Preparation of NiPt/Ti2O3 nanocatalyst and its catalytic performance for hydrogen production from hydrazine hydrate
-
摘要: 本研究利用H2还原制备Ti2O3载体后,通过湿化学浸渍-还原法制备NiPt/Ti2O3纳米催化剂进行催化水合肼研究。研究表明,在催化剂的制备过程中,Ni和Pt之间形成了一种合金,该合金的形成使催化剂的催化活性升高,Ti2O3与NiPt合金的相互作用提升了催化剂催化性能和循环稳定性。Ni5Pt5/Ti2O3催化剂催化水合肼产氢的反应的TOF值为1076.1 h−1。Abstract: Hydrazine hydrate is the most promising hydrogen storage material with a hydrogen content of 8.0%. In this paper, after Ti2O3 support was prepared by H2 reduction, NiPt/Ti2O3 nanocatalyst was prepared by wet chemistry impregnation reduction method and used to catalyze hydrazine hydrate. The research shows that an alloy is formed between Ni and Pt during the preparation of the catalyst, and the formation of the alloy increases the catalytic activity of the catalyst. The interaction between Ti2O3 and NiPt alloy is helpful for the catalytic performance and cyclic stability of the catalyst. The TOF value of the Ni5Pt5/Ti2O3 catalyst for hydrogen production from hydrazine hydrate is 1076.1 h−1, which is superior to the reported catalyst performance.
-
Key words:
- hydrazine hydrate /
- hydrogen production through decomposition /
- NiPt /
- Ti2O3
-
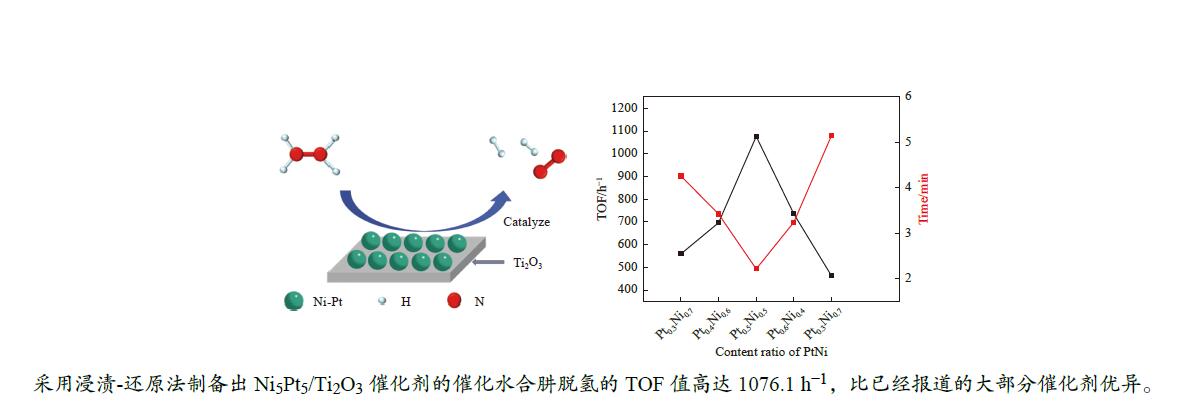
图 6 不同的Ni/Pt物质的量比率下,NiPt/Ti2O3催化剂催化水合肼脱氢性能图(a);不同的Pt/Ni物质的量比率下,NiPt/Ti2O3所对应的催化剂TOF值图(b)
Figure 6 Catalytic performance of NiPt/Ti2O3 catalyst for dehydrogenation of hydrazine hydrate under different Ni/Pt molar ratios (a); TOF values of catalysts corresponding to NiPt/Ti2O3 under different Pt/Ni molar ratios (b)
表 1 Ni0.5Pt0.5/Ti2O3催化剂的ICP-AES分析
Table 1 ICP-AES analysis results of Ni0.5Pt0.5/Ti2O3 catalyst
Catalyst Ni Pt initial ratio Ni Pt finial ratio NiPt/Ti2O3 5∶5 0.497∶0.502 表 2 不同水合肼脱氢催化剂的催化性能
Table 2 Catalytic activities of different catalysts for N2H4·H2O decomposition
Catalyst Selectivity for H2 (100%) TOF/h−1 Ea/(kJ·mol −1) Temperature/℃ Ni0.5Pt0.5/Ti2O3 100 1076.1 47.76 50 Rh55Ni45/Ce(OH) 100 395 38.8 50 Rh34Ni66@ZIF-8 100 140 58.1 50 Pt0.6Ni0.4/PDA-rGO 100 2056 33.39 50 (Ni3Pt7)0.5-(MnOx)0.5/NPC-900 100 706 50.15 50 PtNi/CeO2 100 286 38.7 50 Ni3Pt7/BNG-1000 100 199.4 28.4 30 Ni0.9Pt0.05Rh0.05/La2O3 100 45.9 25 Ni0.58Pt0.42/graphene 100 434 23.9 30 Ni0.9Pt0.1/MIL-101 100 140 48.4 30 (Ni3Pt7)0.5-(MnOx)0.5 100 120 25 Ni/CeO2 100 34 50 CoPt/La(OH)3 100 2400 45.2 50 Ni0.8Pt0.2/DT-Ti3C2Tx 100 1220 64.3 50 Ni-La(OH)3/D-MIL-125 100 2381 36.8 70 -
[1] YIN Y, LI B, YUAN Z M, et al. Enhanced hydrogen storage performance of Mg-Cu-Ni system catalyzed by CeO2 additive[J]. J Rare Earths,2020,38(9):983−993. doi: 10.1016/j.jre.2019.07.010 [2] XU X X, ZHOU Q, YU D H. The future of hydrogen energy: Bio-hydrogen production technology[J]. Int J Hydrogen Energy,2022,47(79):33677−33698. doi: 10.1016/j.ijhydene.2022.07.261 [3] 邹爱华, 徐晓梅, 周浪, 等. 石墨烯负载Co-CeOx纳米复合物的制备及其对氨硼烷水解产氢的催化性能[J]. 燃料化学学报,2021,49(9):1371−1378. doi: 10.1016/S1872-5813(21)60085-3ZOU Aihua, XU Xiaomei, ZHOU Lang, et al. Preparation of graphene-supported Co-CeOx nanocomposites as a catalyst for thehydrolytic dehydrogenation of ammonia borane[J]. J Fuel Chem Technol,2021,49(9):1371−1378. doi: 10.1016/S1872-5813(21)60085-3 [4] ZHAO Y, SOTO LEYTAN K N, MCDONELL V, et al. Investigation of visible light emission from hydrogen-air research flames[J]. Int J Hydrogen Energy,2019,44(39):22347−22354. doi: 10.1016/j.ijhydene.2019.06.105 [5] KARATAIRI E, SARTORI S. Reviving hydrogen as an energy carrier[J]. MRS Bull,2020,45(6):424−426. doi: 10.1557/mrs.2020.157 [6] YANG K, YANG K K, ZHANG S L, et al. Complete dehydrogenation of hydrazine borane and hydrazine catalyzed by MIL-101 supported NiFePd nano-particles[J]. J Alloys Compd,2018,732:363−371. doi: 10.1016/j.jallcom.2017.10.241 [7] 周亮亮. Pt-Ni双金属催化剂的合成与催化水合肼分解制氢性能研究[D]. 广州: 华南理工大学, 2021ZHOU Liangliang. Synthesis of Pt-Ni bimetallic catalysts and performance study of catalytic hydrogen production from hydrous hydrazine decomposition[D]. Guang zhou: South China University of Technology, 2021. [8] SINGH S K, ZHANG X B, XU Q. Room-temperature hydrogen generation from hydrous hydrazine for chemical hydrogen storage[J]. J Am Chem Soc,2009,131(29):9894−9895. doi: 10.1021/ja903869y [9] 雍辉, 季燕全, 胡季帆,等. Mg-Y-Ni 储氢合金吸放氢动力学性能的研究[J]. 稀有金属,2022,46(8):1021−1030.YONG Hui, JI Yanquan, HU Jifan, et al. Absorption and desorption hydrogen kinetic of Mg-Y-Ni based hydrogen storage alloy[J]. Rare Metals,2022,46(8):1021−1030. [10] CHENG Y, WU X, XU H. Catalytic decomposition of hydrous hydrazine for hydrogen production[J]. Sustainable Energy Fuels,2019,3(2):343−365. doi: 10.1039/C8SE00538A [11] YAO Q, CHEN X, LU Z. Catalytic dehydrogenation of NH3BH3, N2H4, and N2H4BH3 for chemical hydrogen storage[J]. Energy Environ Focus,2014,3(3):236−245. doi: 10.1166/eef.2014.1106 [12] WAN C, SUN L, XU L X, et. al. Novel NiPt alloy nanoparticle decorated 2D layered g-C3N4nanosheets: A highly efficient catalyst for hydrogen generatio-n from hydrous hydrazine[J]. J Mater Chem A,2019,7(15):8798−8804. doi: 10.1039/C9TA01535C [13] JIANG Y Y, DAI H B, ZHONG Y J, et. al. Complete and rapid conversion of hydrazine monohydrate to hydrogen oversupported Ni-Pt nanoparticles on mesoporous ceria for chemical hydrogen storage[J]. Chem Eur J,2015,21(43):15439−15445. doi: 10.1002/chem.201502421 [14] WANG J, LI W, WEN Y, et. al. Rh-Ni-B nanoparticles as highly efficient catalysts for hydrogen generation from hydrous hydrazine[J]. Adv Energy Mater,2015,5(10):1401879. doi: 10.1002/aenm.201401879 [15] TUNÇ N, RAKAP M. Preparation and characterization of Ni-M (M: Ru, Rh, Pd) nanoclusters as efficient catalysts for hydrogen evolution from ammonia borane methanolysis[J]. Renewable Energy,2020,155:1222−1230. doi: 10.1016/j.renene.2020.04.079 [16] MEN Y N, DU X Q, CHENG G Z, et al. CeOx-modified NiFe nanodendrits grown on rGO for efficient catalytic hydrogen generation from alkaline soluti-on of hydrazine[J]. Int J Hydrogen Energy,2017,42(44):27165−27173. doi: 10.1016/j.ijhydene.2017.08.214 [17] CHEN J M, ZOU H T, YAO Q L, et al. Cr2O3-modified NiFe nanoparticles as a noble-metal-free catalyst for complete dehydrogenation of hydrazine in a-queous solution[J]. Appl Surf Sci,2020,501:144247. doi: 10.1016/j.apsusc.2019.144247 [18] HE L, HUANG Y Q, WANG A Q, et al. A noble metal-free catalyst derived from Ni-Al hydrotalcite for hydrogen generation from N2H4$H2O decomposi-tion[J]. Angew Chem Int Ed,2012,51:6191−6194. doi: 10.1002/anie.201201737 [19] HE L, HUANG Y Q, LIU X Y, et al. Structural and catalytic properties of supported Ni-Ir alloy catalysts for H2 generation via hydrous hydrazine decom-position[J]. Appl Cata B: Environ,2014,147:779−788. doi: 10.1016/j.apcatb.2013.10.022 [20] LIU T, YU J, BIE H, et al. Highly effificient hydrogen generation from hydrous hydrazine using a reduced graphene oxide-supported NiPtP nanoparticle c-atalyst[J]. J Alloys Compd,2017,690:783−790. doi: 10.1016/j.jallcom.2016.08.113 [21] DAI H, DAI H B, ZHONG Y J, et al. Kinetics of catalytic decomposition of hydrous hydrazine over CeO2-supported bimetallic Ni-Pt nanocatalysts[J]. Int J Hydrogen Energy,2017,42(9):5684−5693. doi: 10.1016/j.ijhydene.2016.10.160 [22] ZHONG Y J, DAI H B, JIANG Y Y, et al. Highly effificient Ni@Ni-Pt/La2O3 catalyst for hydrogen generation from hydrous hydrazine decomposition: Effect of Ni-Pt surface alloying[J]. J Power Sources,2015,300:294−300. doi: 10.1016/j.jpowsour.2015.09.071 [23] DAI H, ZHONG Y, WANG P. Hydrogen generation from decomposition of hydrous hydrazine over Ni-Ir/CeO2 catalyst[J]. Prog Nat Sci Mater,2017,27(1):121−125. [24] WU D, WEN M, GU C, et al. 2D NiFe/CeO2 basic-site-enhanced catalyst via in-situ topotactic reduction for selectively catalyzing the H2 generation from N2H4·H2O[J]. ACS Appl Mater,2017,9:16103−16108. doi: 10.1021/acsami.7b00652 [25] QIU Y P, YIN H, DAI H, et al. Tuning the surface composition of Ni/meso-CeO2 with iridium as an effificient catalyst for hydrogen generation from hydr-ous hydrazine[J]. Chem Eur J,2018,24:4902−4908. doi: 10.1002/chem.201705923 [26] LANG C, JIA Y, YAO X, et al. Recent advances in liquid-phase chemical hydrogen storage[J]. Energy Storage Mater,2020,26:290−312. [27] QIU Y, ZHOU L, SHI Q, et al. Free-standing Pt-Ni nanowires catalyst for H2 generation from hydrous hydrazine[J]. Chem Commun,2021,57(5):623−626. [28] 石张平, 祁晓岚, 李旭光, 等. La2O3助剂对Rh/SiO2催化CO加氢反应性能的影响[J]. 燃料化学学报,2020,48(4):483−489.SHI Zhangping, QI Xiaolan, LI Xuguang, et al. Effect of La2O3 addition on the catalytic performance of Rh/SiO2 for CO hydrogenation[J]. J Fuel Chem Technol,2020,48(4):483−489. [29] WU D, WEN M, LIN X, et al. A NiCo/NiO-CoOx ultrathin layered catalyst with strong basic sites for high-performance H2 generation from hydrous hydr-azine[J]. J Mater Chem A,2016,4(17):6595−6602. doi: 10.1039/C6TA01092J [30] WANG Q, GUAN S Y, LI B. 2D graphitic-C3N4 hybridized with 1D flux-grown Na-modified K2Ti6O13 nanobelts for enhanced simulated sunlight and vi-sible-light photocatalytic performance[J]. Catal Sci Technol, 7(18): 4064–4078. [31] QING S, QIU Y P, DAI H, et al. Study of formation mechanism of Ni-Pt/CeO2 catalyst for hydrogen generation from hydrous hydrazine[J]. Catal Sci Technol,2019,787:1187−1194. [32] LU R, HU M, XU C L, et al. Hydrogen evolution from hydrolysis of ammonia boranecatalyzed by Rh/g-C3N4 under mild conditions[J]. Int J Hydrogen Energy,2018,43(14):7038−7045. doi: 10.1016/j.ijhydene.2018.02.148 [33] ALSAWAT M, ALTALHI T, SANTOS A, et al. Facile and controllable route for nitrogen doping of carbon nanotubes composite membranes by catalyst-free chemical vapour deposition[J]. Carbon,2016,106:295−305. doi: 10.1016/j.carbon.2016.05.043 [34] MAJEED A, NOORI F M, ZEESHAN A, et al. Analysis of activation energy in magnetohydrodynamic flow with chemical reaction and second or-der momentum slip model[J]. Case Stud Therm Eng,2018,12:765−773. doi: 10.1016/j.csite.2018.10.007 [35] LAHNEMAN D J, KIM H, JIANG H, et al. Electronic and optical properties of strain-locked metallic Ti2O3 films[J]. Curr Appl Phys, 2023, 47: 9−14. [36] DU X, DU C, CAI P, et al. NiPt nanocata-lysts supported on boron and nitrogen Co-doped graphene for superior hydrazine dehydrogenation and methanol oxidation[J]. ChemCatChem,2016,8(7):1410. doi: 10.1002/cctc.201501405 [37] WANG C, WANG H L, GAO D W, et al. Amorphous NiCoPt/Ce2O3 nanoparticles as highly efficient catalyst for hydrogen generation from hydrous hyd-razine[J]. Mater Sci Forum,2017,898:1862. doi: 10.4028/www.scientific.net/MSF.898.1862 [38] CHEN J M, LU Z H, HUANG W, et al. Galvanic replacement synthesis of NiPt/graphene as highly efficient catalysts for hydrogen release from hydrazin-e and hydrazine borane[J]. J Alloys Compd,2017,695:3036−3043. doi: 10.1016/j.jallcom.2016.11.351 [39] SONG F Z, YANG X C, XU Q. Ultrafine bimetallic Pt-Ni nanoparticles achieved by metal-organic framework templated zirconia/porous carbon/reduced graphene oxide: Remarkable catalytic activity in dehydrogenation of hydrous hydrazine[J]. Small Methods,2020,4(1):1900707. doi: 10.1002/smtd.201900707 -





 下载:
下载: