Special Petrochem,2019,36(1):20−25. doi: 10.3969/j.issn.1003-9384.2019.01.005 [21] 雷艳秋. Pr和Sm改性的镍基和铜基催化剂在甲醇水蒸气重整制氢中的研究[D]. 昆明: 昆明理工大学, 2017.LEI Qiu-yan. Study on Pr and Sm modified nickel-based and copper-based catalysts for hydrogen production from methanol steam reforming[D]. Kunming: Kunming University of Science and Technology, 2017. [22] 杨淑倩, 贺建平, 张娜, 隋晓伟, 张磊, 杨占旭. 稀土掺杂改性对Cu/ZnAl水滑石衍生催化剂甲醇水蒸气重整制氢性能的影响[J]. 燃料化学学报,2018,46(2):179−188. doi: 10.3969/j.issn.0253-2409.2018.02.007YANG Shu-qian, HE Jian-ping, ZHANG Na, SUI Xiao-wei, ZHANG Lei, YANG Zhan-xu. Effect of rare earth doping modification on the performance of Cu/ZnAl hydrotalcite derived catalysts for methanol steam reforming to hydrogen[J]. J Fuel Chem Technol,2018,46(2):179−188. doi: 10.3969/j.issn.0253-2409.2018.02.007 [23] 黄玉辉, 任国卿, 孙蛟, 王重庆, 陈晓荣, 梅华. 沉淀剂对CuZnAl催化剂糠醛气相加氢制糠醇选择性的影响[J]. 燃料化学学报,2016,44(6):726−731. doi: 10.3969/j.issn.0253-2409.2016.06.013HUANG Yu-hui, REN Guo-qing, SUN Jiao, WANG Chong-qing, CHEN Xiao-rong, MEI Hua. Effect of precipitant on the selectivity of CuZnAl catalyst for gas phase hydrogenation of furfural to furfuryl alcohol[J]. J Fuel Chem Technol,2016,44(6):726−731. doi: 10.3969/j.issn.0253-2409.2016.06.013 [24] TAKEHIRA K. “Intelligent” reforming catalysts: Trace noble metal-doped Ni/Mg(Al)O derived from hydrotalcites[J]. J Nat Gas Chem,2009,3(18):237−259. [25] LIU G, BAO G R, WANG H, LUO J, HUI S, HUANG Y, MA Y W. Ce modified Cu/Zn/Al catalysts for direct liquefaction of microcrystalline cellulose in supercritical methanol[J]. Cellulose,2019,26(15):8291−8300. doi: 10.1007/s10570-019-02565-z [26] VELUS, SUZUKI K, OKAZAKI M, KAPOOR M P, OSAKI T, OHASHI F. Oxidative steam reforming of methanol over CuZnAl(Zr)-oxide catalysts for the selective production of hydrogen for fuel cells: Catalyst characterization and performance evaluation[J]. J Catal,2000,194(2):373−384. doi: 10.1006/jcat.2000.2940 [27] 许川, 马爱琼, 刘民生, 高云琴. 固相反应法合成锌铝尖晶石[J]. 硅酸盐通报,2012,31(2):455−463.XU Chuan, MA Ai-qiong, LIU Min-sheng, GAO Yun-qin. Synthesis of zinc aluminum spinel by solid state reaction method[J]. J Chin Cera Soc,2012,31(2):455−463. [28] 方书农, 姜明, 伏义路, 林培琰, 乔山, 谢亚宁. 不同焙烧温度对Cu/γ-Al2O3催化剂铜物种结构的影响[J]. 物理化学学报,1994,10(7):623−627. doi: 10.3866/PKU.WHXB19940709FANG Shu-nong, JIANG Ming, FU Yi-lu, LIN Pei-yan, QIAO Shan, XIE Ya-ning. The effect of different calcination temperatures on the copper species structure of Cu/γ-Al2O3 catalyst[J]. Acta Phys Chim Sin,1994,10(7):623−627. doi: 10.3866/PKU.WHXB19940709 [29] MASOUD S N, FATEMEH D, MASOUD F K. Bright blue pigment CoAl2O4 nanocrystals prepared by modified sol-gel method[J]. J Sol-Gel Sci Technol,2009,52(3):321−327. doi: 10.1007/s10971-009-2050-y [30] 刘文艳, 王华, 高文桂, 张明宇, 张逢杰. 不同助剂对合成甲醇工业催化剂二氧化碳加氢性能的影响[J]. 材料导报,2012,26(3):96−99. doi: 10.3969/j.issn.1005-023X.2012.03.019LIU Wen-yan, WANG Hua, GAO Wen-gui, ZHANG Feng-jie. Effects of different promoters on the performance of industrial catalysts for the hydrogenation of carbon dioxide in methanol synthesis[J]. Mater Rev,2012,26(3):96−99. doi: 10.3969/j.issn.1005-023X.2012.03.019 [31] GAO W G, WANG H, WANG Y H, GUO W, JIA M R. Dimethyl ether synthesis from CO2 hydrogenation on La-modified CuO-ZnO-Al2O3/HZSM-5 bifunctional catalysts[J]. J Rare Earth,2013,31(5):470−476. doi: 10.1016/S1002-0721(12)60305-6 [32] SHEN M Q, XU L L, WANG J Q, LI C X, WANG W L, WANG J, ZHAI Y P. Effect of synthesis methods on activity of V2O5/CeO2/WO3-TiO2 catalyst for selective catalytic reduction of NOx with NH3[J]. J Rare Earth,2016,34(3):259−267. doi: 10.1016/S1002-0721(16)60023-6 [33] LI M M J, ZENG Z Y, LIAO F L, HONG X L, TSANG S C E. Enhanced CO2 hydrogenation to methanol over CuZn nanoalloy in Ga modified Cu/ZnO catalysts[J]. J Catal,2016,343:157−167. doi: 10.1016/j.jcat.2016.03.020 [34] BYOUNG K K, DAE S P, YANG S Y, JONGHEOP Y. Preparation and characterization of nanocrystalline CuAl2O4 spinel catalysts by sol-gel method for the hydrogenolysis of glycerol[J]. Catal Commun,2012,24:90−95. doi: 10.1016/j.catcom.2012.03.029 [35] 覃发玠, 刘雅杰, 庆绍军, 侯晓宁, 高志贤. 甲醇制氢铜铝尖晶石缓释催化剂的研究-不同铜源合成的影响[J]. 燃料化学学报,2017,45(12):1481−1488. doi: 10.3969/j.issn.0253-2409.2017.12.010QIN Fa-jie, LIU Ya-jie, QING Shao-jun, HOU Xiao-ning, GAO Zhi-xian. Study on the slow-release copper-aluminum spinel catalyst for the production of hydrogen from methanol-the influence of the synthesis of different copper sources[J]. J Fuel Chem Technol,2017,45(12):1481−1488. doi: 10.3969/j.issn.0253-2409.2017.12.010 [36] AUNBAMRUNG P, WONGKAEW A. Effect of Cu loading tocatalytic selective CO oxidation of CuO/CeO-CoO[J]. Adv Chem Eng Sci,2013,3(4):15−19. [37] 李忠, 牛燕燕, 郑华燕, 付廷俊, 朱琼芳, 阴丽华. 表面改性对Cu/活性炭催化剂表面 Cu物种和催化活性的影响[J]. 无机化学学报,2011,27(7):1277−1284.LI Zhong, NIU Yan-yan, ZHENG Hua-yan, FU Ting-jun, ZHU Qiong-fang, YIN Li-hua. Effect of surface modification on Cu species and catalytic activity of Cu/activated carbon catalyst[J]. Chin J Inorg Chem,2011,27(7):1277−1284. [38] CHENG S Y, KOU J W, GAO Z H, HUANG W. Preparation of complexant-modified Cu/ZnO/Al2O3catalysts via hydrotalcite-like precursors and its highly efficient application in direct synthesis of isobutanol and ethanol from syngas[J]. Appl Catal A: Gen,2018,556:113−120. doi: 10.1016/j.apcata.2018.02.027 [39] FRANCO P, RICCARDO P. Catalytic behavior and surface chemistry of Copper/ZnO/Al2O3 catalysts for the decomposition of 2-propanol[J]. J Catal,1992,136:86−95. doi: 10.1016/0021-9517(92)90108-T [40] 王冬蕾. 微波碳热还原法制备Cu/AC催化合成碳酸二甲酯性能研究[D]. 太原: 太原理工大学, 2014.WANG Dong-lei. Preparation of Cu/AC by microwave carbothermal reduction method for catalytic synthesis of dimethyl carbonate[D]. Taiyuan: Taiyuan University of Science and Technology, 2014. [41] GAO P, LI F, ZHAN H, ZHAO N, XIAO F K, WEI W, ZHONG L S, WANG H, SUN Y H. Influence of Zr on the performance of Cu/Zn/Al/Zr catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol[J]. J Catal,2013,298:51−60. doi: 10.1016/j.jcat.2012.10.030 [42] HE J P, YANG Z X, ZHANG L, LI Y, PAN L W. Cu supported on ZnAl-LDHs precursor prepared by in-situ synthesis method on γ-Al2O3 as catalytic material with high catalytic activity for methanol steam reforming[J]. Int J Hydrogen Energy,2017,42(15):9930−9937. doi: 10.1016/j.ijhydene.2017.01.229 [43] ZHANG L, PAN L W, NI C J, SUN T J, ZHAO S S, WANG S D, WANG A J, HU Y K. CeO 2-ZrO2-promoted CuO/ZnO catalyst for methanol steam reforming[J]. Int J Hydrogen Energy,2013,38(11):4397−4406. doi: 10.1016/j.ijhydene.2013.01.053 [44] LI Y, FENG J T, LI D Q. Preparation and characterization of spherical mesoporous ZrO2-Al2O3 composites with high thermal stability[J]. Sci Chain Chem,2011,54(7):1032−1038. doi: 10.1007/s11426-011-4282-2 [45] YAP M H, FOW K L, CHEN G Z. Synthesis and applications of MOF-derived porous nanostructures[J]. Green Energy Environ,2017,2(3):218−245. doi: 10.1016/j.gee.2017.05.003 [46] AGARWAL V, PATEL S, PANT K K. H2 production by steam reforming of methanol over Cu/ZnO/Al2O3 catalysts: Transient deactivation kinetics modeling[J]. Appl Catal A: Gen,2005,279(1):155−164. [47] 张磊, 雷俊腾, 田园, 胡鑫, 白金, 刘丹, 杨义, 潘立卫. 前驱体和沉淀剂浓度对CuO/ ZnO/CeO2-ZrO2甲醇水蒸气重整制氢催化剂性能的影响[J]. 燃料化学学报,2015,43(11):1366−1374. doi: 10.3969/j.issn.0253-2409.2015.11.012ZHANG Lei, LEI Jun-teng, TIAN Yuan, HU Xin, BAI Jin, LIU Dan, YANG Yi, PAN Li-wei. Effectof precursor and precipitant concentration on the performance of CuO/ZnO/CeO2-ZrO2 catalyst for methanol steam reforming[J]. J Fuel Chem Technol,2015,43(11):1366−1374. doi: 10.3969/j.issn.0253-2409.2015.11.012 [48] GAO Z H, LI S S, TIAN H H, DONG W B, LIU Y, JIA L, HUANG W. Synthesis of ethanol from syngas over CuZnAl catalysts with different Cu/Zn/Al molar ratios in polyethylene glycol 600 medium[J]. React Kinet Mech Catal,2017,122(2):1117−1127. doi: 10.1007/s11144-017-1270-3 [49] DAS D, LLORCA J, DOMINGUEZ M, COLUSSI S, TROVARELLI A, GAYEN A. Methanol steam reforming behavior of copperimpregnated over CeO2-ZrO2 derived from a surfactant assisted coprecipitation route[J]. Int J Hydrogen Energy,2015,40(33):10463−10479. doi: 10.1016/j.ijhydene.2015.06.130 [50] SHI L M, GAO C L, GUO F H, WANG Y J, ZHANG T B. Catalytic performance of Zr-doped CuO-CeO2 oxides for CO selective oxidation in H2-rich stream[J]. J Rare Earth,2019,37:720−725. doi: 10.1016/j.jre.2019.01.003 [51] 张国强, 郭天玉, 郑华艳, 李忠. 焙烧温度对CuCe/Ac催化剂甲醇氧化羰基化性能的影响[J]. 燃料化学学报,2016,44(6):674−679. doi: 10.3969/j.issn.0253-2409.2016.06.006ZHANG Guo-qiang, GUO Tian-yu, ZHENG Hua-yan, LI Zhong. Effect of calcination temperature on the performance of CuCe/Ac catalyst for methanol oxidative carbonylation[J]. J Fuel Chem Technol,2016,44(6):674−679. doi: 10.3969/j.issn.0253-2409.2016.06.006 [52] 余启炎, 郝雪松, 杨晓红, 顾申, 闫丽梅, 石翠. 仲丁醇脱氢制甲乙酮催化剂的研究[J]. 石油化工,2005,34(9):818−821. doi: 10.3321/j.issn:1000-8144.2005.09.003YU Qi-yan, HAO Xue-song, YANG Xiao-hong, GU Shen, YAN Li-mei, SHI Cui. Study on the catalyst for the dehydrogenation of sec-butanol to methyl ethyl ketone[J]. Petrochem Technol,2005,34(9):818−821. doi: 10.3321/j.issn:1000-8144.2005.09.003 [53] 何奋彪. 共沉淀法仲丁醇脱氢制甲乙酮催化剂的研究[J]. 上海化工,2011,36(2):12−14. doi: 10.3969/j.issn.1004-017X.2011.02.004HE Fen-biao. Study on the catalyst for the dehydrogenation of sec-butanol to methyl ethyl ketone by co-precipitation[J]. Shanghai Chem Ind,2011,36(2):12−14. doi: 10.3969/j.issn.1004-017X.2011.02.004 [54] 迟德旭, 房德仁. 仲丁醇脱氢催化剂性能试验及工业应用[J]. 工业催化,2012,20(11):65−68. doi: 10.3969/j.issn.1008-1143.2012.11.015CHI De-xu, FANG De-ren. Performance test and industrial application of sec-butanol dehydrogenation catalyst[J]. Ind Catal,2012,20(11):65−68. doi: 10.3969/j.issn.1008-1143.2012.11.015 -





 下载:
下载: