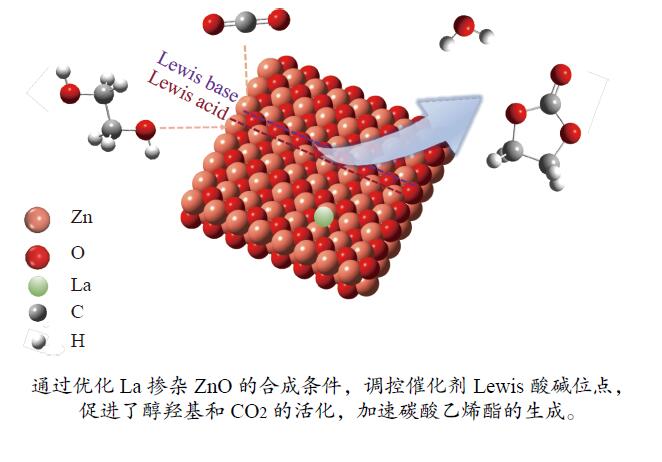
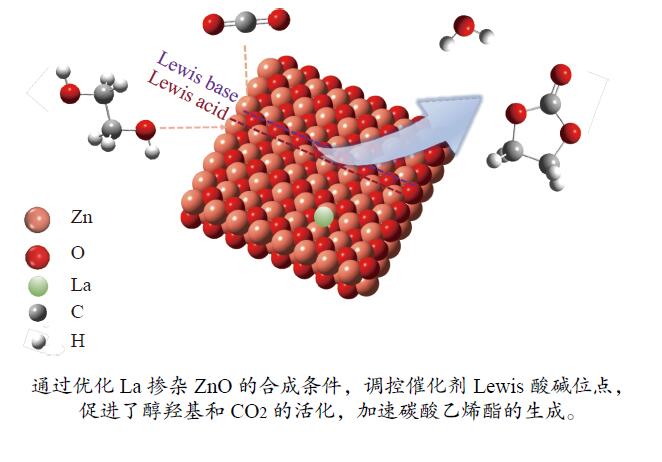
Lewis acid-base modulated lanthanum-doped zinc oxide catalyzed CO2 conversion to ethylene carbonate
-
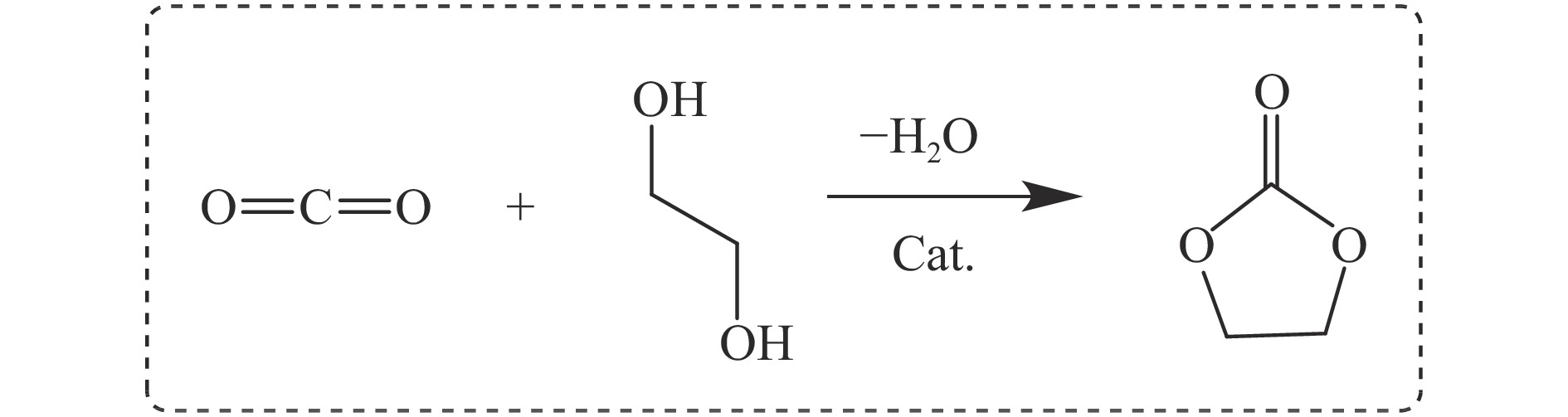
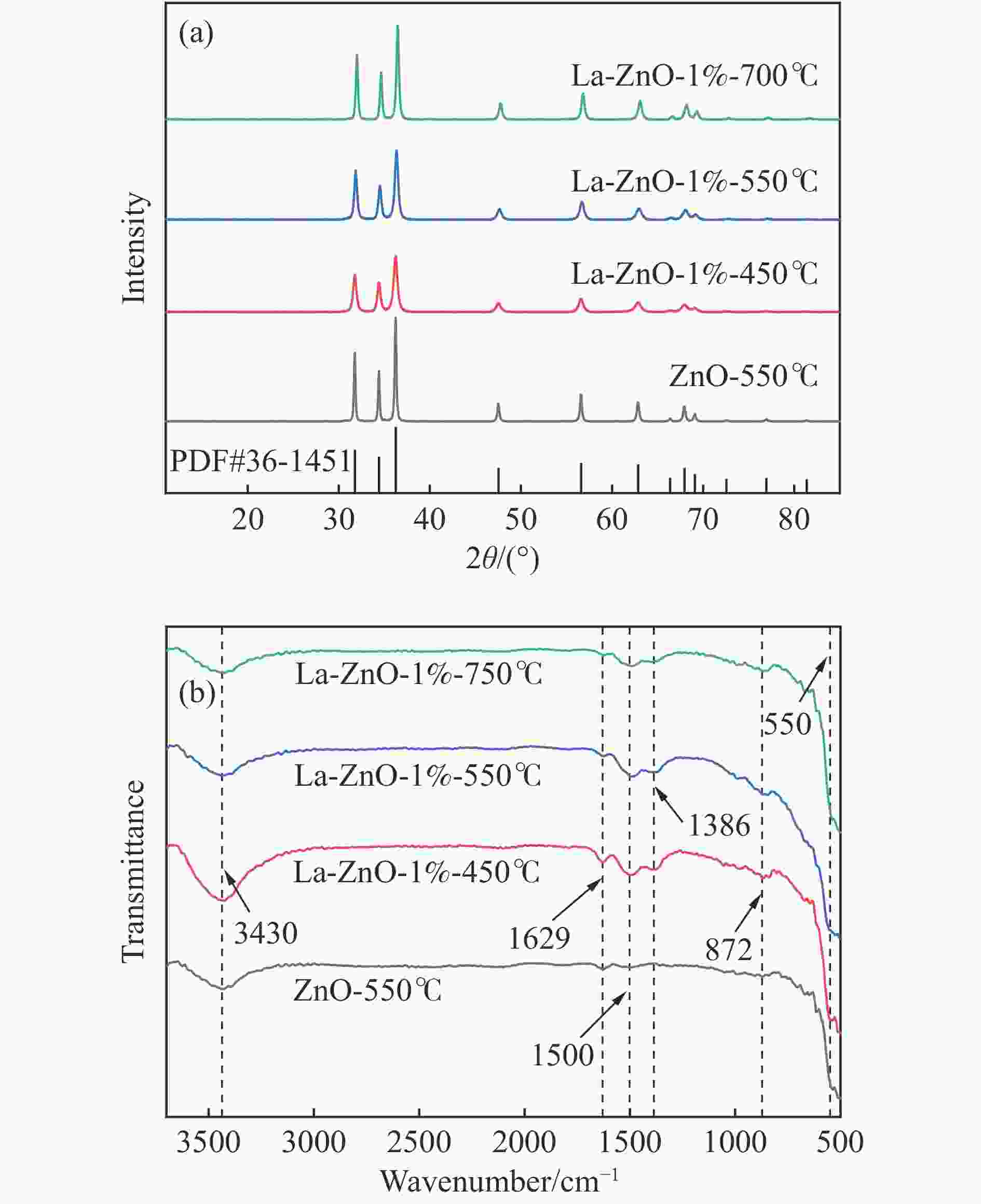
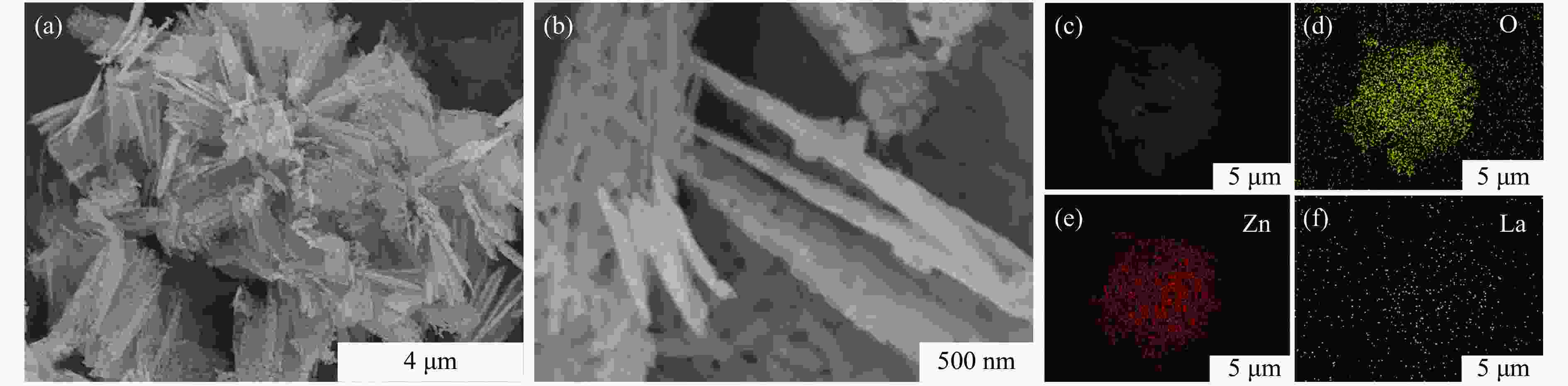
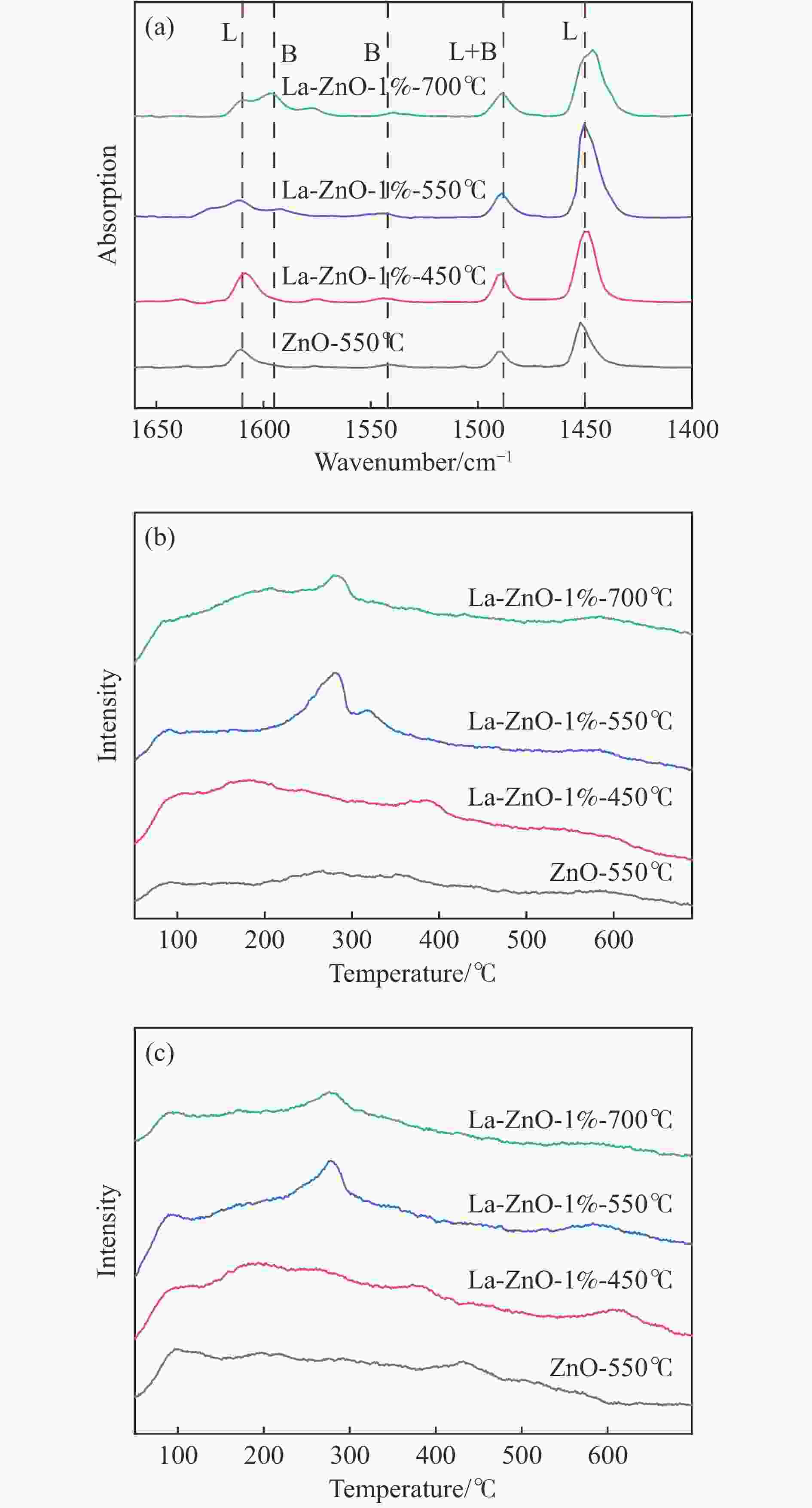
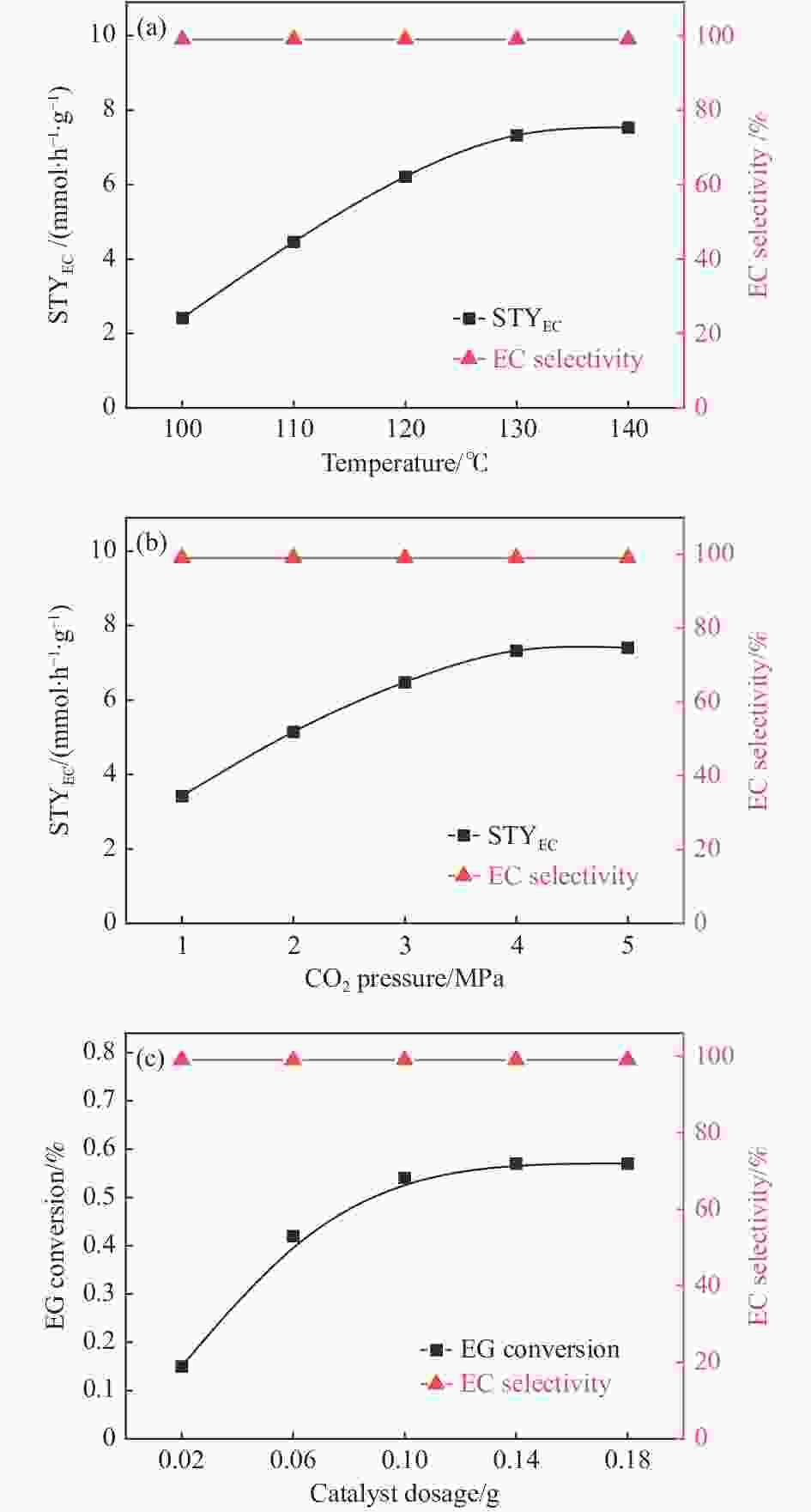
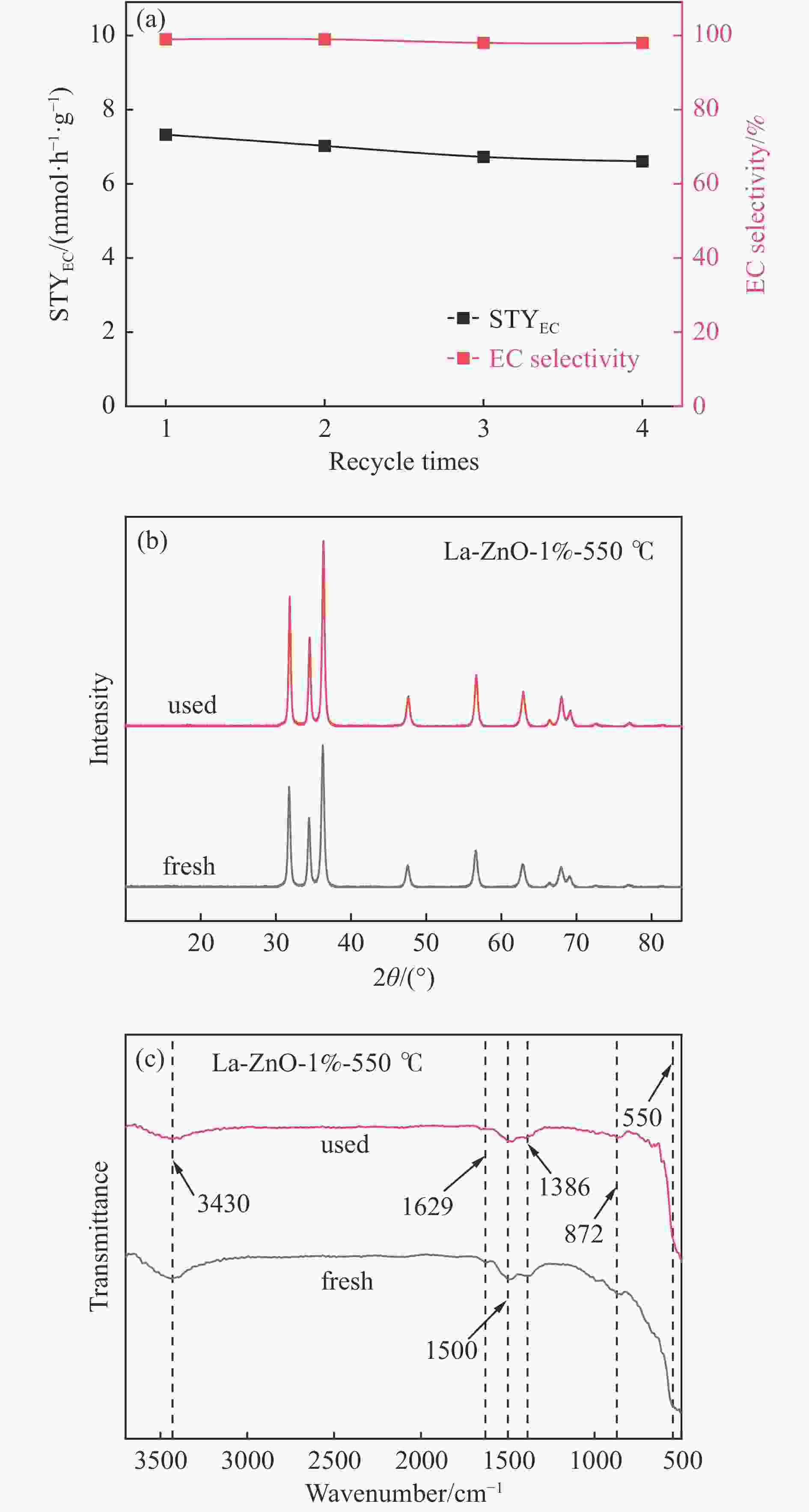
摘要: 本研究以CO2和乙二醇(EG)合成碳酸乙烯酯(EC)为目标,设计合成一系列La掺杂ZnO催化剂,可对ZnO表面Lewis酸碱性位点调控,并在无助剂条件下研究了催化剂活性。La-ZnO-1%-550℃具有最好的催化活性,在130 ℃、4 MPa CO2、1 h条件下,EG的转化率为0.54%,EC的时空收率和选择性分别为7.326 mmol/(h∙g)和99%,并具有良好的稳定性。结合对催化剂的晶体结构、形貌和表面酸碱性等分析,结果显示,La均匀分布在ZnO中空纳米片中,经过550 ℃煅烧的La掺杂ZnO的表面具有最多的Lewis酸碱性位点,催化剂的催化活性随中强Lewis酸碱性位点增多而升高。Abstract: The massive emission of the greenhouse gas CO2 has caused problems such as global warming and ecological damage. How to effectively utilize CO2 as a resource and create economic benefits has attracted much attention in recent years. In this paper, a series of La-doped ZnO catalysts were designed and synthesized targeting the synthesis of ethylene carbonate (EC) from CO2 and ethylene glycol (EG), which could modulate the Lewis acid-base sites on the ZnO surface, and the catalyst activity was investigated under additive-free conditions. La-ZnO-1%-550℃ had the best catalytic activity with 0.54% conversion of EG, 7.326 mmol/(h∙g) and 99% space-time yield and selectivity of EC at 130 ℃, 4 MPa CO2, and 1 h with good stability. Combined with the analysis of the crystal structure, morphology and surface acid-base of the catalysts, the results showed that La was uniformly distributed in the ZnO hollow nanosheets, and the surface of the La-doped ZnO calcined at 550 ℃ had the most Lewis acid-base sites, and the catalytic activity of the catalysts increased with the increase of moderate to strong Lewis acid-base sites.
-
Key words:
- CO2 /
- ZnO /
- ethylene carbonate /
- Lewis acid-base catalysis
-
表 1 催化剂的各种状态氧占比和La占Zn的比例
Table 1 The proportion of oxygen in various states of the catalyst and the proportion of La to Zn
Catalyst OL/% OV/% OC/% La/Zn/% ZnO-550℃ 19.28 54.53 26.20 0.0 La-ZnO-1%-450℃ 24.43 66.44 9.13 1.2 La-ZnO-1%-550℃ 26.76 58.02 15.18 1.1 La-ZnO-1%-700℃ 19.96 69.19 10.85 1.1 表 2 Py-FTIR和TPD结果定量酸碱位点数量
Table 2 Number of acid-base sites quantified by Py-FTIR and TPD
Catalyst Acid/(mmol∙g−1) Base/(mmol∙g−1) B/L
acidweak moderate weak moderate ZnO-550℃ 0.0352 0.0992 0.0504 0.0747 0.0424 La-ZnO-1%-450℃ 0.0921 0.1425 0.0836 0.1263 0.0582 La-ZnO-1%-550℃ 0.0816 0.3786 0.0785 0.2316 0.0315 La-ZnO-1%-700℃ 0.0965 0.2718 0.0671 0.1759 0.0894 表 3 不同催化剂的催化活性
Table 3 Catalytic activity of different catalysts
Catalyst xEG/% STYEC/
(mmol∙h−1∙g−1)sEC/% − 0 0 0 ZnO-550℃ 0.16 1.141 99 Ce-ZnO-1%-550℃ 0.11 0.970 99 Ni-ZnO-1%-550℃ 0.07 0.446 99 Co-ZnO-1%-550℃ 0.28 1.995 99 La-ZnO-1%-550℃ 0.64 4.711 99 La-ZnO-1%-450℃ 0.23 1.651 99 La-ZnO-1%-700℃ 0.32 2.864 99 CeO2-ZrO2a[4] 1.33 1.330 100 Reaction conditions: 150 mmol EG, 0.1 g catalyst, 130 ℃, 4 MPa CO2 and 2 h; a: 100 mmol EG, 120 mmol acetonitrile (dehydrating agent), 0.5 g CeO2-ZrO2, 150 ℃, 3.5 MPa CO2 and 2 h. 表 4 反应时间对合成EC的影响
Table 4 Effect of reaction time on the synthesis of EC
Reaction time/h xEG/% STYEC/(mmol∙h−1∙g−1) sEC/% 0.5 0.31 8.461 99 1 0.54 7.326 99 2 0.64 4.511 99 Reaction conditions: 150 mmol EG, 0.1 g La-ZnO-1%-550℃, 130 ℃ and 4 MPa CO2. -
[1] MÜLLER L J, KÄTELHÖN A, BRINGEZU S, et al. The carbon footprint of the carbon feedstock CO2[J]. Energy Environ Sci,2020,13(9):2979−2992. doi: 10.1039/D0EE01530J [2] DAS S, PEREZ-RAMIREZ J, GONG J, et al. Core-shell structured catalysts for thermocatalytic, photocatalytic, and electrocatalytic conversion of CO2[J]. Chem Soc Rev,2020,49(10):2937−3004. doi: 10.1039/C9CS00713J [3] LIU W, WANG Y, ZHANG J, et al. Large particle spherical poly-ionic liquid-solid base catalyst for high-efficiency transesterification of ethylene carbonate to prepare dimethyl carbonate[J]. Fuel,2022,324:124580−124590. doi: 10.1016/j.fuel.2022.124580 [4] TOMISHIGE K, YASUDA H, YOSHIDA Y, et al. Catalytic performance and properties of ceria based catalysts for cyclic carbonate synthesis from glycol and carbon dioxide[J]. Green Chem,2004,6(4):201−214. [5] LIM Y N, LEE C, JANG H-Y. Metal-free synthesis of cyclic and acyclic carbonates from CO2 and alcohols[J]. Eur J Org Chem,2014,2014(9):1823−1826. doi: 10.1002/ejoc.201400031 [6] HUANG S, LIU S, LI J, et al. Effective synthesis of propylene carbonate from propylene glycol and carbon dioxide by alkali carbonates[J]. Catal Lett,2006,112(3−4):187−191. doi: 10.1007/s10562-006-0201-0 [7] MEHTA S, JOSHI K. From molecular adsorption to decomposition of methanol on various ZnO facets: A periodic DFT study[J]. Appl Surf Sci,2022,602:154150−154157. doi: 10.1016/j.apsusc.2022.154150 [8] XUE L, ZHANG C, SHI T, et al. Unraveling the improved CO2 adsorption and COOH* formation over Cu-decorated ZnO nanosheets for CO2 reduction toward CO[J]. Chem Eng J,2023,452:139701−139714. doi: 10.1016/j.cej.2022.139701 [9] LI P, ZHU S, HU H, et al. Influence of defects in porous ZnO nanoplates on CO2 photoreduction[J]. Catal Today,2019,335:300−305. doi: 10.1016/j.cattod.2018.11.068 [10] ZHU H, YUAN Z, SHEN Y, et al. Conductometric acetic anhydride gas sensors based on S-doped porous ZnO microspheres with enhanced Lewis base interaction[J]. Sensors Actuat B-Chem,2022,373:132726−132741. doi: 10.1016/j.snb.2022.132726 [11] JADHAV N H, SHINDE D R, SAKATE S S, et al. Ti(IV) doping: An effective strategy to boost Lewis acidic performance of ZnO catalyst in fluorescein dye synthesis[J]. Catal Commun,2019,120:17−22. doi: 10.1016/j.catcom.2018.11.008 [12] SINGH K, NANCY, KAUR H, et al. ZnO and cobalt decorated ZnO NPs: Synthesis, photocatalysis and antimicrobial applications[J]. Chemosphere,2023,313:137322−137342. doi: 10.1016/j.chemosphere.2022.137322 [13] TOMAZETT V K, CHACON G, MARIN G, et al. Ionic liquid confined spaces controlled catalytic CO2 cycloaddition of epoxides in BMIm. ZnCl3 and its supported ionic liquid phases[J]. J CO2 Util,2023,69:102400−102409. doi: 10.1016/j.jcou.2023.102400 [14] ZONG X, JIN Y, LI Y, et al. Morphology-controllable ZnO catalysts enriched with oxygen-vacancies for boosting CO2 electroreduction to CO[J]. J CO2 Util,2022,61:102051−102060. doi: 10.1016/j.jcou.2022.102051 [15] CHENG F, YANG J, YAN L, et al. Enhancement of La2O3 to Li-Mn/WO3/TiO2 for oxidative coupling of methane[J]. J Rare Earth,2020,38(2):167−174. doi: 10.1016/j.jre.2019.03.023 [16] POORNAPRAKASH B, CHALAPATHI U, SUBRAMANYAM K, et al. Wurtzite phase Co-doped ZnO nanorods: Morphological, structural, optical, magnetic, and enhanced photocatalytic characteristics[J]. Ceram Int,2020,46(3):2931−2939. doi: 10.1016/j.ceramint.2019.09.289 [17] AL-SULTAN F S, BASAHEL S N, NARASIMHARAO K. Yttrium oxide supported La2O3 nanomaterials for catalytic oxidative cracking of n-propane to olefins[J]. Catal Lett,2019,150(1):185−195. [18] RANJBARI A, DEMEESTERE K, KIM K-H, et al. Oxygen vacancy modification of commercial ZnO by hydrogen reduction for the removal of thiabendazole: Characterization and kinetic study[J]. Appl Catal B: Environ,2023,324:122265−122283. doi: 10.1016/j.apcatb.2022.122265 [19] SUN K, ZHAN G, ZHANG L, et al. Highly sensitive NO2 gas sensor based on ZnO nanoarray modulated by oxygen vacancy with Ce doping[J]. Sensor Actuat B-Chem,2023,379:133294−133304. doi: 10.1016/j.snb.2023.133294 [20] YANG J, WANG H, YANG H, et al. Efficient electroreduction of CO2 to syngas over ZIF-8 derived oxygen vacancy-rich ZnO nanomaterials[J]. New J Chem,2023,47(10):4992−4998. doi: 10.1039/D2NJ05378K [21] SCOTTI N, DANGATE M, GERVASINI A, et al. Unraveling the role of low coordination sites in a Cu metal nanoparticle: A step toward the selective synthesis of second generation biofuels[J]. ACS Catal,2014,4(8):2818−2826. doi: 10.1021/cs500581a [22] LIN L, LIU J, ZHANG X, et al. Effect of zeolitic hydroxyl nests on the acidity and propane aromatization performance of zinc nitrate impregnation-modified HZSM-5 zeolite[J]. Ind Eng Chem Res,2020,59(37):16146−16160. doi: 10.1021/acs.iecr.0c02596 [23] JU F, WU T, WANG M, et al. Effect of nitrogen compounds on reactive adsorption desulfurization over NiO/ZnO-Al2O3-SiO2 adsorbents[J]. Ind Eng Chem Res,2019,58(29):13401−13407. doi: 10.1021/acs.iecr.9b01682 [24] JIN L, WANG Y. Surface chemistry of methanol on different ZnO surfaces studied by vibrational spectroscopy[J]. Phys Chem Chem Phys,2017,19(20):12992−13001. doi: 10.1039/C7CP01715D [25] GONG Z-J, LI Y-R, WU H-L, et al. Direct copolymerization of carbon dioxide and 1, 4-butanediol enhanced by ceria nanorod catalyst[J]. Appl Catal B: Environ,2020,265:118524−118536. doi: 10.1016/j.apcatb.2019.118524 -




 下载:
下载: