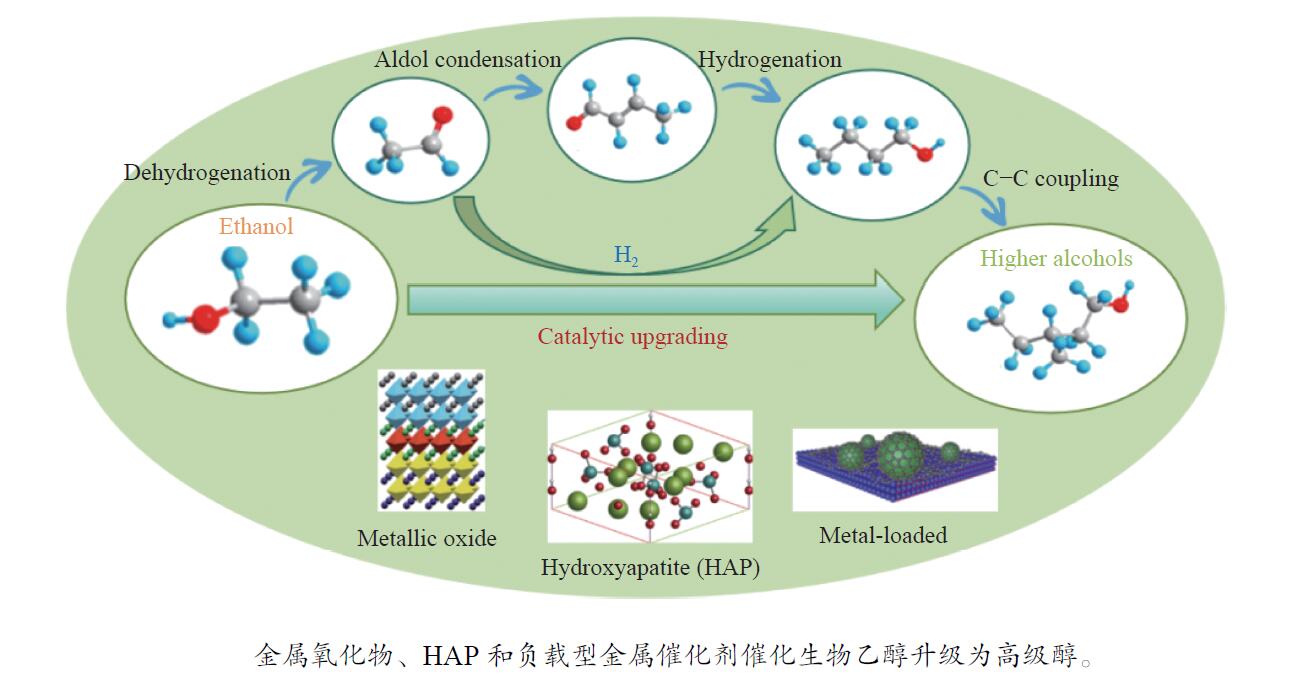
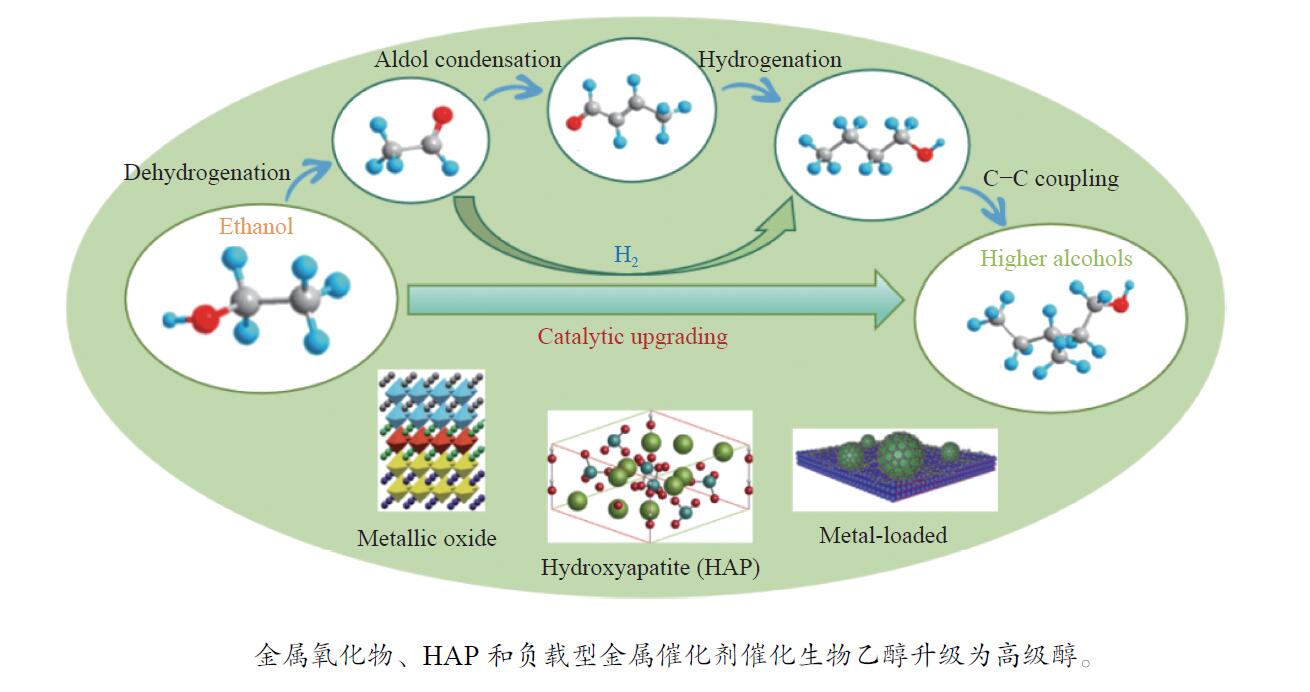
Research progress in catalysts for producing higher alcohols from bioethanol
-
摘要: 与乙醇相比,高级醇具有高的十六烷值、高能量密度、对发动机部件无腐蚀性、与水不混溶、稳定性好等直接作为燃料或燃料添加剂的优势,将发酵产生的生物乙醇转化为更有价值的高级醇受到了广泛关注。本工作综述了近年来世界各国有关生物乙醇制高级醇的研究进展,包括金属氧化物、羟基磷灰石(HAP)和负载型金属催化剂的研究开发现状,并比较了不同类型催化剂参与下的乙醇转化率和高级醇选择性,结合乙醇经缩合反应制备高级醇的机理进行了讨论,最后对当前生物乙醇制高级醇的挑战以及未来研究趋势进行了总结与展望,指出多功能催化剂的开发是未来研究重点,羟醛缩合是进一步提高生物乙醇制高级醇转化率与选择性的有效策略。Abstract: Compared with ethanol, higher alcohols have the advantages of high cetane number, high energy density, non corrosiveness to engine parts, immiscibility with water, good stability, and other advantages as fuel or fuel additive directly. The conversion of fermentation bioethanol into more valuable higher alcohols has attracted widespread attention. This paper reviewed the research progress of bioethanol to higher alcohols at home and abroad in recent years, including the research and development of metal oxides, hydroxyapatite (HAP) and supported metal catalysts. Finally, the current challenges and future research trends of bioethanol to higher alcohols are summarized and prospected, pointing out that the development of multifunctional catalysts is the focus of future research, and Aldol condensation is an effective strategy to further improve the conversion and selectivity of bioethanol to higher alcohols.
-
Key words:
- bioethanol /
- higher alcohol /
- catalysts /
- reaction mechanism
-
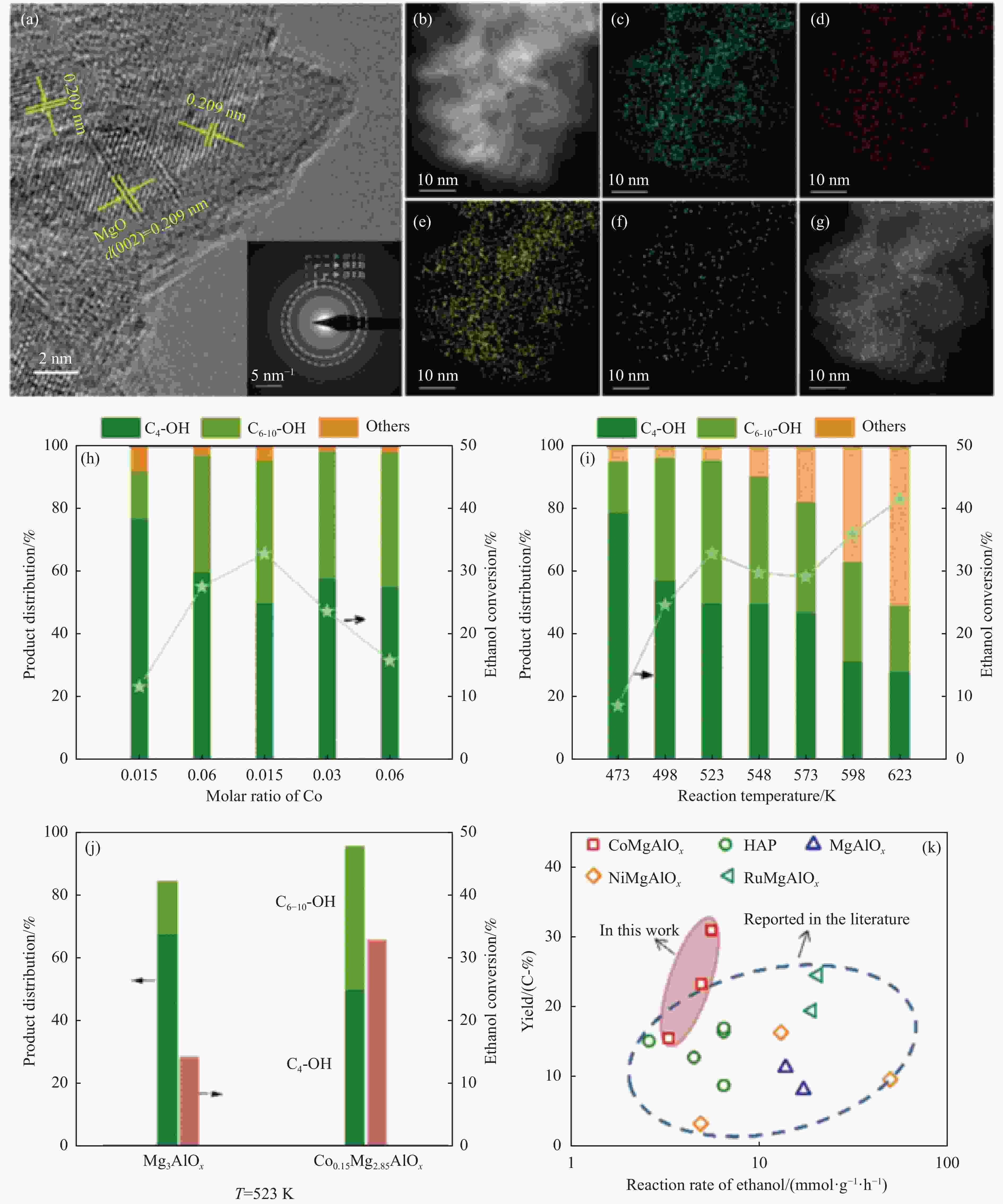
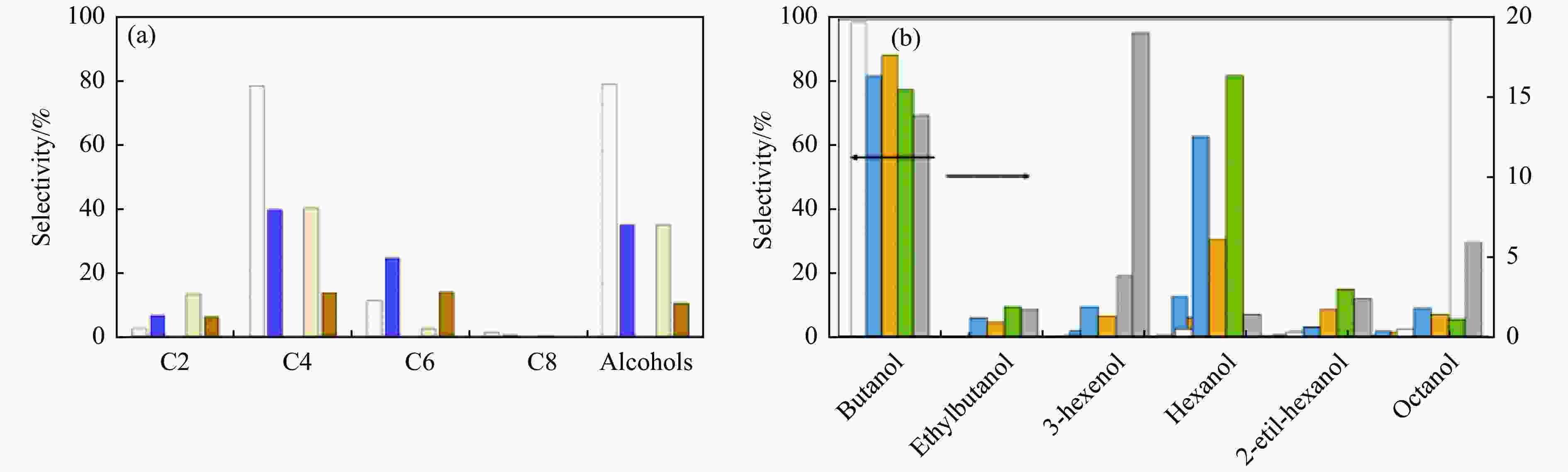
图 8 (a) 8 h 后的产物选择性:使用MgAl(2/1)负载量分别为 2.5 g/L(白色)和 10 g/L(紫色)或HAP 2.5 g/L(黄色)和10 g/L(棕色);(b) 8 h 后的产物分布:MgAl (2/1) (白色);Cu/MgAl (蓝色);Ru/MgAl (黄色);Pd/MgAl (绿色);Pt/MgAl (灰色)[42]
Figure 8 (a) Product selectivity after 8 h: Using MgAl (2/1) with loading amounts of 2.5 g/L (white) and 10 g/L (purple) or HAP 2.5 g/L (yellow) and 10 g/L (brown); (b) Product distribution after 8 h: MgAl (2/1) (white); Cu/MgAl (blue); Ru/MgAl (yellow); Pd/MgAl (green); Pt/MgAl (gray)[42] (with permission from RSC Publications)
表 1 金属氧化物催化剂
Table 1 Metal oxide catalysts
Catalyst Reaction conditions Conv./% Sel./% Reference MgO 450 ℃, 0.5 g catalyst, N2 10 mL/min, 7 h 56.1 32.7 [15] MgO 400 ℃, 0.2 g catalyst, 6% ethanol, 1.3 atm 23.0 34.0 [16] Mg-ZrO2 400 ℃ 52.0 35.0 [17] Mg-Al(Mg/Al=3) 350 ℃, 0.3 g catalyst, 12% ethanol,
atmospheric pressure, 12 h35.0 40.0 [18] Cu1MgAl3O 200 ℃, 0.5 g catalyst, 39.5 g ethanol,
autogenic pressure, 5 h2.5 43.0 [20] Cu5MgAl3O 200 ℃, 0.5 g catalyst, 39.5 g ethanol,
autogenic pressure, 5 h4.1 40.0 [20] Cu10MgAl3O 200 ℃, 0.5 g catalyst, 39.5 g ethanol,
autogenic pressure, 5 h4.5 28.0 [20] Cu20MgAl3O 200 ℃, 0.5 g catalyst, 39.5 g ethanol,
autogenic pressure, 5 h3.8 18.0 [20] Pd5MgAlO 200 ℃, 0.5 g catalyst, 39.5 g ethanol,
autogenic pressure, 5 h3.8 72.7 [20] Ag5MgAlO 200 ℃, 0.5 g catalyst, 39.5 g ethanol,
autogenic pressure, 5 h1.6 38.8 [20] Mn5MgAlO 200 ℃, 0.5 g catalyst, 39.5 g ethanol,
autogenic pressure, 5 h0.7 53.3 [20] Fe5MgAlO 200 ℃, 0.5 g catalyst, 39.5 g ethanol,
autogenic pressure, 5 h0.3 39.2 [20] Sm5MgAlO 200 ℃, 0.5 g catalyst, 39.5 g ethanol,
autogenic pressure, 5 h1.3 66.3 [20] Yb5MgAlO 200 ℃, 0.5 g catalyst, 39.5 g ethanol,
autogenic pressure, 5 h1.2 53.0 [20] CuMgAlOx 260 ℃, 0.1 MPa, GHSV=750 mL/(g·h),
LHSV=2 mL/(g·h)43.9 48.0 [21] CuMgAlOx 350 ℃, 0.15 g catalyst, 5 h 79.6 32.0 [22] Co0.15Mg2.85AlOx 250 ℃, 0.1 MPa, 0.2 g catalyst, WHSV=0.96 h−1 32.9 95.4 [23] OM-Cu4La2.6Al100 260 ℃, 3 MPa (N2), LHSV=2 mL/(h·g), 12 h 52.2 72.2 [24] Conv.: conversion of ethanol; Sel.: selectivity of higher alcohol. 表 2 羟基磷灰石(HAP)催化剂
Table 2 Hydroxyapatite (HAP) catalyst
Catalyst Reaction conditions Conv./% Sel./% Reference HAP Ca/P=1.64 320 ℃, 0.21 g catalyst, GHSV=10000 h−1 22.7 62.4 [26] HAP (Ca+Sr)/P=1.67 400 ℃, flow=50 mL/min,
GHSV=5000 mL/(g·h), 4 h13.0 76.4 [27] Ca-HAP-1(1.59) 400 ℃, atmospheric pressure, GHSV=10000 h−1 16.2 22.2 [28] Ca-HAP-2(1.62) 400 ℃, atmospheric pressure, GHSV=10000 h−1 20.8 50.4 [28] Ca-HAP-3(1.65) 400 ℃, atmospheric pressure, GHSV=10000 h−1 21.2 62.4 [28] Ca-HAP-4(1.67) 400 ℃, atmospheric pressure, GHSV=10000 h−1 15.8 56.2 [28] Sr-HAP-1(1.58) 300 ℃, atmospheric pressure, W/Fethanol=130 (h·g)/mol 1.1 69.0 [28] Sr-HAP-2(1.64) 300 ℃, atmospheric pressure, W/Fethanol=130 (h·g)/mol 5.9 78.1 [28] Sr-HAP-3(1.67) 300 ℃, atmospheric pressure, W/Fethanol=130 (h·g)/mol 7.9 81.7 [28] Sr-HAP-4(1.70) 300 ℃, atmospheric pressure, W/Fethanol=130 (h·g)/mol 11.3 86.4 [28] Cu-HAP 250 ℃, 0.1 g catalyst,
H2 or N2 30 mL/min, 0.5 h36.6 86.7 [29] Ni-HAP 400 ℃, 0.25 g catalyst, 0.5 mL ethanol, 0.1 MPa N2, 24 h 55.6 67.7 [30] Conv.: conversion of ethanol; Sel.: selectivity of higher alcohol. 表 3 单金属负载催化剂
Table 3 Monometal Supported Catalysts
Catalyst Reaction conditions Conv./% Sel./% Reference 5%Ru/Al2O3 300 ℃, 0.01−0.05 g catalyst, 1.2 g ethanol, autogenic pressure, 3 h 12.0 9.0 [34] 5%Rh/Al2O3 300 ℃, 0.01−0.05 g catalyst, 1.2 g ethanol, autogenic pressure, 3 h 5.0 35.0 [34] 5%Pd/Al2O3 300 ℃, 0.01−0.05 g catalyst, 1.2 g ethanol, autogenic pressure, 3 h 9.0 21.0 [34] 5%Pt/Al2O3 300 ℃, 0.01−0.05 g catalyst, 1.2 g ethanol, autogenic pressure, 3 h 3.0 37.0 [34] 0.8%Au/Al2O3 300 ℃, 0.01−0.05 g catalyst, 1.2 g ethanol, autogenic pressure, 3 h 6.0 35.0 [34] 6%Ag/Al2O3 300 ℃, 0.01−0.05 g catalyst, 1.2 g ethanol, autogenic pressure, 3 h 2.0 20.0 [34] Ag/Mg-Al 250 ℃ 53.7 13.8 [35] Ni/γ-Al2O3 230 ℃, WHSV=1.42 h−1, 100 bar, 10 h 41.0 47.5 [36] Ni/γ-Al2O3 240 ℃, 2 g catalyst, 70 bar, LHSV=0.1 h−1, 10 h 14.0 69.0 [37] Cu/γ-Al2O3 240 ℃, 2 g catalyst, 70 bar, LHSV=0.1 h−1, 10 h 14.0 64.0 [37] Cu/CeO2 260 ℃, 1 mL/min CO2 and 0.05 mL/min EtOH, LHSV=1.97 h−1 39.0 35.0 [38] Ru/MgO 400 ℃ 43.0 9.0 [39] Au/mTiO2 250 ℃ 74.0 10.0 [40] Co/MgAlO 350 ℃ 55.0 33.0 [41] Cu/MgAl 230 ℃, 0.5 or 2 g catalyst, 200 mL ethanol, 30 bar N2, 8 h − 81.9 [42] Ru/Mg3Al1-LDO 350 ℃, 0.5 g catalyst, p(N2)=0.1 MPa, WHSV=3.2 h−1 29.6 82.6 [43] Ni@C 0.5 g catalyst, 5 MPa H2 initial pressure, 10 h 61.7 85.7 [44] Conv.: conversion of ethanol; Sel.: selectivity of higher alcohol. 表 4 多金属负载催化剂
Table 4 Multimetal supported catalysts
Catalyst Reaction conditions Conv./% Sel./% Reference Cu-CeO2/AC 250 ℃, 1.0 g catalyst, 2 MPa (N2), LHSV=4 mL/(h·g) 39.1 55.2 [45] 5Cu1Ce/AC 250 ℃, 1.0 g catalyst, 2 MPa (N2), LHSV=4 mL/(h·g) 46.2 61.8 [45] 4Cu1Ce/AC 250 ℃, 1.0 g catalyst, 2 MPa (N2), LHSV=4 mL/(h·g) 45.6 62.7 [45] 3Cu1Ce/AC 250 ℃, 1.0 g catalyst, 2 MPa (N2), LHSV=4 mL/(h·g) 46.2 60.0 [45] 2Cu1Ce/AC 250 ℃, 1.0 g catalyst, 2 MPa (N2), LHSV=4 mL/(h·g) 46.3 58.7 [45] 1Cu1Ce/AC 250 ℃, 1.0 g catalyst, 2 MPa (N2), LHSV=4 mL/(h·g) 44.0 45.5 [45] 3Cu1Ce/SiO2 250 ℃, 1.0 g catalyst, 2 MPa (N2), LHSV=4 mL/(h·g) 23.3 12.3 [45] 3Cu1Ce/A2O3 250 ℃, 1.0 g catalyst, 2 MPa (N2), LHSV=4 mL/(h·g) 46.9 17.4 [45] Ni-Cu/HT 310 ℃, Ni:Cu=1:1 62.4 34.8 [46] Ni/La2O3/Al2O3 230 ℃, 30 g catalyst,
reactor pressure=100 bar41.0 74.0 [47] Ni/Cu/La2O3/β-Al2O3 230 ℃, 30 g catalyst, WHSV=2.06 h−1 15.0 78.0 [48] NiSn/MgAlO 250 ℃, 1 g NaOH, 10 g H2O, 10 g ethanol, 12 h 66.9 93.8 [49] NiSn@C 250 ℃, 0.5 g catalyst, EtOH/H2O=1, 24 h 47.0 36.0 [50] NiSn@NC 250 ℃, 0.5 g catalyst, 15 g ethanol,
15 g H2O, 1 g NaOH, 24 h68.5 31.8 [51] NiZn@NC 250 ℃, 0.5 g catalyst, 15 g ethanol,
15 g H2O, 1 g NaOH, 24 h75.2 − [52] Sn-Ni/CS 230 ℃, 0.3 g catalysts, 0.87 g NaOH,
10 g EtOH, 10 g H2O, 12 h60.0 85.0 [53] NiMo@C 240 ℃, 0.6 g catalyst, 13.5 g C2H5OH,
1.5 g fusel, 15.0 g H2O, 0.9 g NaOH, 12 h89.4 44.7 [54] NiSn@C-MgO 250 ℃, 0.5 g catalyst, 10 g ethanol,
10 g H2O, 0.5 g NaOH, 12 h73.3 60.9 [55] Cu-La2O3/Al2O3 250 ℃, 1.0 g catalyst, 3 MPa (N2), LHSV=2 mL/(g·h) 56.7 76.1 [9] Conv.: conversion of ethanol; Sel.: selectivity of higher alcohol. -
[1] KLINGER J L, WESTOVER T L, EMERSON R M, et al. Effect of biomass type, heating rate, and sample size on microwave-enhanced fast pyrolysis product yields and qualities[J]. Appl Energy,2018,228:535−545. doi: 10.1016/j.apenergy.2018.06.107 [2] SARKODIE SA, STREZOV V. Effect of foreign direct investments, economic development and energy consumption on greenhouse gas emissions in developing countries[J]. Sci Total Environ,2019,646:862−871. doi: 10.1016/j.scitotenv.2018.07.365 [3] SHEN F, XIONG X, FU J, et al. Recent advances in mechanochemical production of chemicals and carbon materials from sustainable biomass resources[J]. Renewable Sustainable Energy Rev,2020,130:109944. doi: 10.1016/j.rser.2020.109944 [4] CALIGIURI C, RENZI M, BIETRESATO M, et al. Experimental investigation on the effects of bioethanol addition in diesel-biodiesel blends on emissions and performances of a micro-cogeneration system[J]. Energy Convers Manag,2019,185:55−65. doi: 10.1016/j.enconman.2019.01.097 [5] HOSSEINZADEH-BANDBAFHA H, TABATABAEI M, AGHBASHLO M, et al. A comprehensive review on the environmental impacts of diesel/biodiesel additives[J]. Energy Convers Manag,2018,174:579−614. doi: 10.1016/j.enconman.2018.08.050 [6] MACHADO C F R, ARAÚJO O Q F, DE MEDEIROS J L, et al. Carbon dioxide and ethanol from sugarcane biorefinery as renewable feedstocks to environment-oriented integrated chemical plants[J]. J Cleaner Prod,2018,172:1232−1242. doi: 10.1016/j.jclepro.2017.10.234 [7] NASSAR H N, EL-AZAB W I M, EL-GENDY N S. Sustainable ecofriendly recruitment of bioethanol fermentation lignocellulosic spent waste biomass for the safe reuse and discharge of petroleum production produced water via biosorption and solid biofuel production[J]. J Hazard Mater,2022,422:126845. doi: 10.1016/j.jhazmat.2021.126845 [8] MOORE C M, STAPLES O, JENKINS R W, et al. Acetaldehyde as an ethanol derived bio-building block: An alternative to Guerbet chemistry[J]. Green Chem,2017,19(1):169−174. [9] HE J, LI X, KOU J, et al. Catalytic upgrading of ethanol to higher alcohols over nickel-modified Cu-La2O3/Al2O3 catalysts[J]. Catal Sci Technol,2023,13(1):170−177. doi: 10.1039/D2CY01554D [10] DAMODHARAN D, SATHIYAGNANAM A P, RANA D, et al. Effective utilization of waste plastic oil in a direct injection diesel engine using high carbon alcohols as oxygenated additives for cleaner emissions[J]. Energy Convers Manag,2018,166:81−97. doi: 10.1016/j.enconman.2018.04.006 [11] EAGAN N M, KUMBHALKAR M D, BUCHANAN J S, et al. Chemistries and processes for the conversion of ethanol into middle-distillate fuels[J]. Nat Rev Chem,2019,3(4):223−249. doi: 10.1038/s41570-019-0084-4 [12] CAO Y, ZHANG C, TSANG D C W, et al. Hydrothermal liquefaction of lignin to aromatic chemicals: Impact of lignin structure[J]. Ind Eng Chem Res,2020,59(39):16957−16969. doi: 10.1021/acs.iecr.0c01617 [13] CAO Y, HE M, DUTTA S, et al. Hydrothermal carbonization and liquefaction for sustainable production of hydrochar and aromatics[J]. Renewable Sustainable Energy Rev,2021,152:111722. doi: 10.1016/j.rser.2021.111722 [14] MALIK K, SHARMA P, YANG Y, et al. Lignocellulosic biomass for bioethanol: Insight into the advanced pretreatment and fermentation approaches[J]. Ind Crops Prod,2022,188:115569. doi: 10.1016/j.indcrop.2022.115569 [15] NDOU A S, PLINT N, COVILLE N J. Dimerisation of ethanol to butanol over solid-base catalysts[J]. Appl Catal A: Gen,2003,251(2):337−345. doi: 10.1016/S0926-860X(03)00363-6 [16] BIRKY T W, KOZLOWSKI J T, DAVIS R J. Isotopic transient analysis of the ethanol coupling reaction over magnesia[J]. J Catal,2013,298:130−137. doi: 10.1016/j.jcat.2012.11.014 [17] KOZLOWSKI J T, DAVIS R J. Heterogeneous catalysts for the Guerbet coupling of alcohols[J]. ACS Catal,2013,3(7):1588−1600. doi: 10.1021/cs400292f [18] CARVALHO D L, DE AVILLEZ R R, RODRIGUES M T, et al. Mg and Al mixed oxides and the synthesis of n-butanol from ethanol[J]. Appl Catal A: Gen,2012,415−416:96−100. doi: 10.1016/j.apcata.2011.12.009 [19] CARVALHO D L, BORGES L E P, APPEL L G, et al. In situ infrared spectroscopic study of the reaction pathway of the direct synthesis of n-butanol from ethanol over MgAl mixed-oxide catalysts[J]. Catal Today,2013,213(18):115−121. [20] MARCU I C, TICHIT D, FAJULA F, et al. Catalytic valorization of bioethanol over Cu-Mg-Al mixed oxide catalysts[J]. Catal Today,2009,147(3/4):231−238. doi: 10.1016/j.cattod.2009.04.004 [21] 程福龙, 郭荷芹, 崔静磊, 等. CuMgAlOx复合氧化物催化甲醇乙醇Guerbet反应: Mg2+/Al3+比的影响[J]. 燃料化学学报,2018,46(12):1472−1481. doi: 10.1016/S1872-5813(18)30061-6CHENG Fulong, GUO Heqin, CUI Jinglei, et al. Guerbet reaction of methanol and ethanol catalyzed by CuMgAlOx mixed oxides: Effect of M2+/Al3+ ratio[J]. J Fuel Chem Technol,2018,46(12):1472−1481. doi: 10.1016/S1872-5813(18)30061-6 [22] SIQUEIRA M R, PERRONE O M, METZKER G, et al. Highly selective 1-butanol obtained from ethanol catalyzed by mixed metal oxides: Reaction optimization and catalyst structure behavior[J]. Mol Catal,2019,476:110516. doi: 10.1016/j.mcat.2019.110516 [23] LV W L, HE L, LI W C, et al. Atomically dispersed Co2+ on MgAlOx boosting C4–10 alcohols selectivity of ethanol valorization[J]. Green Chem,2023,25(7):2653−2662. doi: 10.1039/D3GC00078H [24] ZHAO H, SHEN X, HE J, et al. Catalytic upgrading of ethanol to higher alcohols over ordered mesoporous Cu-La-Al composite metal oxides[J]. Appl Surf Sci,2022,599:153851. doi: 10.1016/j.apsusc.2022.153851 [25] YERGAZIYEVA G Y, DOSSUMOV K, MAMBETOVA M M, et al. Effect of Ni, La, and Ce oxides on a Cu/Al2O3 catalyst with low copper loading for ethanol non-oxidative dehydrogenation[J]. Chem Eng Technol,2021,44(10):1890−1899. doi: 10.1002/ceat.202100112 [26] OGO S, ONDA A, IWASA Y, et al. 1-butanol synthesis from ethanol over strontium phosphate hydroxyapatite catalysts with various Sr/P ratios[J]. J Catal,2012,296:24−30. doi: 10.1016/j.jcat.2012.08.019 [27] TSUCHIDA T, YOSHIOKA T, SAKUMA S, et al. Synthesis of biogasoline from ethanol over hydroxyapatite catalyst[J]. Ind Eng Chem Res,2015,47(5):1443−1452. [28] SILVESTER L, LAMONIER J F, LAMONIER C, et al. Guerbet reaction over strontium-substituted hydroxyapatite catalysts prepared with various (Ca+Sr)/P ratios[J]. ChemCatChem,2017,9(12):2250−2261. doi: 10.1002/cctc.201601480 [29] ZHOU B C, LI W C, LV W L, et al. Enhancing ethanol coupling to produce higher alcohols by tuning H2 partial pressure over a copper-hydroxyapatite catalyst[J]. ACS Catal,2022,12(19):12045−12054. doi: 10.1021/acscatal.2c03327 [30] XUE M, YANG B, XIA C, et al. Upgrading ethanol to higher alcohols via biomass-derived Ni/bio-apatite[J]. ACS Sustainable Chem Eng,2022,10(11):3466−3476. doi: 10.1021/acssuschemeng.1c07189 [31] WANG Q N, SHI L, LU A H. Highly selective copper catalyst supported on mesoporous carbon for the dehydrogenation of ethanol to acetaldehyde[J]. ChemCatChem,2015,7(18):2846−2852. doi: 10.1002/cctc.201500501 [32] ZAFFRAN J, MICHEL C, DELBECQ F, et al. Trade-off between accuracy and universality in linear energy relations for alcohol dehydrogenation on transition metals[J]. J Phys Chem C,2015,119(23):12988−12998. doi: 10.1021/acs.jpcc.5b01703 [33] ZHANG J, SHI K, AN Z, et al. Acid-base promoted dehydrogenation coupling of ethanol on supported Ag particles[J]. Ind Eng Chem Res,2020,59(8):3342−3350. doi: 10.1021/acs.iecr.9b06778 [34] RIITTONEN T, TOUKONIITTY E, MADNANI D K, et al. One-pot liquid-phase catalytic conversion of ethanol to 1-butanol over aluminium oxide-the effect of the active metal on the selectivity[J]. Catalysts,2012,2(4):68−84. [35] KOZLOWSKI J T, DAVIS R J. Sodium modification of zirconia catalysts for ethanol coupling to 1-butanol[J]. J Energy Chem,2013,22(1):58−64. doi: 10.1016/S2095-4956(13)60007-8 [36] JORDISON T L, LIRA C T, MILLER D J. Condensed-phase ethanol conversion to higher alcohols[J]. Ind Eng Chem Res,2015,54(44):10991−11000. doi: 10.1021/acs.iecr.5b02409 [37] RIITTONEN T, ERANEN K, MAKIARVELA P, et al. Continuous liquid-phase valorization of bio-ethanol towards bio-butanol over metal modified alumina[J]. Renewable Energy,2015,74:369−378. doi: 10.1016/j.renene.2014.08.052 [38] EARLEY J H, BOURNE R A, WATSON M J, et al. Continuous catalytic upgrading of ethanol to n-butanol and >C4 products over Cu/CeO2 catalysts in supercritical CO2[J]. Green Chem,2015,17:3018−3025. doi: 10.1039/C4GC00219A [39] CIMINO S, LISI L, ROMANUCCI S, et al. Catalysts for conversion of ethanol to butanol: Effect of acid-base and redox properties[J]. Catal Today,2017,304:58−63. [40] QUESADA J, ARREOLA- SÁNCHEZ R, FABA L, et al. Effect of Au nanoparticles on the activity of TiO2 for ethanol upgrading reactions[J]. Appl Catal A: Gen,2018,551(5):23−33. [41] QUESADA J, FABA L, DÍAZ, EVA, et al. Tuning the selectivities of Mg-Al mixed oxides for ethanol upgrading reactions through the presence of transition metals[J]. Appl Catal A: Gen,2018,559(5):167−174. [42] FABA L, CUETO J, PORTILLO M Á, et al. Effect of catalyst surface chemistry and metal promotion on the liquid-phase ethanol condensation to higher alcohols[J]. Appl Catal A: Gen,2022,643:118783. doi: 10.1016/j.apcata.2022.118783 [43] YUAN B, ZHANG J, AN Z, et al. Atomic Ru catalysis for ethanol coupling to C4+ alcohols[J]. Appl Catal B: Environ,2022,309:121271. doi: 10.1016/j.apcatb.2022.121271 [44] ZHONG Q, LIAO J, ZHONG Q, et al. Aqueous upgrading of ethanol to higher alcohol diesel blending and jet fuel precursors over Na-doped porous Ni@C nanocomposite[J]. Fuel,2022,324:124507. [45] JIANG D, WU X, MAO J, et al. Continuous catalytic upgrading of ethanol to n-butanol over Cu-CeO2/AC catalysts[J]. ChemComm,2016,52(95):13749−13752. [46] ZACCHERIA F, SCOTTI N, RAVASIO N. The role of copper in the upgrading of bioalcohols[J]. ChemCatChem,2018,10(7):1526−1535. doi: 10.1002/cctc.201701844 [47] NEZAM I, PEEREBOOM L, MILLER D J. Continuous condensed-phase ethanol conversion to higher alcohols: Experimental results and techno-economic analysis[J]. J Cleaner Prod,2019,209:1365−1375. doi: 10.1016/j.jclepro.2018.10.276 [48] NEZAM I, ZAK J, MILLER D J. Condensed-phase ethanol conversion to higher alcohols over bimetallic catalysts[J]. Ind Eng Chem Res,2020,59(31):13906−13915. [49] WU X, CAI X, ZHANG Q, et al. Upgrading of aqueous bioethanol to higher alcohols over NiSn/MgAlO catalyst[J]. ACS Sustainable Chem Eng,2021,9(33):11269−11279. [50] LIU W, CHEN B, ZHANG Q, et al. One-pot synthesis of high-carbon bio-alcohols from aqueous ethanol upgrading over water-tolerance NiSn@ C catalyst[J]. Energy Convers Manag,2021,249:114822. [51] FEI X, XU Q, XUE L, et al. Aqueous phase catalytic conversion of ethanol to higher alcohols over NiSn bimetallic catalysts encapsulated in nitrogen-doped biorefinery lignin-based carbon[J]. Ind Eng Chem Res,2021,60(49):17959−17969. [52] LIN X, FEI X, CHEN D, et al. Efficient catalytic upgrading of ethanol to higher alcohols via inhibiting C–C cleavage and promoting C–C coupling over biomass-derived NiZn@ NC catalysts[J]. ACS Catal,2022,12(19):11573−11585. [53] CHEN B, ZHENG X, GU J, et al. Engineering Sn Sn doping Ni/chitosan to boost higher alcohols synthesis from direct coupling of aqueous ethanol: Modifying adsorption of aldehyde intermediates for C-C bond cleavage suppressing[J]. Appl Catal B: Environ, 2023, 321: 122048. [54] LIAO J, LIU Z, LING Y, et al. Electronic and surface engineering of Mo doped Ni@ C nanocomposite boosting catalytic upgrading of aqueous bio-ethanol to bio-jet fuel precursors[J]. Chem Eng J,2023,461:141888. doi: 10.1016/j.cej.2023.141888 [55] CAI X, LI X, DANG M, et al. Boosting the selectivity of higher alcohols in upgrading of aqueous bioethanol over amphiphilic NiSn@ C-MgO catalyst[J]. Fuel,2023,335:126971. [56] YANG C, MENG Z Y. Bimolecular condensation of ethanol to 1-butanol catalyzed by alkali cation zeolites[J]. J Catal,1993,142(1):37−44. doi: 10.1006/jcat.1993.1187 [57] SMITH K J, ANDERSON R B. A chain growth scheme for the higher alcohols synthesis[J]. J Catal,1984,85(2):428−436. [58] DI COSIMO J I, APESTEGUYÍA CR, GINÉS M J L, et al. Structural requirements and reaction pathways in condensation reactions of alcohols on MgyAlOx catalysts[J]. J Catal,2000,190:261−275. doi: 10.1006/jcat.1999.2734 [59] SCALBERT J, THIBAULT-STARZYk F, JACQUOT R, et al. Ethanol condensation to butanol at high temperatures over a basic heterogeneous catalyst: How relevant is acetaldehyde self-aldolization?[J]. J Catal,2014,311:28−32. doi: 10.1016/j.jcat.2013.11.004 [60] CHIEREGATO A, VELASQUEZ OCHOA J, BANDINELLI C, et al. On the chemistry of ethanol on basic oxides: Revising mechanisms and intermediates in the Lebedev and Guerbet reactions[J]. ChemSusChem,2015,8(2):377−388. doi: 10.1002/cssc.201402632 [61] WU X, FANG G, TONG Y, et al. Catalytic upgrading of ethanol to n‐butanol: Progress in catalyst development[J]. ChemSusChem,2018,11(1):71−85. [62] HANSPAL S, YOUNG Z D, PRILLAMAN J T, et al. Influence of surface acid and base sites on the Guerbet coupling of ethanol to butanol over metal phosphate catalysts[J]. J Catal,2017,352:182−190. [63] MANOJVEER S, SALAHI S, WENDT O F, et al. Ru-catalyzed cross-dehydrogenative coupling between primary alcohols to guerbet alcohol derivatives: With relevance for fragrance synthesis[J]. J Org Chem,2018,83(18):10864−10870. doi: 10.1021/acs.joc.8b01558 [64] GABRIËLS D, HERNÁNDEZ W Y, SELS B, et al. Review of catalytic systems and thermodynamics for the Guerbet condensation reaction and challenges for biomass valorization[J]. Catal Sci Technol,2015,5(8):3876−3902. doi: 10.1039/C5CY00359H -




 下载:
下载: