Effect of Si/Al ratio of β zeolite on propane dehydrogenation without H2 over PtZn catalyst
-
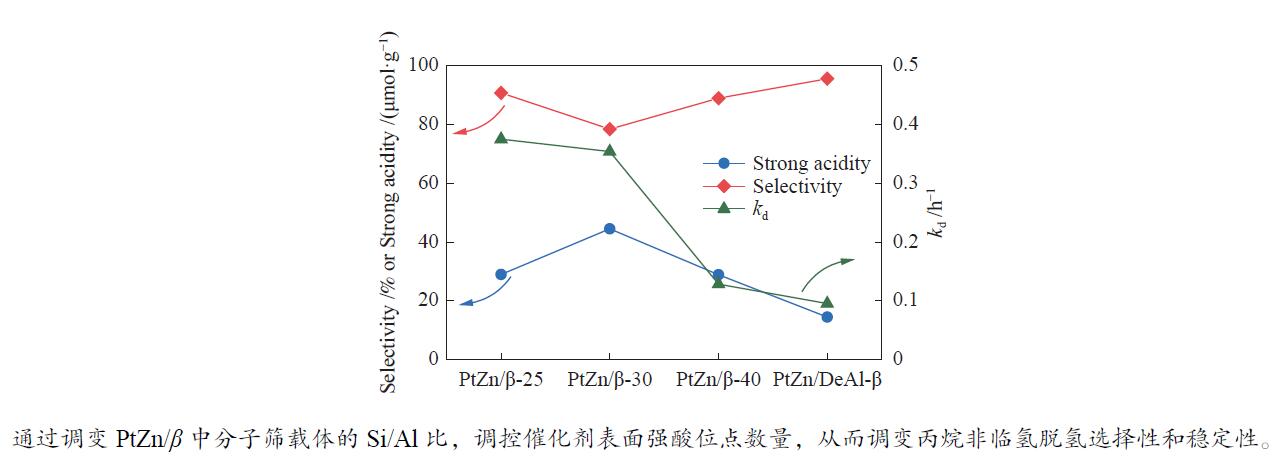
摘要: 采用共浸渍法制备PtZn/β-x(x为SiO2/Al2O3物质的量比)分子筛双金属催化剂,探究了β分子筛中硅铝比对丙烷非临氢脱氢反应性能的影响。采用XRD、BET、HAADF-STEM、NH3-TPD、C3H6-TPD等一系列表征技术对催化剂的物相结构、表面性质及其对丙烷非临氢脱氢反应性能的影响进行了研究。结果表明,催化稳定性随载体中Si/Al比的增大而提高(稳定性顺序:PtZn/DeAl-β > PtZn/β-40 > PtZn/β-30 > PtZn/β-25),而催化剂的强酸位点数量(PtZn/β-30 > PtZn/β-40 > PtZn/β-25 > PtZn/DeAl-β),在一定程度上受硅铝比的影响,与丙烯选择性顺序相反。因此,分子筛的Si/Al比对催化剂的性质有重要的调变作用,当催化剂强酸位点较少、丙烯吸附较弱、比表面积较大时,有助于提高丙烷转化率、丙烯选择性和催化稳定性。
-
关键词:
- 硅铝比 /
- PtZn双金属催化剂 /
- β分子筛 /
- 丙烷非临氢脱氢
Abstract: In this study, the PtZn/β-x catalysts (x refers to SiO2/Al2O3 mole ratio) were synthesized by co-impregnation method and the effect of Si/Al ratio of the β zeolite on propane non-hydrogen dehydrogenation was explored. A series of characterization such as XRD, BET, HAADF-STEM, NH3-TPD, C3H6-TPD were applied to investigate the phase structure, surface properties of catalysts, and their effects on the reaction performances. The results showed that, with the increase of Si/Al ratio, the catalytic stability would be enhanced following the order of PtZn/DeAl-β > PtZn/β-40 > PtZn/β-30 > PtZn/β-25, while the number of strong acid sites of the catalyst (PtZn/β-30 > PtZn/β-40 > PtZn/β-25 > PtZn/DeAl-β) was affected by the Si/Al ratio to some extent, which was opposite to the order of the propylene selectivity. Therefore, the control of the Si/Al ratio of zeolite is very important for optimizing the properties of the catalysts for propane dehydrogenation reaction. The catalysts with few strong acid sites, weak adsorption of propylene and large specific surface area are contributed to higher propane conversion, propylene selectivity and catalytic stability.-
Key words:
- Si/Al ratio /
- PtZn bimetallic catalyst /
- β zeolite /
- propane non-hydrogen dehydrogenation
-
图 8 (a) 不同目数和(b) 不同线速下PtZn/DeAl-β催化剂的丙烷脱氢催化性能比较
Figure 8 Comparison of PDH performance of PtZn/DeAl-β catalysts (a) with different mesh numbers and (b) at different linear velocities (Reaction conditions: 600 ℃, WHSV = 1.8 h−1, (a) 0.1 g catalyst, 20−40 or 60−80 mesh, 30 mL/min 5%C3H8/He(b) 0.02 g or 0.05 g or 0.1 g catalyst, total mass is 0.1 g, 60−80 mesh, 6 or 15 or 30 mL/min 5%C3H8/He)
表 1 PtZn/β-x系列催化剂的织构参数和Pt、Zn元素含量
Table 1 Textural parameters and Pt, Zn element content of PtZn/β-x catalysts
Catalyst $S_{{\rm{BET}}}^{\rm{a}} $ /
(m2·g−1)$S_{\rm{micro}}^{\rm{b}} $ /(m2·g−1) $S_{\rm{external}}^{\rm{b}} $ /(m2·g−1) $v_{\rm{micro}}^{\rm{b}} $ /
(cm3·g−1)$v_{\rm{meso}}^{\rm{c}} $ /
(cm3·g−1)Ptd w/% Znd w/% PtZn/β-25 436.4 305.6 130.7 0.16 0.28 0.50 2.60 PtZn/β-30 482.8 383.7 99.1 0.20 0.18 0.55 2.84 PtZn/β-40 438.6 345.2 93.4 0.18 0.17 0.57 2.83 PtZn/DeAl-β 499.5 329.5 170.0 0.17 0.48 0.56 2.83 a: Calculated by BET method, b: Calculated by t-plot method, c: Calculated by BJH method, d: Characterized by ICP-OES 表 2 PtZn/β-x系列催化剂的H2-TPR过程耗氢量
Table 2 Hydrogen consumption in H2-TPR process of PtZn/β-x catalysts
Catalyst Theoretical H2 consumption of
Ptδ + /(mmol·g−1)(1) /
(mmol·g−1)(2) /
(mmol·g−1)(3) /
(mmol·g−1)(4) /
(mmol·g−1)Reduction temperature of
Ptδ + /℃PtZn/β-25 0.025 0.039 0.002 0.012 0 −30.7 PtZn/β-30 0.028 0.043 0.003 0 0.13 −31.2 PtZn/β-40 0.029 0.034 0.010 0 0 −27.1 PtZn/DeAl-β 0.029 0.024 0.002 0.026 0 −26.6 表 3 PtZn/β-x系列催化剂反应3 h后的热重参数和失活速率常数(kd)
Table 3 Thermogravimetric parameters and deactivation rate constant (kd) of PtZn/β-x catalysts after 3 h propane dehydrogenation reaction without H2
Catalyst Weight /% Temp. range /℃ kd /h−1 PtZn/β-25 14.4 387−610 0.38 PtZn/β-30 12.5 397−610 0.35 PtZn/β-40 11.5 415−618 0.13 PtZn/DeAl-β 1.50 505−681 0.10 -
[1] LIU Y L, ADAM T, DANG Y L, ANNE M, STEVEN L S, PRASHANT D. Roles of enhancement of C-H activation and diminution of C-O formation within M1-phase pores in propane selective oxidation[J]. ChemCatChem,2020,13(3):882−899. [2] ZHANG H C, LI C S, LU Q, CHENG M J, WILLIAM A G. Selective activation of propane using intermediates generated during water oxidation[J]. J Am Chem Soc,2021,143(10):3967−3974. doi: 10.1021/jacs.1c00377 [3] HU Y Q, ZHAO F Y, WEI F, JIN Y. Ammoxidation of propylene to acrylonitrile in a bench-scale circulating fluidized bed reactor[J]. Chem Eng Process,2007,46(10):918−923. doi: 10.1016/j.cep.2007.05.009 [4] XI Z W, ZHOU N, SUN Y, LI K L. Reaction-controlled phase-transfer catalysis for propylene epoxidation to propylene oxide[J]. Science,2001,292(5519):1139−1141. doi: 10.1126/science.292.5519.1139 [5] LEI Y, MEHMOOD F, LEE S, GREELEY J, LEE B, SEIFERT S, WINANS R E, ELAM J W, MEYER R J, REDFERN P C, TESCHNER D, SCHLOGL R, PELLIN M J, CURTISS L A, VAJDA S. Increased silver activity for direct propylene epoxidation via subnanometer size effects[J]. Science,2010,328(5975):224−228. doi: 10.1126/science.1185200 [6] MATTEO M, MARIANNA G, SIPPAKORN W, BERT M W. Propane to olefins tandem catalysis: A selective route towards light olefins production[J]. Chem Soc Rev,2021,50(20):11503−11529. doi: 10.1039/D1CS00357G [7] ALI H M, RAWAN A, JAMES W, VALENTINA O I, SULJO L. Stable and selective catalysts for propane dehydrogenation operating at thermodynamic limit[J]. Science,2021,373(6551):217−222. doi: 10.1126/science.abg7894 [8] STEPHANIE S, MAARTEN K S, VLADIMIR V G, EVGENIY A R, STEPHANIE S, MARIE F R, GUY B M. The positive role of hydrogen on the dehydrogenation of propane on Pt (111)[J]. ACS Catal,2017,7:7495−7508. doi: 10.1021/acscatal.7b01584 [9] FENG B H, WEI Y C, SONG W Y, XU C M. A review on the structure-performance relationship of the catalysts during propane dehydrogenation reaction[J]. Pet Sci,2022,19(2):819−838. doi: 10.1016/j.petsci.2021.09.015 [10] SAJJAD R, CHEN L W, ANTONIO M, SIBUDJING K, ARMANDO B. Enhanced selectivity and stability of Pt-Ge/Al2O3 catalysts by Ca promotion in propane dehydrogenation[J]. Chem Eng J,2021,405:1−10. [11] WU X P, ZHANG Q, CHEN L G, LIU Q Y, ZHANG X H, ZHANG Q, MA L L, WANG C G. Enhanced catalytic performance of PtSn catalysts for propane dehydrogenation by a Zn-modified Mg(Al)O support[J]. Fuel Process Technol,2020,198:1−9. [12] 余长林, 徐恒泳, 陈喜蓉, 葛庆杰, 李文钊. PtZn-Sn/SBA-15合成、表征及对丙烷催化脱氢性能[J]. 燃料化学学报,2010,38(3):308−312. doi: 10.1016/S1872-5813(10)60036-9YU Chang-lin, XU Heng-yong, CHEN Xi-rong, GE Qing-jie, LI Wen-zhao. Preparation, characterization and catalytic performance of PtZn-Sn/SBA-15 catalyst for propane dehydrogenation[J]. J Fuel Chem Technol,2010,38(3):308−312. doi: 10.1016/S1872-5813(10)60036-9 [13] ZHOU H L, GONG J J, XU B L, DENG S C, DING Y H, YU L, FAN Y N. PtSnNa/SUZ-4: An efficient catalyst for propane dehydrogenation[J]. Chin J Catal,2017,38(3):529−536. doi: 10.1016/S1872-2067(17)62750-5 [14] SUN Q M, WANG N, FAN Q Y, ZENG L, ALVARO M, MIAO S, YANG R O, JIANG Z, ZHOU W, ZHANG J C, ZHANG T J, XU J, ZHANG P, CHENG J, YANG D C, JIA R, LI L, ZHANG Q H, WANG Y, OSAMU T, YU J H. Subnanometer bimetallic platinum-zinc clusters in zeolites for propane dehydrogenation[J]. Angew Chem Int Ed,2020,59(44):19450−19459. doi: 10.1002/anie.202003349 [15] QI L, MELIKE B, ZHANG Y F, ALICIA L, LIU L M, LI J W, CHEN Y Z, ADAM S H, SIMON R B, HAN Y, BRUCE C G, ALEXIS T B. Propane dehydrogenation catalyzed by isolated pt atoms in identical with ≡SiOZn-OH nests in dealuminated zeolite beta[J]. J Am Chem Soc,2021,143(50):21364−21378. doi: 10.1021/jacs.1c10261 [16] 薛琳, 刘红梅, 刘东兵, 张明森. 丙烷脱氢 Pt 催化剂稳定性研究进展[J]. 石油化工,2019,48(7):731−735.XUE Lin, LIU Hong-mei, LIU Dong-bing, ZHANG Ming-sen. Research progress in the stability of Pt-based catalysts for propane dehydrogenation[J]. Pet Technol,2019,48(7):731−735. [17] SHI L, DENG G M, LI W G, MIAO S, WANG Q N, ZHANG W P, LU A H. Al2O3 nanosheets rich in pentacoordinate Al3 + ions stabilize Pt-Sn clusters for propane dehydrogenation[J]. Angew Chem Int Ed,2015,54(47):13994−13998. doi: 10.1002/anie.201507119 [18] ZHU X Y, WANG T H, XU Z K, YUE Y Y, LIN M G, ZHU H B. Pt-Sn clusters anchored at Alpenta3 + sites as a sinter-resistant and regenerable catalyst for propane dehydrogenation[J]. J Energy Chem,2022,65:293−301. doi: 10.1016/j.jechem.2021.06.002 [19] YUKI N, XING F L, HYUNGWON H, KEN-ICHI S, SHINYA F. Doubly decorated platinum-gallium intermetallics as stable catalysts for propane dehydrogenation[J]. Angew Chem Int Ed,2021,60(36):19715−19719. doi: 10.1002/anie.202107210 [20] CHEN S, ZHAO Z J, MU R T, CHANG X, LUO J, STEPHEN C P, A JEREMY K, SUN G D, PEI C L, JEFFREY T M, ZHOU X H, EVGENY V, YANG Y, GONG J L. Propane dehydrogenation on single-site [PtZn4] intermetallic catalysts[J]. Chem,2021,7(2):387−405. doi: 10.1016/j.chempr.2020.10.008 [21] XU Z K, YUE Y Y, BAO X J, XIE Z L, ZHU H B. Propane dehydrogenation over Pt clusters localized at the Sn single-site in zeolite framework[J]. ACS Catal,2020,10:818−828. doi: 10.1021/acscatal.9b03527 [22] ZHANG B F, LI G Z, ZHAI Z W, CHEN D L, TIAN Y J, YANG R O, WANG L, ZHANG X W, LIU G Z. PtZn intermetallic nanoalloy encapsulated in silicalite‐1 for propane dehydrogenation[J]. AIChE J,2021,67(7):1−12. [23] TREACY M M NEWSAM J, J M. Two new three-dimensional twelve-ring zeolite frameworks of which zeolite beta is a disordered intergrowth[J]. Nature,1988,332(17):249−251. [24] WEI S, DAI H, LONG J P, LIN H Q, GU J K, ZONG X P, YANG D, TANG Y, YANG Y H, DAI Y H. Nonoxidative propane dehydrogenation by isolated Co2 + in BEA zeolite: Dealumination-determined key steps of propane C-H activation and propylene desorption[J]. Chem Eng J, 2022. [25] PHUOC H H, JUNGWON W, ROJIN F I, MUHAMMAD A S, DEREK C, LOUISE O. The effect of Si/Al ratio on the oxidation and sulfur resistance of beta zeolite-supported Pt and Pd as diesel oxidation catalysts[J]. ACS Eng Au,2021,2(1):27−45. [26] FAN X Q, LI J M, ZHAO Z, WEI Y C, LIU J, DUAN A J, JIANG G Y. Dehydrogenation of propane over PtSnAl/SBA-15 catalysts: Al addition effect and coke formation analysis[J]. Catal Sci Technol,2015,5:339−350. doi: 10.1039/C4CY00951G [27] 吴玉帅, 尤晴, 董旭杰, 朱子麒, 王旭, 陈汇勇, 马晓迅. 杂原子掺杂beta分子筛的烯烃环氧化催化性能[J]. 化工进展,2022,41(8):4192−4203.WU Yu-shuai, YOU Qing, DONG Xu-jie, ZHU Zi-qi, WANG Xu, CHEN Hui-yong, MA Xiao-xun. Synthesis of heteroatom-substituted beta zeolites for catalytic epoxidation of cyclic olefins[J]. Chem Ind Eng Prog,2022,41(8):4192−4203. [28] HO L W, HWANG C P, LEE J F, WANG I, YEH C T. Reduction of platinum dispersed on dealuminated beta zeolite[J]. J Mol Catal A-Chem,1998,136:293−299. doi: 10.1016/S1381-1169(98)00081-8 [29] CHEN C, SUN M L, HU Z P, REN J T, ZHANG S M, YUAN Z Y. New insight into the enhanced catalytic performance of ZnPt/HZSM-5 catalysts for direct dehydrogenation of propane to propylene[J]. Catal Sci Technol,2019,9(8):1979−1988. doi: 10.1039/C9CY00237E [30] WANG H Z, SUN L L, SUI Z J, ZHU Y A, YE G H, CHEN D, ZHOU X G, YUAN W K. Coke formation on Pt-Sn/Al2O3 catalyst for propane dehydrogenation[J]. Ind Eng Chem Res,2018,57:8647−8654. doi: 10.1021/acs.iecr.8b01313 [31] ZHANG Y W, ZHOU Y M, LIU H, WANG Y, XU Y, WU P C. Effect of La addition on catalytic performance of PtSnNa/ZSM-5 catalyst for propane dehydrogenation[J]. Appl Catal A: Gen,2007,333(2):202−210. doi: 10.1016/j.apcata.2007.07.049 [32] CHEN S, CHANG X, SUN G D, ZHANG T T, XU Y Y, WANG Y, PEI C L, GONG J L. Propane dehydrogenation: catalyst development, new chemistry, and emerging technologies[J]. Chem Soc Rev,2021,50:3315−3354. doi: 10.1039/D0CS00814A [33] ZHANG Y W, ZHOU Y M, HUANG L, ZHOU S J, SHENG X L, WANG Q L, ZHANG C. Structure and catalytic properties of the Zn-modified ZSM-5 supported platinum catalyst for propane dehydrogenation[J]. Chem Eng J,2015,270:352−361. doi: 10.1016/j.cej.2015.01.008 [34] YU J F, WANG R, REN S Y, SUN X Y, CHEN C L, GE Q J, FANG W, ZHANG J, XU H Y, SU D S. The unique role of CaO in stabilizing the Pt/Al2O3 catalyst for the dehydrogenation of cyclohexane[J]. ChemCatChem,2012,4:1376−1381. doi: 10.1002/cctc.201200067 [35] LIU L C, MIGUEL L H, CHRISTIAN W L, SERGIO R B, PATRICIA C, RAMON M, LAURA S, AARON S, PEDRO S, JOSE J C, AVELINO C. Structural modulation and direct measurement of subnanometric bimetallic PtSn clusters confined in zeolites[J]. Nat Catal,2020,3:628−638. doi: 10.1038/s41929-020-0472-7 -





 下载:
下载: