Synthesis of propylene carbonate from CO2 catalyzed by supported imidazoles ionic liquids
-
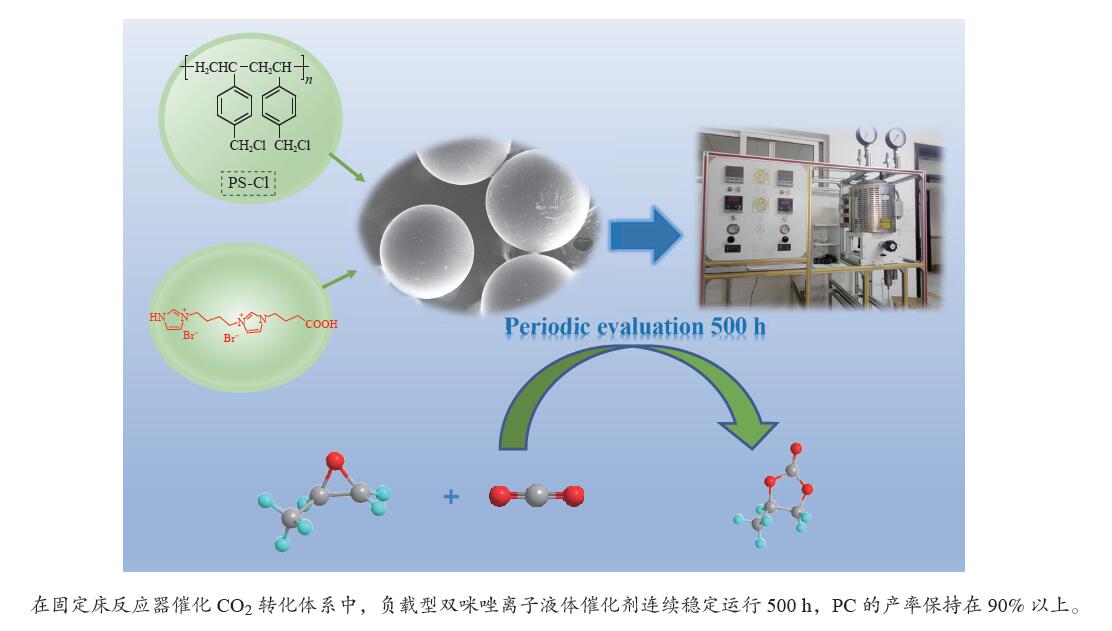
摘要: CO2是导致全球气候变暖的主要温室气体之一,但同时又是丰富的C1资源,故其高值化利用受到了人们关注。环状碳酸酯是电池及电容器的优良介质,在工业生产中应用极为广泛。因此,从环境保护和资源利用的角度看,将CO2转化为环状碳酸酯具有重要的意义。本工作合成了一系列聚苯乙烯树脂负载的咪唑类非均相催化剂,考察了此类催化剂在高压反应釜中催化二氧化碳环加成反应的活性。结果表明,催化剂PS-TBIM-PCIMBr2表现出优秀且稳定的催化活性,PS-TBIM-PCIMBr2在固定床连续反应器上可以连续运行500 h,反应依旧可以获得91%的产率。Abstract: Carbon dioxide is one of the most main greenhouse gases causing the global warming, however, as a rich C1 resource, the high value utilization of CO2 has attracted wide attention. Cyclic carbonate is an excellent medium for batteries and capacitors, which is widely used in industrial production. Therefore, it is of great significance to convert CO2 into cyclic carbonate from the viewpoint of environmental protection and resource utilization. In this paper, we synthesized a series of imidazole heterogeneous catalysts supported on polystyrene resin, and the catalytic activity for cycloaddition reaction of CO2 in high pressure reactor was studied. The results showed that PS-TBIM-PCIMBr2 exhibited the excellent and stable catalytic activity. PS-TBIM-PCIMBr2 was also used to prepare propylene carbonate in the continuous fixed-bed reactor and the yield of PC was still 91% after 500 h.
-
Key words:
- carbon dioxide /
- propylene oxide /
- cyclic carbonate /
- imidazole ionic liquid /
- heterogeneous catalyst
-
表 1 催化剂的元素分析
Table 1 Elemental analysis data of catalysts
Entry Catalyst N /% C /% H /% IL grafting amount
/(mmol·g−1)1 PS-MIMCl 6.25 83.94 7.35 2.23 2 PS-CPIMBr 7.71 60.13 5.63 2.75 3 PS-TBIM-PCIMBr2 7.95 60.19 6.71 1.42 4 PS-TBIM-IMPCOOHTMGBr2 9.53 55.44 6.11 0.97 表 2 CO2制备碳酸丙烯酯反应的催化活性a
Table 2 Catalytic activity of the preparation of propylene carbonate from CO2a
Entry Catalyst Selectivityb /% Yieldb /% 1 − − 0 2 PS-Cl − 0 3 PS-MIMCl 99 40 4 PS-CPIMBr 99 83 5 PS-TBIM-PCIMBr2 99 90 6 PS-TBIM-IMPCOOHTMGBr2 99 91 a: Reaction conditions: PO 0.137 mol, catalyst 20%, 120 ℃, CO2 2 MPa, 4 h; b: Determined by GC analysis 表 3 各种环氧化物与二氧化碳反应的催化活性a
Table 3 Catalytic activity of various epoxides with carbon dioxidea
Entry Epoxide Cyclic carbonate Selectivityb /% Yieldb/% 1 

99 99 2 

99 99 3 

99 98 4 

99 96 5 

99 68 6 

99 95 a: Reaction conditions: Epoxide (0.137 mol), PS-TBIM-PCIMBr2 (20%), 120 ℃, CO2 2 MPa, 5 h, b: Determined by GC analysis -
[1] GUO L, DENG L, JIN X, WU H, YIN L. Catalytic conversion of CO2 into propylene carbonate in a continuous fixed bed reactor by immobilized ionic liquids[J]. RSC Adv,2018,8(47):26554−26562. doi: 10.1039/C8RA03952F [2] CAO H, LIU S, WANG X. Environmentally benign metal catalyst for the ring-opening copolymerization of epoxide and CO2: State-of-the-art, opportunities, and challenges[J]. Green Chem Eng,2022,3(2):111−124. doi: 10.1016/j.gce.2021.11.005 [3] REIS N V D, ARRON C, ROSETTO, GLORIA. Heterodinuclear Mg(II)M(II) (M=Cr, Mn, Fe, Co, Ni, Cu and Zn) complexes for the ring opening copolymerization of carbon dioxide/epoxide and anhydride/epoxide[J]. Chem,2022,28(14):e202104198. [4] RAZAGHI M, KHORASANI M. Boosting the quaternary ammonium halides catalyzed CO2 coupling with epoxides on the hollow mesoporous silica sphere[J]. J CO2 Util,2022,61:102028. [5] GENG H, ZHANG C, TAO M, MA N, ZHANG W. Ionic microenvironment constructed in quaternary ammonium modified polyacrylonitrile fiber for efficient CO2 fixation[J]. J CO2 Util,2021,49:101559. doi: 10.1016/j.jcou.2021.101559 [6] ZHANG W Y, LUO R C, XU Q H, CHEN Y J, LIN X W, ZHOU X T, JI H B. Transformation of carbon dioxide into valuable chemicals over bifunctional metallosalen catalysts bearing quaternary phosphonium salts[J]. Chin J Catal,2017,38(4):736−744. doi: 10.1016/S1872-2067(17)62802-X [7] ZHANG W, LUO R, XU Q, CHEN Y, LIN X, ZHOU X, JI H. Alkali metal salt as catalyst for direct synthesis of carbamate from carbon Dioxide[J]. ACS Sustainable Chem Eng,2018,6(5):6675−6681. doi: 10.1021/acssuschemeng.8b00449 [8] KANG Y R, WANG B N, NAN R X, LI Y W, ZHU Z L, XIAO X Q. Cyclic carbonate synthesis from epoxides and CO2 catalyzed by aluminum-salen complexes bearing a nido-C2B9 carborane ligand[J]. Inorg Chem,2022,61(23):8806−8814. doi: 10.1021/acs.inorgchem.2c00797 [9] SANG Y F, CAO Y W, WANG L Z, YAN W, CHEN T, HUANG J W, LIU Y N. N-rich porous organic polymers based on Schiff base reaction for CO2 capture and mercury(II) adsorption[J]. J Colloid Interface Sci,2021,587:121−130. doi: 10.1016/j.jcis.2020.12.002 [10] YANG G Y, YU J L, PENG S W, SHENG K, ZHANG H N. Poly(ionic liquid)-modified metal organic framework for carbon dioxide adsorption[J]. Polym,2020,12:370. doi: 10.3390/polym12020370 [11] ZHANG H, HUO J H, YANG H W, LI F, DUAN C X, XI H X. Green and rapid preparation of hierarchically porous metal-organic zeolites and simulation of their growth[J]. J Mater Chem A,2019,7(3):1022−1029. doi: 10.1039/C8TA08702D [12] ZHANG Y, LIU L, XU W G, HAN Z B. MOF@POP core-shell architecture as synergetic catalyst for high-efficient CO2 fixation without cocatalyst under mild conditions[J]. J CO2 Util,2021,46:101463. doi: 10.1016/j.jcou.2021.101463 [13] HE W F, WEN M S, SHI L J, WANG R M, LI F W. Porous polymeric metalloporphyrin obtained through sonogashira coupling: Catalytic performance at CO2 cycloaddition to epoxides[J]. J Solid State Chem,2022,309:122965. [14] ZHAO Y M, PENG Y L, SHAN C, LU Z, WOJTAS L, ZHANG Z, ZHANG B, FENG Y, MA S. Metallocorrole-based porous organic polymers as a heterogeneous catalytic nanoplatform for efficient carbon dioxide conversion[J]. Nano Res,2021,15:1145−1152. [15] WANG J J, WANG L Z, WANG Y, YANG F, LI J W, GUAN X Y, ZONG J J, ZHOU F, HUANG J H, LIU Y N. Covalently connected core-shell NH2-UiO-66@Br-COFs hybrid materials for CO2 capture and I2 vapor adsorption[J]. Chem Eng J,2022,438:135555. doi: 10.1016/j.cej.2022.135555 [16] LI C, LIU F, ZHAO T X, GU J R, CHEN P, CHEN T. Highly efficient CO2 fixation into cyclic carbonate by hydroxyl-functionalized protic ionic liquids at atmospheric pressure[J]. Mol Catal,2021,511:24688231. [17] AL-GARNI T, AL-JALLAL N, AOUISSI A. Synthesis of propylene carbonate from epoxide and CO2 catalyzed by carbon nanotubes supported Fe1.5PMo12O40[J]. J Chem,2017,5641604. [18] ILIUTA I, LARACHI F, FONTAINE F G. Performance of catalytic cycloaddition of CO2 to styrene oxide in three-phase co-current (micro)fixed-bed and monolith reactors[J]. J CO2 Util,2022,60:101977. [19] LONG G C, SU K, DONG H N, ZHAO T X, YANG C L, LIU F, HU X B. Straightforward construction of amino-functionalized ILs@SBA-15 catalysts via mechanochemical grafting for one-pot synthesis of cyclic carbonates from aromatic olefins and CO2[J]. J CO2 Util,2022,59:101962. doi: 10.1016/j.jcou.2022.101962 [20] 张建, 张志智, 孙潇磊, 孙万付, 方向晨. CO2与环氧丙烷合成碳酸丙烯酯的多相催化剂研究[J]. 天然气化工(C1 化学与化工),2015,40(3):41−44.ZHANG Jiang, ZHANG Zhi-zhi, SUN Xiao-lei, SUN Wan-fu, FANG Xiang-cheng. Synthesis of propylene carbonate from CO2 and propylene oxide over a novel heterogeneous catalyst[J]. Nat Gas Chem Ind,2015,40(3):41−44. [21] LIU Y, HU Y H, ZHOU J S, ZHU Z Y, ZHANG Z K, LI Y Y, WANG L, ZHANG J L. Polystyrene-supported novel imidazolium ionic liquids: Highly efficient catalyst for the fixation of carbon dioxide under atmospheric pressure[J]. Fuel,2021,305:00162361. [22] ZHANG X, GENG W H, YUE C T, WU W, XIAO L F. Multilayered supported ionic liquids bearing a carboxyl group: Highly efficient catalysts for chemical fixation of carbon dioxide[J]. J Environ Chem Eng,2016,4(2):2565−2572. doi: 10.1016/j.jece.2016.05.001 [23] GUO L Y, DENG L L, JIN X C, WU Hao, YIN L Z. Composite ionic liquids immobilized on MCM-22 as efficient catalysts for the cycloaddition reaction with CO2 and propylene oxide[J]. Catal Lett,2017,147(9):2290−2297. doi: 10.1007/s10562-017-2137-y [24] JADHAV A H, THORAT G M, LEE K, LIM A C, KANG H, SEO J G. Effect of anion type of imidazolium based polymer supported ionic liquids on the solvent free synthesis of cycloaddition of CO2 into epoxide[J]. Catal Today,2016,265:56−67. doi: 10.1016/j.cattod.2015.09.048 [25] ZHANG X, SU D, XIAO L F, WU W. Immobilized protic ionic liquids: Efficient catalysts for CO2 fixation with epoxides[J]. J CO2 Util,2017,17:37−42. doi: 10.1016/j.jcou.2016.11.005 [26] ZHANG Z Z, SUN X L, ZHANG X W, FANG X C. Catalytic synthesis of propylene carbonate from CO2 and propylene oxide on fixed bed[J]. Catal Lett,2016,146(10):2098−2104. doi: 10.1007/s10562-016-1808-4 [27] MENG X L, NIE Y, SUN J, CHENG W G, WANG J Q, HE H Y, ZHANG S J. Functionalized dicyandiamide–formaldehyde polymers as efficient heterogeneous catalysts for conversion of CO2 into organic carbonates[J]. Green Chem,2014,16(5):2771−2778. doi: 10.1039/C3GC42331J -





 下载:
下载: