Study on hydrogen transfer reaction in C5 hydrocarbons catalytic pyrolysis
-
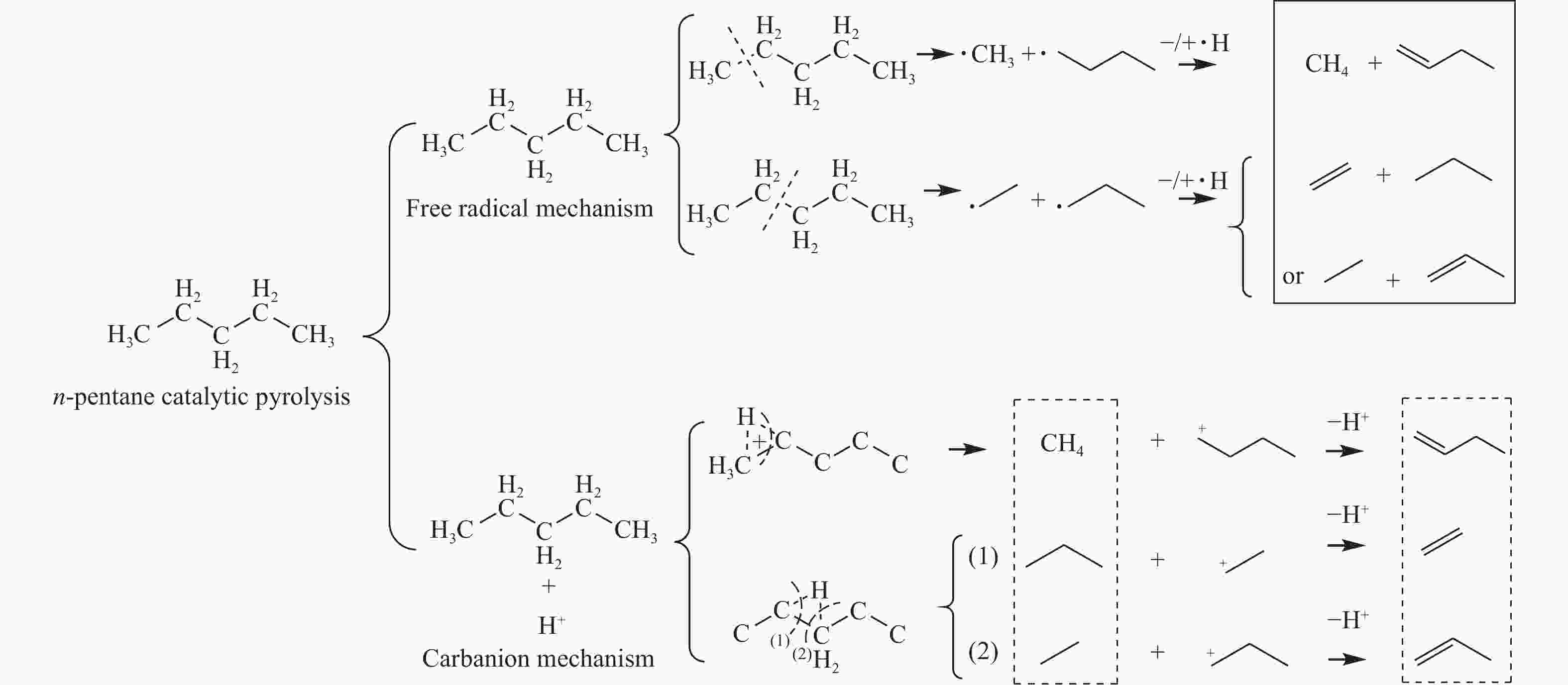
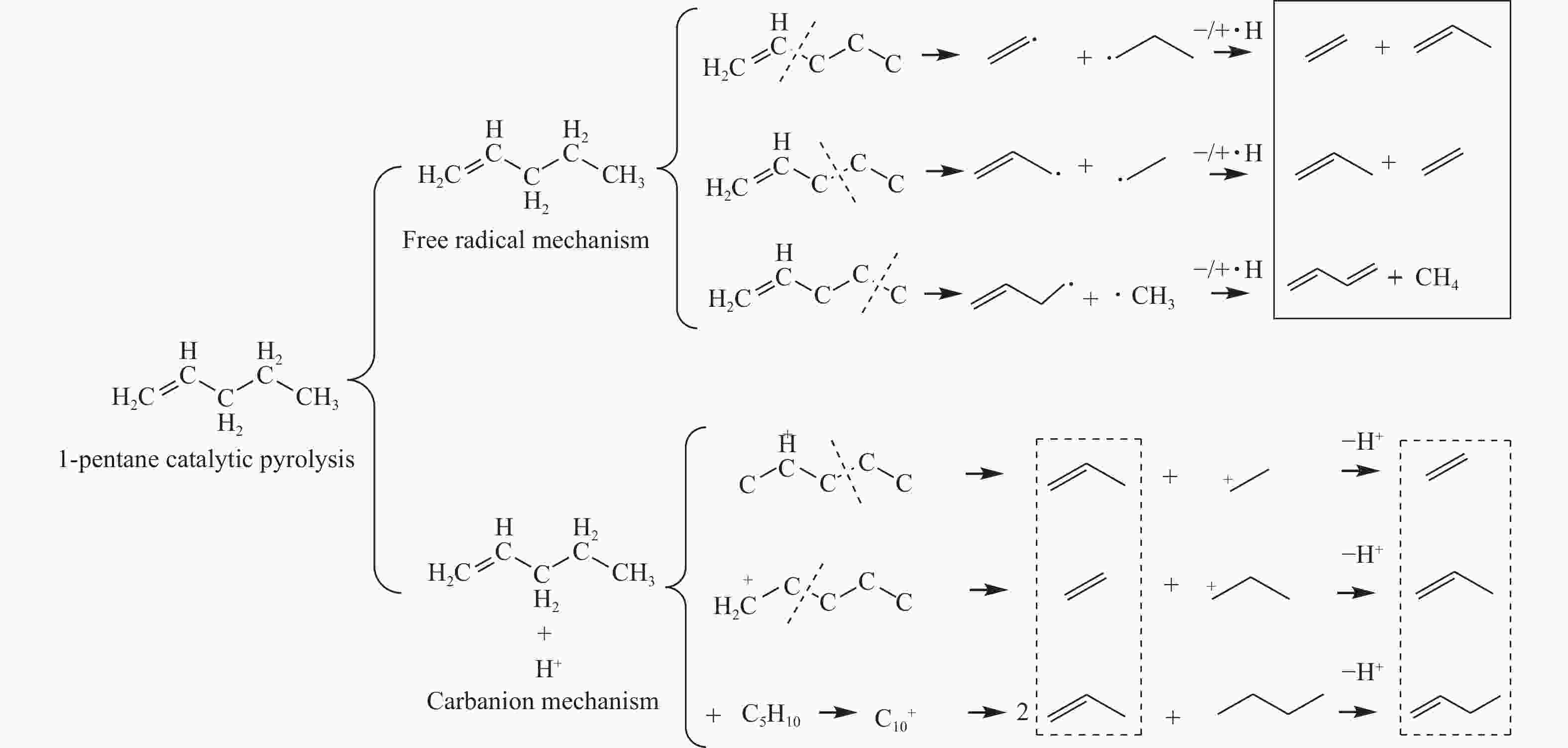
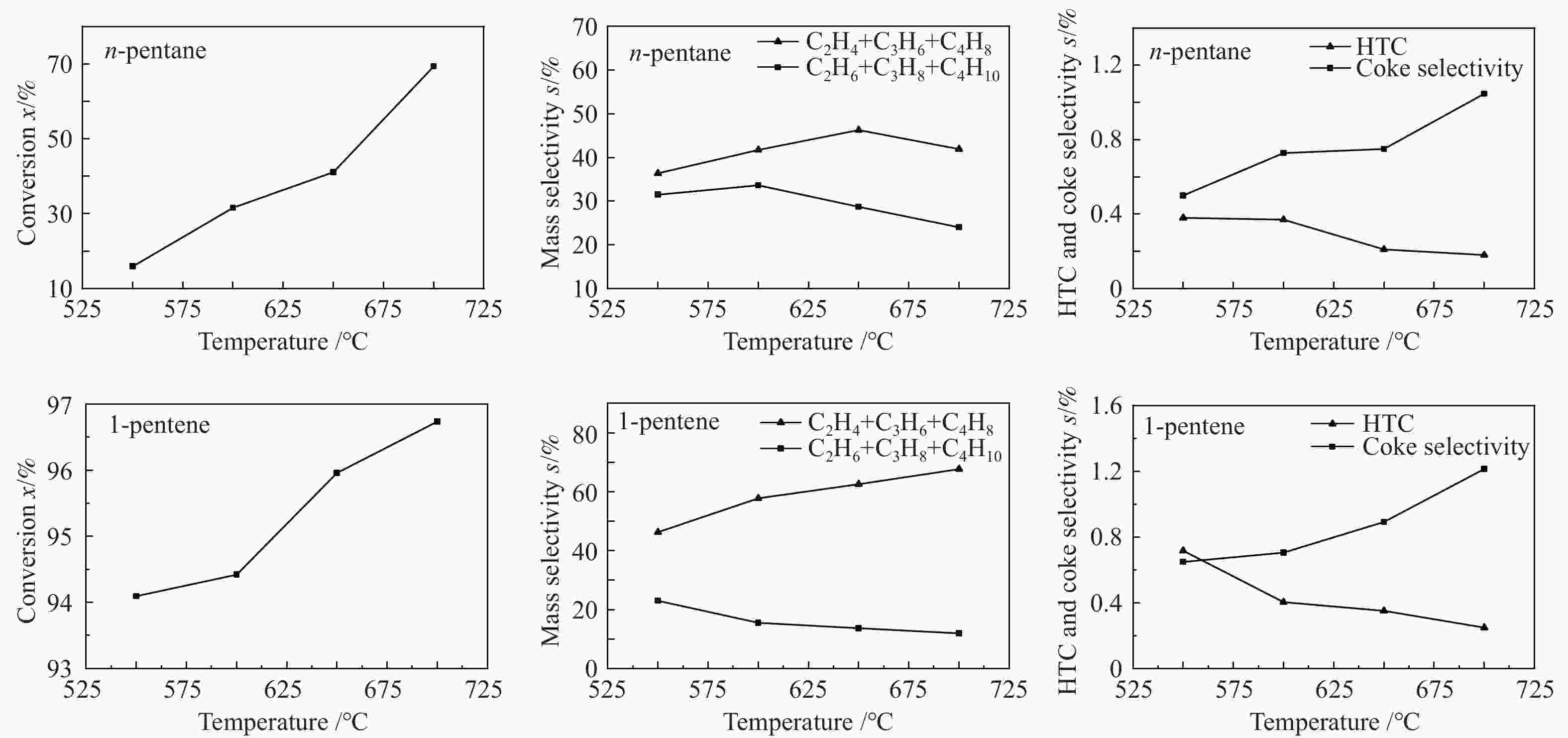
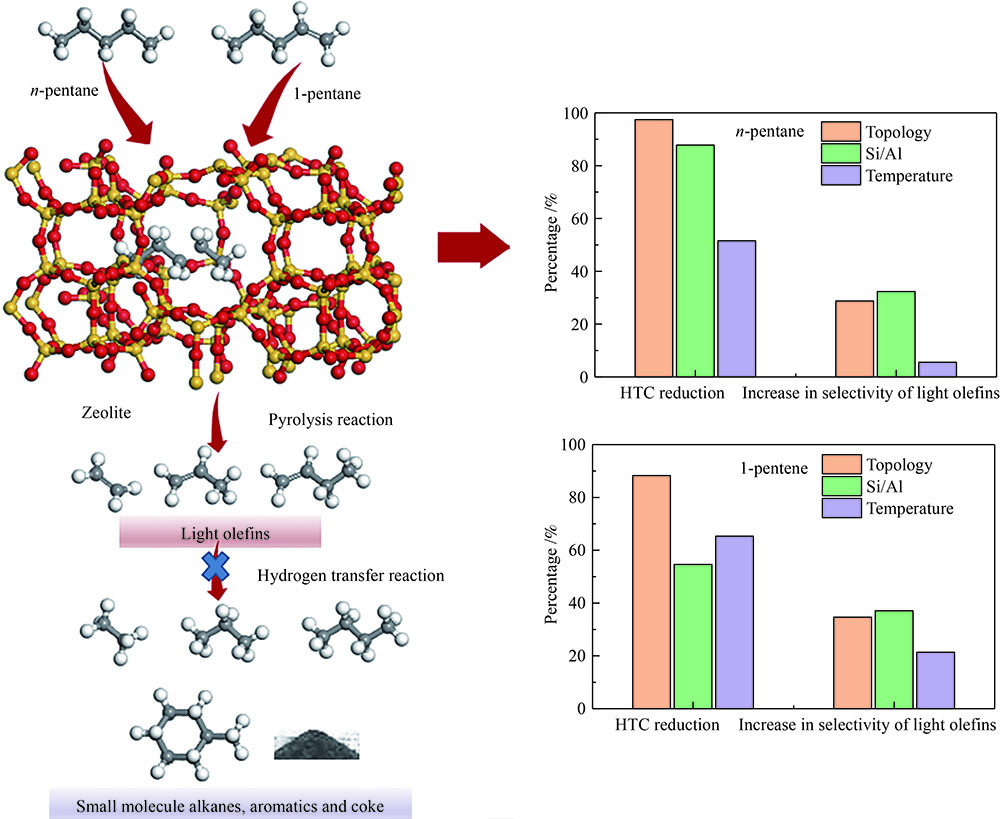
摘要: 对C5烃(正戊烷、1-戊烯)的裂解反应产物进行分析,按照理想正碳离子和自由基反应机理,正戊烷和1-戊烯裂解生成低碳烯烃(C2H4+C3H6+C4H8)的摩尔选择性分别达到50%和100%。但是使用MFI-30分子筛,在650 ℃反应条件下,正戊烷和1-戊烯催化裂解生成低碳烯烃的摩尔选择性分别为23.41%和56.79%,说明分别有26.59%和43.21%的低碳烯烃发生了氢转移反应。进一步考察了不同类型分子筛和关键反应温度对C5烃催化裂解过程中氢转移反应的影响,研究发现,小孔结构、低酸密度的分子筛和较高反应温度,可以不同程度地抑制氢转移反应,提高低碳烯烃的选择性。在650 ℃条件下,当分子筛由大孔结构、高酸量的FAU更换为小孔结构、低酸量的MFI-120时,正戊烷和1-戊烯催化裂解的氢转移系数HTC分别减小96.86%和50.58%,焦炭选择性分别由11.91%和20.77%减小到0.75%和0.89%,低碳烯烃(C2H4+C3H6+C4H8)的选择性分别由14.25%和25.14%增加到46.28%和62.58%。Abstract: The cracking reaction mechanism of C5 hydrocarbons(n-pentane, 1-pentene) was analyzed. It is found that according to the ideal carbanion reaction mechanism and free radicals reaction mechanism, the molar selectivity of the cracking of n-pentane and 1-pentene to lower olefins (C2H4+C3H6+C4H8) is 50% and 100%, respectively. However, using MFI-30 zeolite, the molar selectivity of catalytic cracking of n-pentane and 1-pentene to light olefins at 650 ℃ is 23.41% and 56.79%, respectively, suggesting that 26.59% and 43.21% of light olefins have undergone hydrogen transfer reactions. The effects of different zeolites and key reaction temperature on the hydrogen transfer reaction during the catalytic pyrolysis of C5 hydrocarbons were further investigated. The results show that the zeolite with small pore structure and low acid density and higher reaction temperature can inhibit the hydrogen transfer reaction to varying degrees, thereby increasing the selectivity of light olefins. At 650 ℃, as the zeolite changes from the FAU with a large pore structure and high acid content to the MFI-120 with a small pore structure and low acid content, the hydrogen transfer coefficient HTC of the catalytic pyrolysis of n-pentane and 1-pentene is reduced by 96.86% and 50.58%, respectively, and the coke selectivity is reduced from 11.91% and 20.77% to 0.75% and 0.89%, respectively. However, the selectivity of the lower olefins increases from 14.25% and 25.14% to 46.28% and 62.58%, respectively.
-
Key words:
- C5 /
- catalytic pyrolysis /
- hydrogen transfer /
- light olefins
-
表 1 不同类型分子筛的物化性质
Table 1 Properties of different zeolites
Topology Si/Al2 Channel dimension Maximum spherical
diameter contained
in zeolite[12]/nmSBET/
(m2·g−1)vmicro/
(cm3·g−1)Pore size/nm Weak acid/
(mmol·g−1)Strong acid/
(mmol·g−1)Total acid/
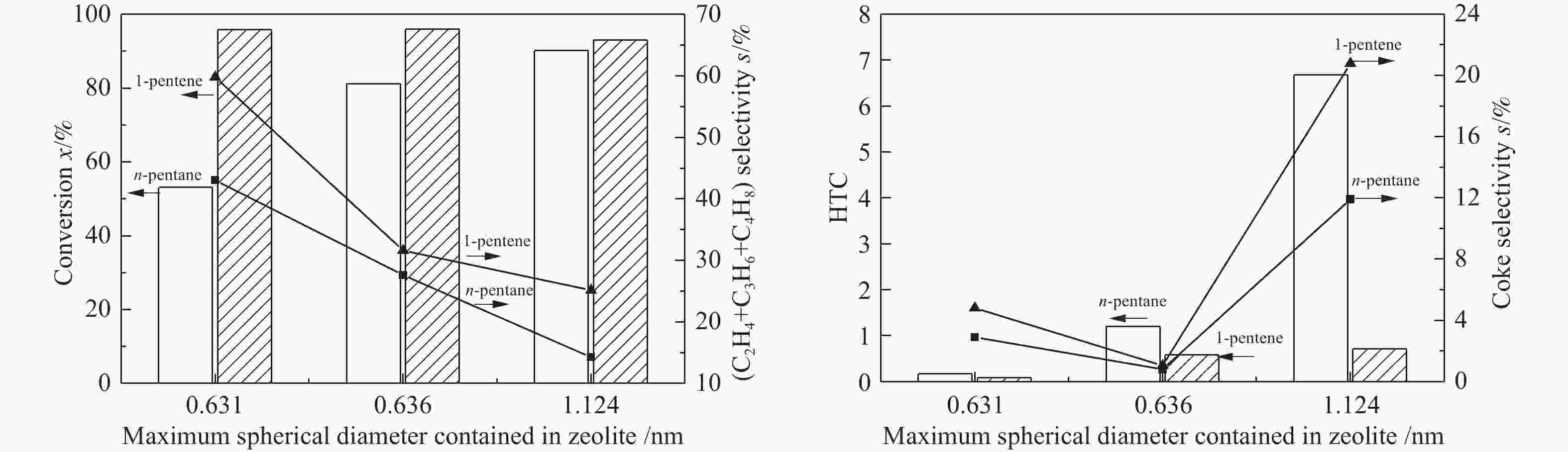
(mmol·g−1)MFI 30 3 0.636 397.650 0.142 0.520 0.214 0.208 0.422 MFI 40 3 0.636 397.411 0.141 0.520 0.173 0.189 0.362 MFI 120 3 0.636 397.520 0.141 0.520 0.116 0.102 0.218 FER 25 2 0.631 401.698 0.142 0.513 0.149 0.207 0.356 FAU 6 3 1.124 793.403 0.272 0.735 0.127 0.227 0.354 表 2 C5烃催化裂解条件下的产品分布
Table 2 Product distribution of C5 hydrocarbons pyrolysis under catalytic conditions
n-pentane thermal
cracking1-pentene thermal
crackingn-pentane catalytic
pyrolysis1-pentene catalytic
pyrolysisConversion w/% 8.83 25.49 97.41 99.78 Product yield w/% H2 0.03 0.03 1.26 1.37 CH4 0.60 2.36 11.35 11.65 C2H6 0.96 2.18 16.00 8.42 C2H4 1.30 3.27 9.40 13.60 C3H8 0.10 0.51 24.70 11.52 C3H6 2.01 3.95 8.23 9.58 C4H10 0.33 0.32 4.70 1.82 C4H8 0.64 2.51 2.48 2.27 1-C5H10 0.79 74.51 0.44 0.22 n-C5H12 91.17 3.24 2.59 0.01 Aromatics 0.02 0.68 13.63 31.32 Other components 1.89 6.27 4.44 7.16 Coke 0.16 0.16 0.78 1.06 表 3 C5烃主要裂解产物的摩尔选择性(650 ℃,MFI-30分子筛,重时空速220 h−1)
Table 3 Molar selectivity of the main products of C5 hydrocarbons cracking (650 ℃, MFI-30 zeolite, weight hourly space velocity 220 h−1)
Molar selectivity/% n-pentane 1-pentene CH4 28.81 − C2H4+C3H6+C4H8 23.41 56.79 C2H6+C3H8+C4H10 47.78 43.21 表 4 C5烃在不同分子筛上的催化裂解(650 ℃)
Table 4 The catalytic pyrolysis reaction of C5 hydrocarbons on different zeolites (650 ℃)
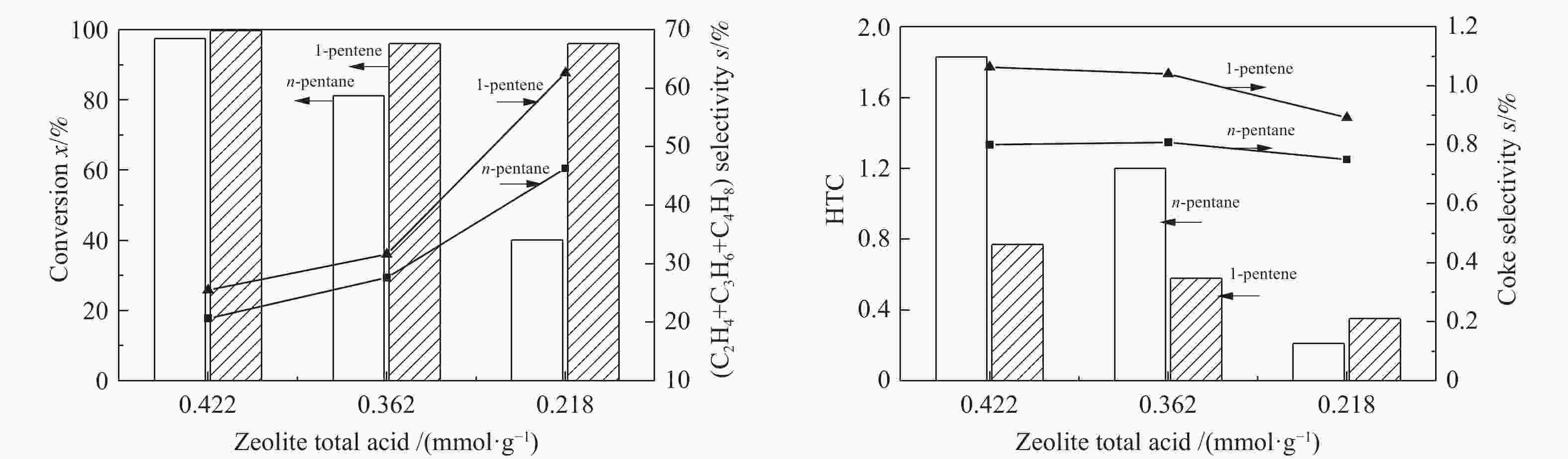
Reactant n-pentane 1-pentene Zeolite FAU MFI-120 FAU MFI-120 HTC 6.68 0.21 0.71 0.35 Coke selectivity s/% 11.91 0.75 20.77 0.89 Light olefins selectivity s/% 14.25 46.28 25.14 62.58 -
[1] LIU Z Y, ZHANG Z D, YANG C H, GAO X H. Domestic technology developments on high-efficiency heavy oil conversion FCC catalysts’ industrialization[J]. Appl Petrochem Res,2015,5(4):269−275. doi: 10.1007/s13203-015-0133-y [2] 王刚, 孙静, 方东, 肖俊, 南杰, 高金森. 分子炼油为导向的催化裂化加工重质油策略[J]. 中国科学: 化学,2018,48(4):362−368. doi: 10.1360/N032017-00169WANG Gang, SUN Jing, FANG Dong, XIAO Jun, NAN Jie, GAO Jin-sen. Molecular-refining oriented strategy of catalytic cracking for processing heavy oil[J]. Sci Sin Chim,2018,48(4):362−368. doi: 10.1360/N032017-00169 [3] LI C Y, YANG C H, SHAN H H. Maximizing propylene yield by two-stage riser catalytic cracking of heavy oil[J]. Ind Eng Chem Res,2007,46(14):4914−4920. doi: 10.1021/ie061420l [4] ZAMOSTNY P, BELOHLAV Z, STARKBAUMOVA L, PATERA J. Experimental study of hydrocarbon structure effects on the composition of its pyrolysis products[J]. J Anal Appl Pyrolysis,2010,87(2):207−216. [5] 王为然, 张文斌, 王刚, 蓝兴英, 徐春明, 高金森. FCC汽油二次裂化增产丙烯过程中主要影响因素的研究[J]. 燃料化学学报,2009,31(1):67−72.WANG Wei-ran, ZHANG Wen-bin, WANG Gang, LAN Xing-ying, XU Chun-ming, GAO Jin-sen. Research on the main influencing factors in the process of FCC gasoline secondary cracking to increase propylene production[J]. J Fuel Chem Technol,2009,31(1):67−72. [6] 许友好, 崔守业, 汪燮卿. FCC汽油烯烃双分子裂化反应及其与双分子氢转移反应之比的研究[J]. 石油炼制与化工,2007,38(9):1−5. doi: 10.3969/j.issn.1005-2399.2007.09.001XU You-hao, CUI Shou-ye, WANG Xie-qing. Study on the bimolecular cracking reaction of FCC gasoline olefins and its ratio to bimolecular hydrogen transfer reaction[J]. Pet Process Petrochem,2007,38(9):1−5. doi: 10.3969/j.issn.1005-2399.2007.09.001 [7] POTAPENKO O V, DORONIN V P, SOROKINA T P, KROL O V, LIKHOLOBOVET V A. A study of intermolecular hydrogen transfer from naphthenes to 1-hexene over zeolite catalysts[J]. Appl Catal A: Gen, 2016, 516, 153-159. [8] BORTNOVSKY O, SAZAMA P, WICHTERLOVA B. Cracking of pentenes to C2-C4 light olefins over zeolites and zeotypes[J]. Appl Catal A: Gen,2005,287(2):203−213. doi: 10.1016/j.apcata.2005.03.037 [9] HOU X, QIU Y, ZHANG X W, LIU G Z. Analysis of reaction pathways forn-pentane cracking over zeolites to produce light olefins[J]. Chem Eng J,2016,307(1):372−381. [10] THIVASASITH A, MAIHOM T, PENGPANICH S, WATTANAKIT C. Nanocavity effects of various zeolite frameworks on n-pentane cracking to light olefins: Combination studies of DFT calculations and experiments[J]. Phys Chem Chem Phys,2019,21(40):22215−22223. doi: 10.1039/C9CP03871J [11] KUBO K, LIDA H, NAMBA S, LGARASHI A. Selective formation of light olefin by n-heptane cracking over HZSM-5 at high temperatures[J]. Microporous Mesoporous Mater,2012,149(1):126−133. doi: 10.1016/j.micromeso.2011.08.021 [12] TREACY M M J, FOSTER M D. Packing sticky hard spheres into rigid zeolite frameworks[J]. Microporous Mesoporous Mater,2009,118(1/3):106−114. doi: 10.1016/j.micromeso.2008.08.039 [13] HOU X, NI N, WANG Y, ZHU W J, QIU Y, DIAO Z H, LIU G Z, ZHANG X W. Roles of the free radical and carbenium ion mechanisms in pentane cracking to produce light olefins[J]. J Anal Appl Pyrolysis,2019,138:270−280. [14] ZHANG R, WANG Z X, LIU H Y, LIU Z C, LIU G L, MENG X H. Thermodynamic equilibrium distribution of light olefins in catalytic pyrolysis[J]. Appl Catal A: Gen,2016,522:165−171. doi: 10.1016/j.apcata.2016.05.009 [15] HAAG W, DESSAU R. Duality of mechanism for acid-catalyzed paraffin cracking[C]// Proceedings of the 8th International Congress on Catalysis, 1984. [16] CORMA A, MONTON J B, ORCHILLES A V. Cracking of n-heptane on a HZSM-5 zeolite. The influence of acidity and pore structure[J]. Appl Catal,1985,16(1):59–74. [17] ZHANG Y, ZHAO R X, SANCHEZSANCHEZ M, HALLER G L, HU J Z, BERMEJODEVAL R, LIU Y, LERCHER J A. Promotion of protolytic pentane conversion on H-MFI zeolite by proximity of extra-framework aluminum oxide and Brønsted acid sites[J]. J Catal,2019,370:424−433. doi: 10.1016/j.jcat.2019.01.006 [18] 罗渝然. 《化学键能数据手册》[J]. 科学通报,2005,50(8):759−759. doi: 10.3321/j.issn:0023-074X.2005.08.021LUO Yu-ran. "Chemical Bond Energy Data Handbook"[J]. Scientia,2005,50(8):759−759. doi: 10.3321/j.issn:0023-074X.2005.08.021 [19] HUANG X, AIHEMAITIJIANG D, XIAO W D. Reaction pathway and kinetics of C3–C7 olefin transformation over high-silicon HZSM-5 zeolite at 400–490 °C[J]. Chem Eng J,2015,280(15):222−232. [20] CNUDDE P, DE WISPELAERE K, VAN D M J, WAROQUIER M, SPEYBROECK V V. Effect of temperature and branching on the nature and stability of alkene cracking intermediates in H-ZSM-5[J]. J Catal,2017,345:53−69. doi: 10.1016/j.jcat.2016.11.010 [21] 朱华元, 何鸣元, 张信, 宋家庆. 正己烷在几种不同分子筛上的氢转移反应[J]. 石油炼制与化工,2001,(9):39−42. doi: 10.3969/j.issn.1005-2399.2001.09.011ZHU Hua-yuan, HE Ming-yuan, ZHANG Xin, SONG Jia-qing. Hydrogen transfer reaction of n-hexane on several different molecular sieves[J]. Pet Process Petrochem,2001,(9):39−42. doi: 10.3969/j.issn.1005-2399.2001.09.011 [22] 许友好. 氢转移反应在烯烃转化中的作用探讨[J]. 石油炼制与化工,2002,(1):38−41. doi: 10.3969/j.issn.1005-2399.2002.01.009XU You-hao. Discussion on the role of hydrogen transfer reaction in olefin conversion[J]. Pet Process Petrochem,2002,(1):38−41. doi: 10.3969/j.issn.1005-2399.2002.01.009 [23] 朱向学, 宋月芹, 李宏冰, 刘盛林, 孙新德, 徐龙伢. 丁烯催化裂解制丙烯/乙烯反应的热力学研究[J]. 催化学报,2005,26(2):22−28.ZHU Xiang-xue, SONG Yue-qin, LI Hong-bing, LIU Sheng-lin, SUN Xin-de, XU Long-ya. Thermodynamic study on the catalytic cracking of butene to propylene/ethylene[J]. Chin J Catal,2005,26(2):22−28. -





 下载:
下载: