Thermodynamic analysis and experimental verification of a new route for direct diethyl oxalate synthesis
-
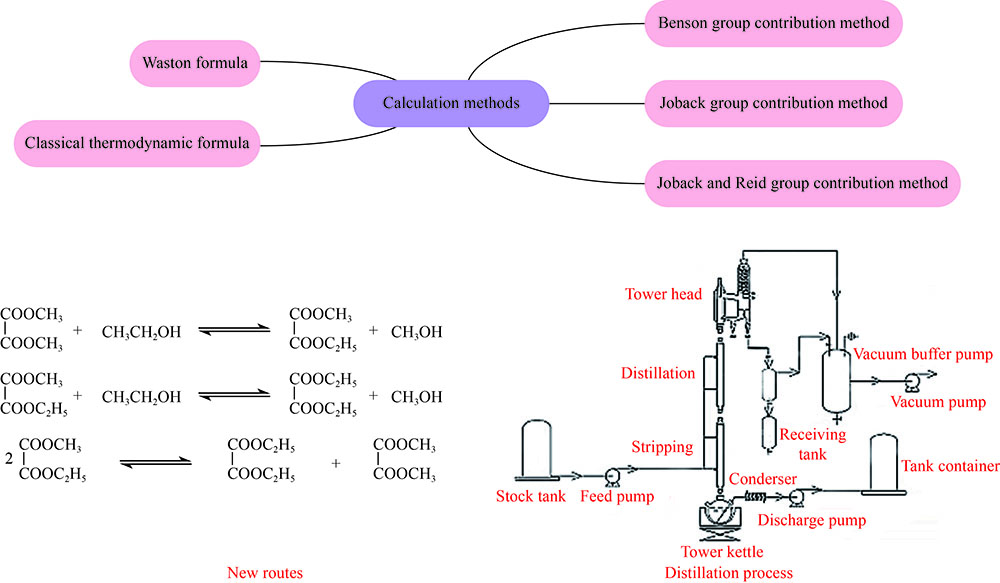
摘要: 报道新颖的草酸二甲酯(DMO)与乙醇(EtOH)通过酯交换路径一步合成高品质草酸二乙酯(DEO)。采用基团贡献法及Watson公式估算过程中各物质的热力学参数,并通过经典热力学公式计算在常压和温度323−368 K时合成DEO各步反应的焓变、熵变、吉布斯自由能及平衡常数。通过实验测定不同温度和原料比例下DMO转化率、产物组成和反应平衡常数并与理论估算值比较。发现实测DMO转化率与估算值误差在1%内,实测平衡常数与估算值基本一致。经过严格的实验验证,证明经热力学方法估算的热力学参数比较可靠。模拟真实催化精馏条件,以塔釜DEO纯度达99.9%为目标计算353 K时塔釜的初始原料和最终产物组成,当塔釜EtOH含量高于2.59%,初始n(EtOH)/n(DMO)大于2.10时可使DEO纯度达到指标,并显著降低整体工艺能耗,是一个高效绿色的DEO生产工艺。Abstract: The synthesis of high quality diethyl oxalate (DEO) via transesterification of dimethyl oxalate (DMO) and ethanol (EtOH) was reported. The thermodynamic data of each substance involved in the reaction were estimated by Benson and Joback's group contribution method and Watson formula, and the enthalpy change, entropy change, Gibbs free energy and equilibrium constant of each step of DEO synthesis were calculated by classical thermodynamic formula under atmospheric pressure and in the temperature range of 323−368 K. The DMO conversion, product composition and reaction equilibrium constant at different temperatures and raw material ratios were measured by experiments and compared with the theoretical data. It is found that the error between the measured DMO conversion and the estimated value is less than 1%, and the measured equilibrium constant is basically consistent with the estimated value. After strict experimental verification, it is proved that the thermodynamic data estimated by thermodynamic analysis are reliable. The actual catalytic distillation conditions were simulated, and the composition of the initial raw materials and the final products at 353 K was calculated with the hypothesis of 99.9% DEO purity at the bottom. When the content of EtOH in the bottom was higher than 2.59% and the molar ratio of initial EtOH to DMO was higher than 2.10, the purity of DEO could reach the target, and the overall process energy consumption was significantly reduced. It would be an efficient and green route for DEO synthesis.
-
表 1 估算标准摩尔生成焓的Benson基团贡献值
Table 1 Group contribution value based on Benson method for standard molar formation enthalpy
Group ni(DMO) ni(MEO) ni(DEO) ${\Delta _f }H_{298}^\Theta$ / (kJ·mol−1) C-(O)(H)3 2 1 0 −42.19 O−(C)(CO) 2 2 2 −180.41 CO−(O)(CO) 2 2 2 −122.65 C−(C)(H)3 0 1 2 −42.19 C−(O)(C)(H)2 0 1 2 −33.91 表 2 Joback估算理想气相比热容的基团贡献值
Table 2 Group contribution value of the specific heat capacity in gas phase derived from Joback method
Group Group contribution value / (J·mol−1·K−1) $\Delta {a_i}$ $\Delta {b_i} \times {10^2}$ $\Delta {c_i} \times {10^4}$ $\Delta {d_i} \times {10^6}$ −CH3 19.5 −0.808 1.53 −0.0967 −CH2− −0.909 9.50 −0.544 0.0119 −OH(alcohol) 25.7 −6.91 1.77 −0.0988 −COO− 24.5 4.02 0.402 −0.0452 表 3 Joback and Reid法估算ΔvHb的基团贡献值
Table 3 Group contribution value of ΔvHb calculatied by Joback and Reid method
Group ni(DMO) ni(MEO) ni(DEO) Δi −CH3 2 2 2 2.373 −CH2− 0 1 2 2.226 −COO− 2 2 2 9.633 表 4 计算的各物质在不同温度下的蒸发焓
Table 4 Vaporization enthalpy values at different temperatures
T/K ΔvHT / (kJ·mol−1) DMO MEO DEO MeOH EtOH 323 47.60 49.89 56.27 36.38 40.55 338 46.44 48.66 54.80 35.26 39.26 353 45.64 47.81 53.78 34.08 37.91 368 44.41 46.92 52.21 32.82 36.47 表 5 计算的各物质在不同温度下的气或液态标准摩尔生成焓和标准熵
Table 5 Calculated standard molar enthalpy of formation and entropy for either gas or liquid phase at different temperatures
T/K DMO MEO DEO MeOH EtOH ${\Delta _f}H_{{\rm{g}},T}^{\Theta}$/
(kJ·mol−1)323 −687.19 −720.51 −753.83 −199.68 −232.99 338 −685.13 −718.07 −751.02 −199.00 −231.94 353 −683.02 −715.57 −748.13 −198.31 −230.86 368 −680.85 −713.00 −745.15 −197.59 −229.74 ${\Delta _f}H_{{\rm{l}},T}^{\Theta}$/
(kJ·mol−1)323 −734.79 −770.40 −810.10 −236.06 −273.54 338 −731.57 −766.73 −805.82 −234.26 −271.20 353 −728.66 −763.38 −801.91 −232.39 −268.77 368 −725.26 −759.92 −797.36 −230.41 −266.21 $S_{{\rm{g}},T}^{\Theta}$ /
(J·mol−1·K−1)323 351.47 400.16 437.33 243.30 286.23 338 357.70 407.53 445.83 245.34 289.40 353 363.83 414.77 454.20 247.36 292.54 368 369.84 421.90 462.45 249.34 295.65 $S_{{\rm{l}},T}^{\Theta}$ /
(J·mol−1·K−1)323 204.10 245.70 263.12 130.67 160.69 338 220.30 263.57 283.70 141.02 173.25 353 234.54 279.33 301.85 150.82 185.15 368 249.16 294.40 320.58 160.16 196.54 表 6 酯交换反应(1)−(4)在不同温度下的ΔrHΘ(kJ/mol)和ΔrSΘJ/(mol·K)
Table 6 Calculated enthalpy ΔrHΘ(kJ/mol)and entropy ΔrSΘJ/(mol·K)values of transesterifications(1)−(4)at different temperatures
T/K Reaction(1) Reaction(2) Reaction(3) Reaction(4) ΔrHΘ ΔrSΘ ΔrHΘ ΔrSΘ ΔrHΘ ΔrSΘ ΔrHΘ ΔrSΘ 323 −0.35 −1.02 1.87 11.58 −2.22 −12.60 −4.09 −24.18 338 −0.37 −1.06 1.78 11.04 −2.15 −12.10 −3.93 −23.14 353 −0.49 −1.35 1.66 10.46 −2.15 −11.81 −3.81 −22.27 368 −0.50 −1.34 1.14 8.86 −1.64 −10.20 −2.78 −19.06 表 7 酯交换反应(1)−(4)在不同温度下的ΔrGΘ(kJ/mol)和KΘ
Table 7 Calculated Gibbs free energy ΔrGΘ(kJ/mol)and equilibrium constant KΘ of reactions(1)−(4)at different temperatures
T/K Reaction(1) Reaction(2) Reaction(3) Reaction(4) ΔrGΘ KΘ ΔrGΘ KΘ ΔrGΘ KΘ ΔrGΘ KΘ 323 −2.054 × 10−2 1.007 −1.870 2.006 1.849 0.502 3.720 0.250 338 −1.172 × 10−2 1.004 −1.952 2.003 1.939 0.502 3.891 0.250 353 −1.345 × 10−2 1.004 −2.032 1.998 2.018 0.503 4.051 0.251 368 −6.880 × 10−3 1.002 −2.120 2.000 2.114 0.501 4.234 0.251 表 8 实验测定的n(DMO)/n(EtOH) = 1/4时各温度下的平衡常数及组成
Table 8 Measured equilibrium constant and composition (initial ratio of n(DMO)/n(EtOH)=1/4)
T/K t/min xDMO
/%sMEO
/%sDEO
/%wMEO
/%wDEO
/%KΘ 323 120 56.79 75.35 24.65 42.79 14.00 180 77.38 63.43 36.57 49.08 28.30 300 82.69 51.63 48.37 42.69 40.00 360 86.35 49.49 50.51 42.73 43.62 480 87.89 47.82 52.18 42.03 45.86 0.96 338 30 55.03 79.93 20.07 43.98 11.04 60 77.67 60.63 39.37 47.09 30.58 120 84.51 51.39 48.61 43.42 41.08 180 86.52 48.92 50.08 42.33 43.33 360 88.15 46.62 52.38 41.10 46.17 1.00 353 30 60.02 77.10 22.90 46.27 13.74 60 80.61 59.80 40.20 48.20 32.04 120 85.46 50.56 49.44 43.21 42.25 180 87.48 49.35 50.65 43.17 44.31 360 88.16 47.65 52.35 42.01 46.15 1.00 表 9 计算的n(DMO)/n(EtOH) = 1/4时各温度下的组成
Table 9 Calculated composition (initial ratio of n(DMO)/n(EtOH) = 1/4) at different temperatures
n(DMO)/
n(EtOH)T/K xDMO
/%sMEO
/%sDEO
/%wMEO
/%wDEO
/%1/4 323 88.95 49.58 50.42 44.10 44.84 338 88.93 49.57 50.43 44.08 44.85 353 88.92 49.52 50.48 44.03 44.89 368 88.92 49.59 50.41 44.10 44.82 表 10 不同物质的量比原料DMO转化率及DEO选择性和产率计算值与实验值比较
Table 10 Comparison between calculated and experimental values of DMO conversion, DEO selectivity and yield of raw materials with different initial molar ratios of reactants
n(DMO)/
n(EtOH)Calculation Experiment xDMO
/%sDEO
/%wDEO
/%xDMO
/%sDEO
/%wDEO
/%KΘ 1/6 93.75 60.00 56.25 93.50 61.75 57.74 1.00 1/12 97.95 75.00 73.46 97.92 75.81 74.23 1.00 1/24 99.40 85.71 85.20 99.38 88.32 87.78 1.01 -
[1] LI Y X, XUE B, YANG Y T. Synthesis of ethylbenzene by alkylation of benzene with diethyl oxalate over HZSM-5[J]. Fuel Process Technol,2009,90(10):1220−1225. doi: 10.1016/j.fuproc.2009.06.001 [2] WILEY R H, SLAYMAKER S C. Carbamylmaleimides from the malonamide-diethyl oxalate reaction[J]. J Am Chem Soc,1958,80(6):1385−1388. doi: 10.1021/ja01539a028 [3] CAMPAIGNE E, VAN VERTH J E. Reaction of diethyl oxalate with some ortho-substituted anilines[J]. J Org Chem,1958,23(9):1344−1346. doi: 10.1021/jo01103a029 [4] CREARY X. Reaction of organometallic reagents with ethyl trifluoroacetate and diethyl oxalate. Formation of trifluoromethyl ketones and a-keto esters via stable tetrahedral adducts[J]. J Org Chem,1987,52(22):5026−5030. doi: 10.1021/jo00231a036 [5] MIYAMURA S, SATOH T, MIURA M. Rhodium-catalyzed diarylation of oxalates using arylboron compounds[J]. J Org Chem,2007,72(6):2255−2257. doi: 10.1021/jo062628j [6] AFROOZ M, DEHGHANI H. First application of diethyl oxalate as efficient additive in high performance dye-sensitized solar cells based on iodide/triiodide electrolyte[J]. Electrochim Acta,2015,174:521−531. doi: 10.1016/j.electacta.2015.06.024 [7] CONTI C, ALIATIS I, CASATI M, COLOMBO C, MATTEINI M, NEGROTTI R, REALINI M, ZERBI G. Diethyl oxalate as a new potential conservation product for decayed carbonatic substrates[J]. J Cult Herit,2014,15(3):336−338. doi: 10.1016/j.culher.2013.08.002 [8] KENEALY W, HORN E, HOUTMAN C. Vapor-phase diethyl oxalate pretreatment of wood chips: Part 1. Energy savings and improved pulps[J]. Holzforschung,2007,61(3):223−229. doi: 10.1515/HF.2007.040 [9] KENEALY W, HORN E, DAVIS M, SWANEY R, HOUTMAN C. Vapor-phase diethyl oxalate pretreatment of wood chips: Part 2. Release of hemicellulosic carbohydrates[J]. Holzforschung,2007,61(3):230−235. doi: 10.1515/HF.2007.041 [10] HAO C Y, WANG S P, MA X B. Gas phase decarbonylation of diethyl oxalate to diethyl carbonate over alkali-containing catalyst[J]. J Mol Catal A: Chem,2009,306(1/2):130−135. doi: 10.1016/j.molcata.2009.02.038 [11] 郝翠英, 王胜平, 李振花, 马新宾. 草酸二乙酯气相脱羰基制碳酸二乙酯产物的气相色谱-质谱分析[J]. 天然气化工,2006,31(3):74−76.HAO Cui-ying, WANG Sheng-ping, LI Zhen-hua, MA Xin-bin. Analysis of diethyl carbonate from diethyl oxalate by GC-MS[J]. Nat Gas Ind,2006,31(3):74−76. [12] UCHIUMI S, ATAKA K, MATSUZAKI T. Oxidative reactions by a palladium-alkyl nitrite system[J]. J Org Chem,1999,576(1/2):279−289. doi: 10.1016/S0022-328X(98)01064-X [13] 马新宾, 许根慧, 陈锦文, 陈洪钫. CO气相催化偶联合成草酸二乙酯动力学[J]. 化工学报,1995,46(1):50−56.MA Xin-bin, XU Gen-hui, CHEN Jin-wen, CHEN Hong-fang. Kinetics of CO gas phase catalytic coupling to diethyl oxalate[J]. CIESC J,1995,46(1):50−56. [14] MENG F D, XU G H, GUO Q R. Kinetics of the catalytic coupling reaction of carbon monoxide to diethyl oxalate over Pd-Fe/a-Al2O3 catalyst[J]. J Mol Catal A: Chem,2003,201(1/2):283−288. doi: 10.1016/S1381-1169(03)00182-1 [15] FANG J G, WANG B W, LI Z H. Study on the reaction of CO coupling to oxalate[J]. React Kinet Catal Lett,2003,80(2):293−301. doi: 10.1023/B:REAC.0000006138.57139.16 [16] GAO Z H, LIU Z C, HE F, XU G H. Combined XPS and in situ DRIRS study of mechanism of Pd-Fe/α-Al2O3 catalyzed CO coupling reaction to diethyl oxalate[J]. J Mol Catal A: Chem,2005,235(1/2):143−149. doi: 10.1016/j.molcata.2005.03.003 [17] GAO X C, ZHAO Y J, WANG S P, YIN Y L, WANG B W, MA X B. A Pd-Fe/a-Al2O3/cordierite monolithic catalyst for CO coupling to oxalate[J]. Chem Eng Sci,2011,66(15):3513−3522. doi: 10.1016/j.ces.2011.04.012 [18] 李振花, 许根慧, 王保伟, 马新宾, 杜葩. H2对CO气相催化偶联制草酸二乙酯反应的失活机理[J]. 化工学报,2003,54(1):59−63. doi: 10.3321/j.issn:0438-1157.2003.01.014LI Zhen-hua, XU Gen-hui, WANG Bao-wei, MA Xin-bin, DU Pa. Deactivation mechanism of H2 on CO gas phase catalytic coupling to diethyl oxalate[J]. CIESC J,2003,54(1):59−63. doi: 10.3321/j.issn:0438-1157.2003.01.014 [19] 石磊, 于悦, 赵福鑫, 夏禹, 范家麒. 由草酸二甲酯及醇类一步合成对称草酸酯的方法: 中国, 108911975A[P]. 2018-11-30.SHI Lei, YU Yue, ZHAO Fu-xin, XIA Yu, FAN Jia-qi. Method for one-step synthesis of symmetrical oxalate from dimethyl oxalate and alcohol: CN, 108911975A[P]. 2018-11-30. [20] PRAUSNITZ J M, POLING B E, O’CONNELL J P. 气液物性估算手册[M]. 5版. 北京: 化学工业出版社, 2006: 48-55.PRAUSNITZ J M, POLING B E, O’CONNELL J P. The Properties of Gases and Liquids[M]. 5nd ed. Beijing: Chemical Industry Press, 2006: 48-55. [21] 马沛生. 有机化合物实验物性数据手册—含碳、氢、氧、卤部分[M]. 北京: 化学工业出版社, 2006: 521.MA Pei-sheng. Handbook of Experimental Physical Properties of Organic Compounds-Carbon, Hydrogen, Oxygen and Halogen[M]. Beijing: Chemical Industry Press, 2006: 521. [22] 天津大学物理化学教研室. 物理化学[M]. 5版. 北京: 高等教育出版社, 2009: 294-295.Department of Physical Chemistry, Tianjin University. Physical Chemistry[M]. 5nd ed. Beijing: Higher Education Press, 2009: 294-295. [23] 杨勇, 汤吉海, 陈献, 费兆阳, 崔咪芬, 乔旭. 甲基苯基碳酸酯合成氨基甲酸甲酯热力学分析[J]. 化学工业与工程,2016,33(1):10−14.YANG Yong, TANG Ji-hai, CHEN Xian, FEI Zhao-yang, CUI Mi-fen, QIAO Xu. Thermodynamic analysis of synthesis of methyl carbamate from methyl phenyl carbonate[J]. Chem Ind Eng,2016,33(1):10−14. [24] 贾晓强, 王桂荣, 赵新强, 王延吉. 合成甲苯二氨基甲酸苯酯的热力学分析[J]. 天然气化工(C1化学与化工),2013,38(2):19−23. doi: 10.3969/j.issn.1001-9219.2013.02.005JIA Xiao-qiang, WANG Gui-rong, ZHAO Xin-qiang, WANG Yan-ji. Thermodynamic analysis of synthesis of toluene diphenyl carbamate[J]. Nat Gas Ind,2013,38(2):19−23. doi: 10.3969/j.issn.1001-9219.2013.02.005 [25] 董新法, 方利国, 陈砺. 物性估算原理及计算机计算[M]. 北京: 化学工业出版社, 2006: 195−196.DONG Xin-fa, FANG Li-guo, CHEN Li. Physical Property Estimation Principle and Computer Calculation[M]. Beijing: Chemical Industry Press, 2006: 195−196. [26] 王丽萍. 碳酸二甲酯与乙醇酯交换反应体系的热力学分析[J]. 天然气化工(C1化学与化工),2012,37(5):23−26. doi: 10.3969/j.issn.1001-9219.2012.05.006WANG Li-ping. Thermodynamic analysis of transesterification reaction system of dimethyl carbonate and ethanol[J]. Nat Gas Ind,2012,37(5):23−26. doi: 10.3969/j.issn.1001-9219.2012.05.006 [27] 马沛生. 化工数据[M]. 北京: 中国石化出版社, 2003: 99−100.MA Pei-sheng. Chemical Data[M]. Beijing: China Petrochemical Press, 2003: 99−100. [28] J A 迪安. 兰氏化学手册[M]. 2版. 北京: 科学出版社, 2003: 58.J A DEAN. Gram's Handbook of Chemistry[M]. 2nd ed. Beijing: Science Press, 2003: 58. [29] 马沛生. 石油化工基础数据手册[M]. 北京: 化学工业出版社, 1993: 27.MA Pei-sheng. Basic Data Manual for Petrochemical Industry[M]. Beijing: Chemical Industry Press, 1993: 27. [30] FISHTINE S H. Hydrocarbon process petro[J]. Ind Eng Chem Res,1963,42(10):140−143. -





 下载:
下载: