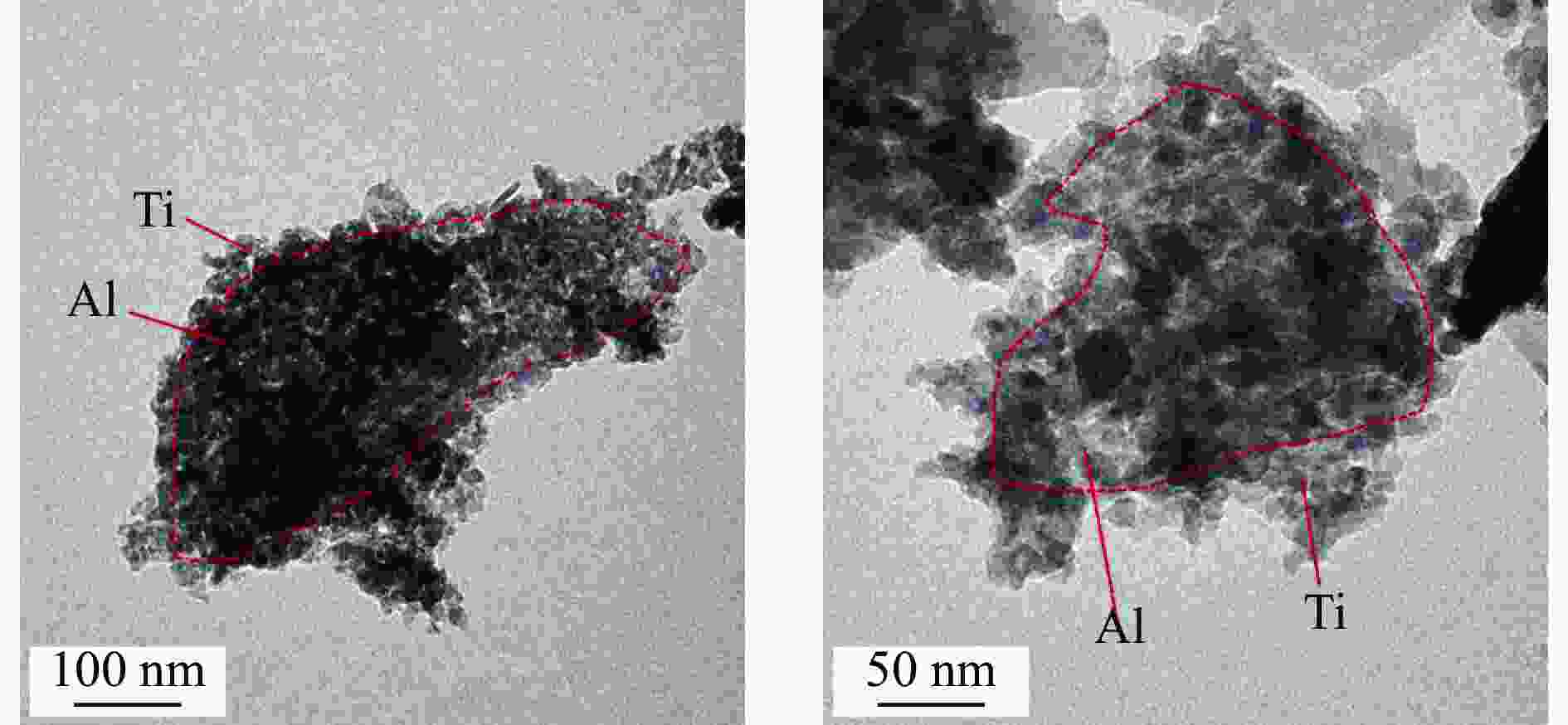
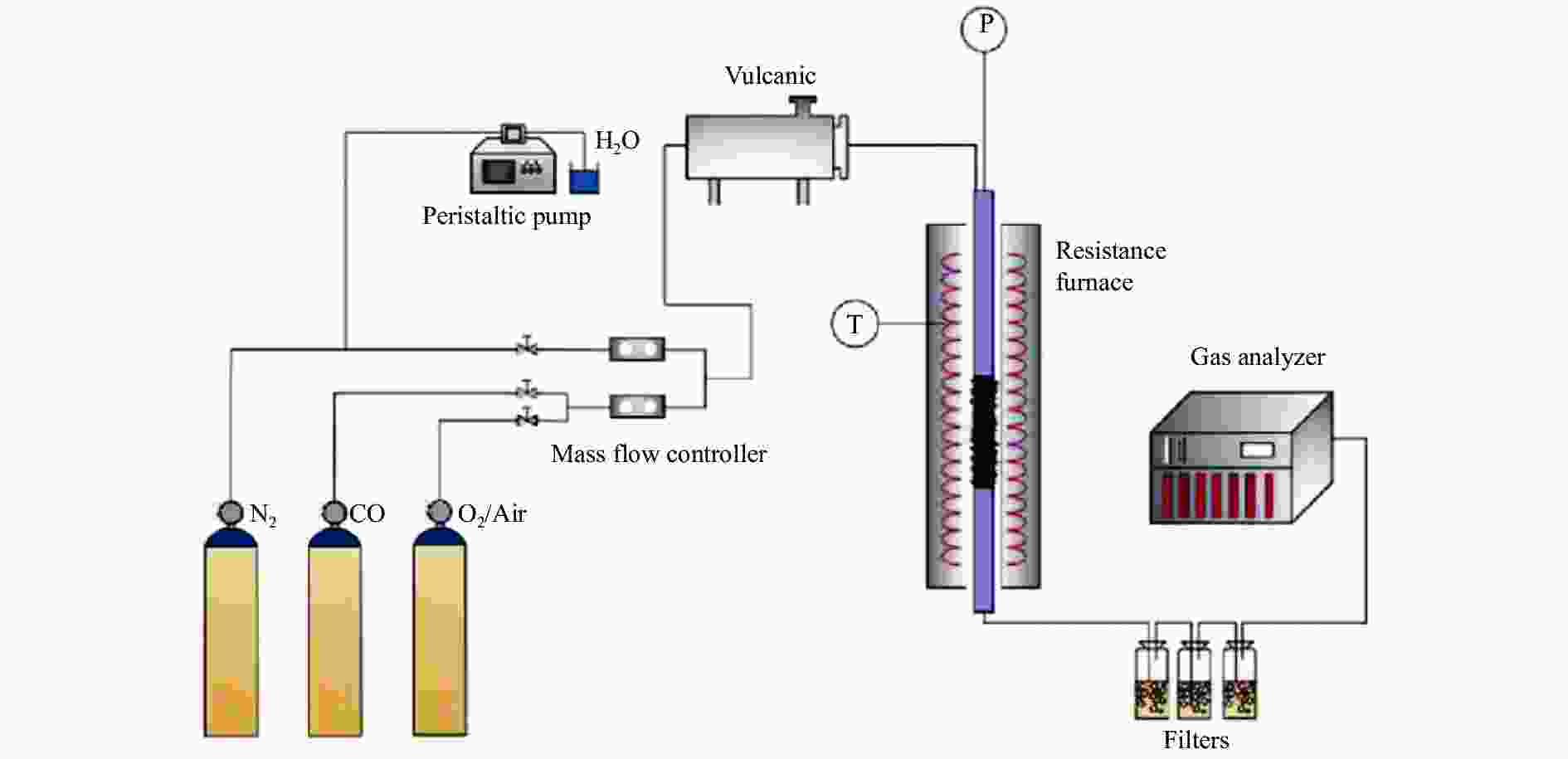
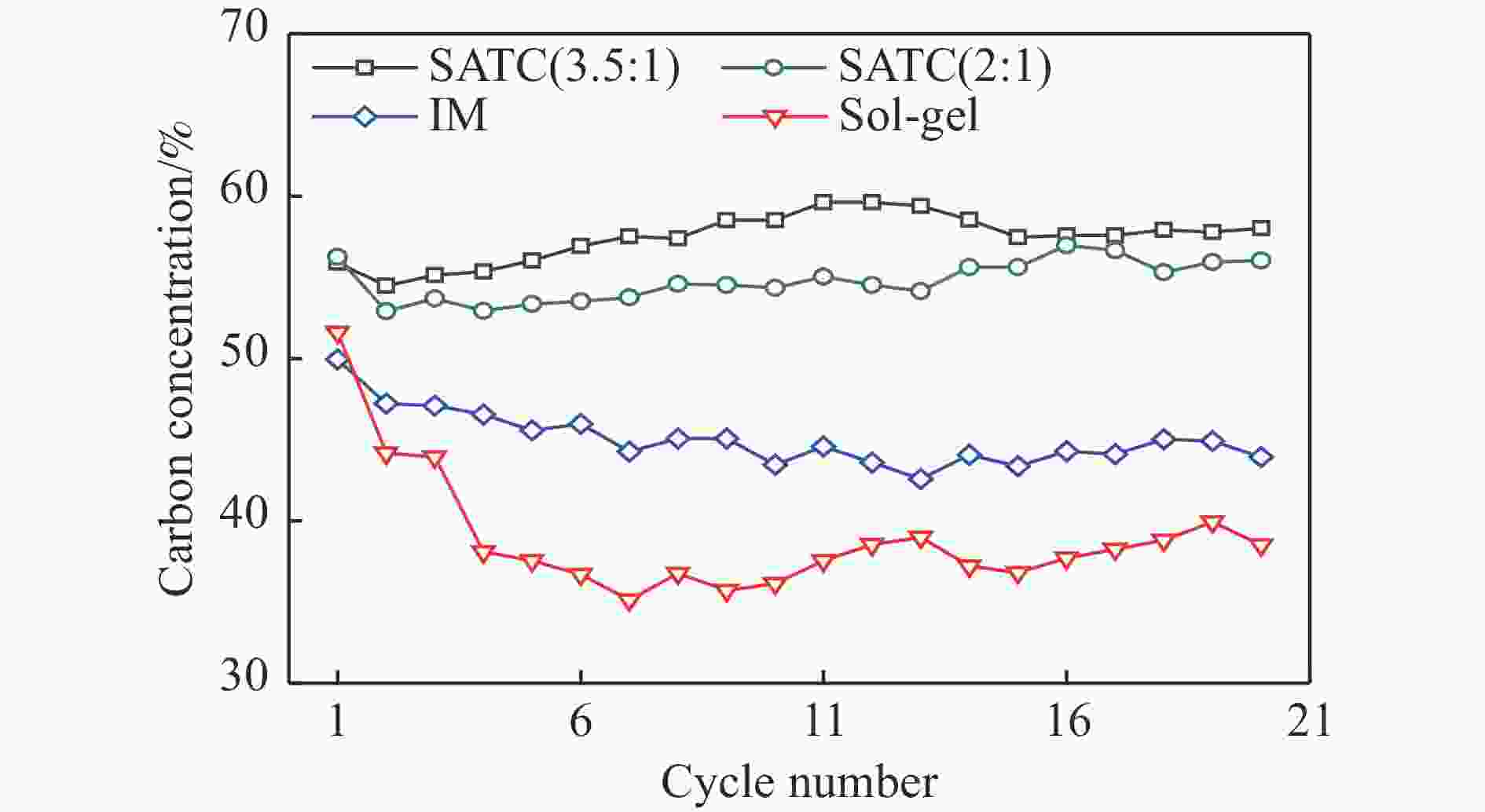
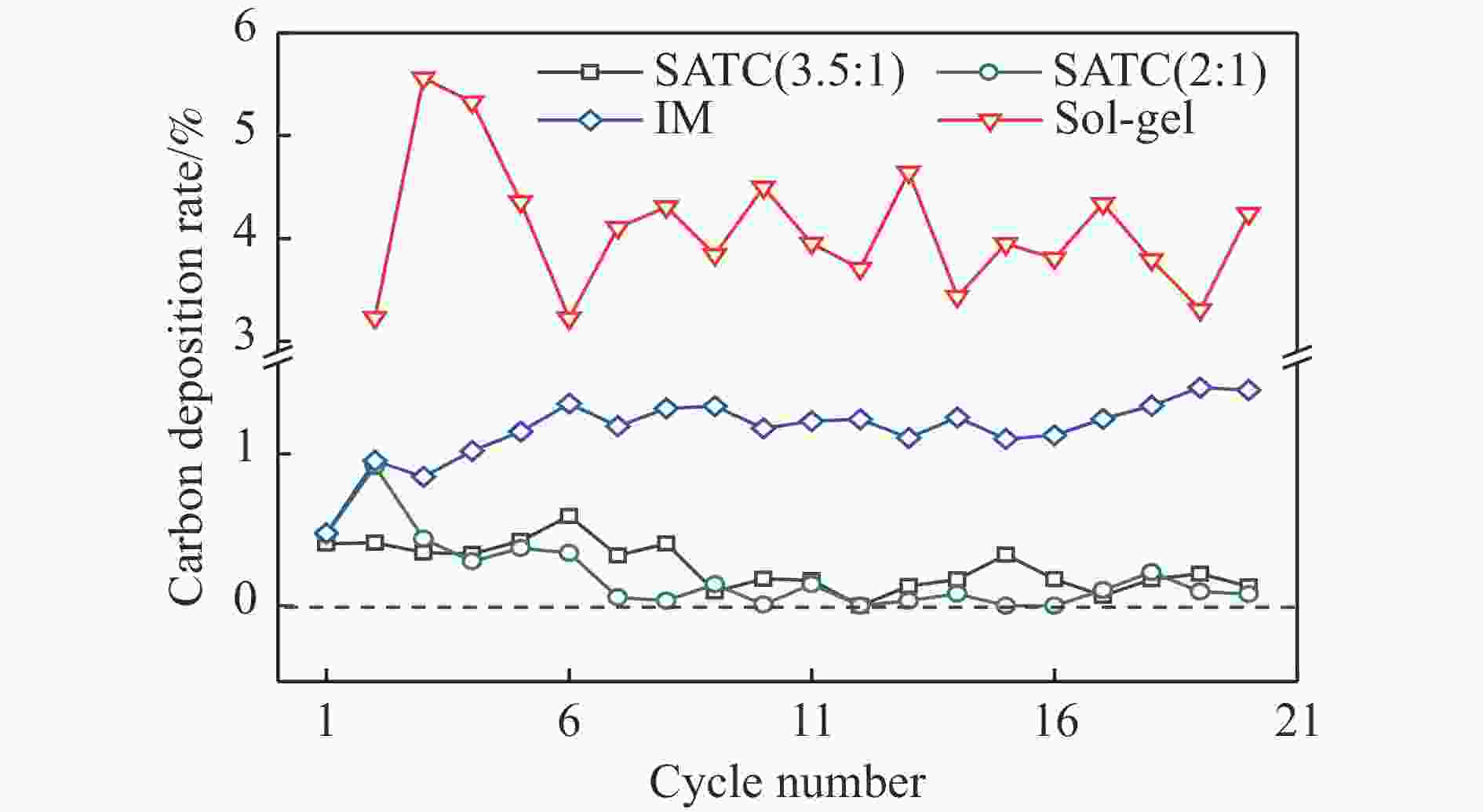
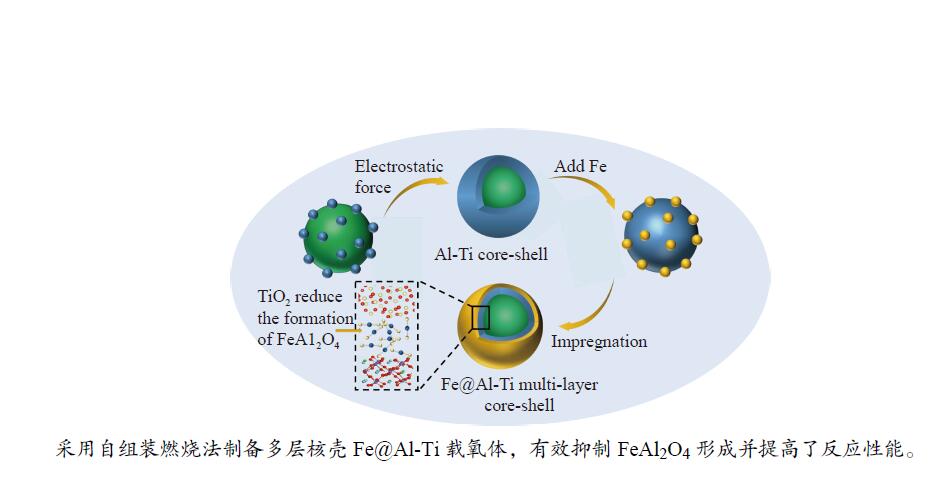
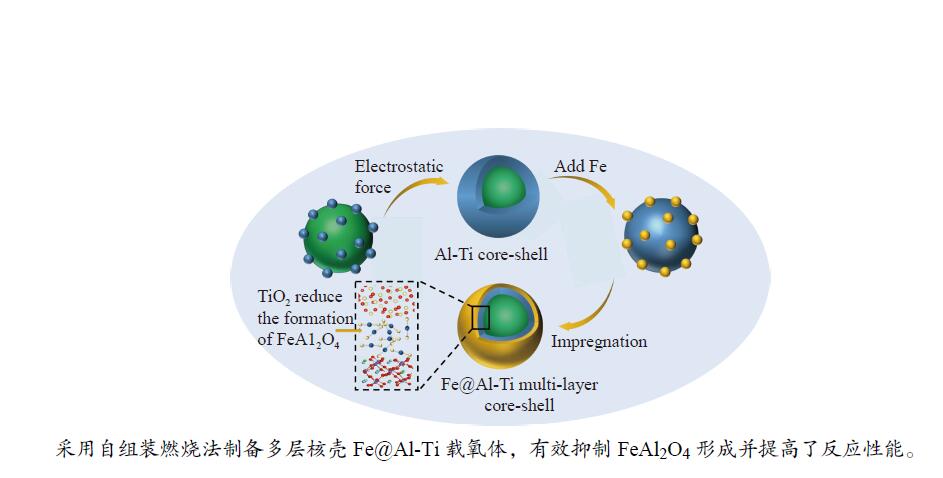
Chemical looping hydrogen generation with multi-layer core-shell oxygen carrier of Fe@Al-Ti
-
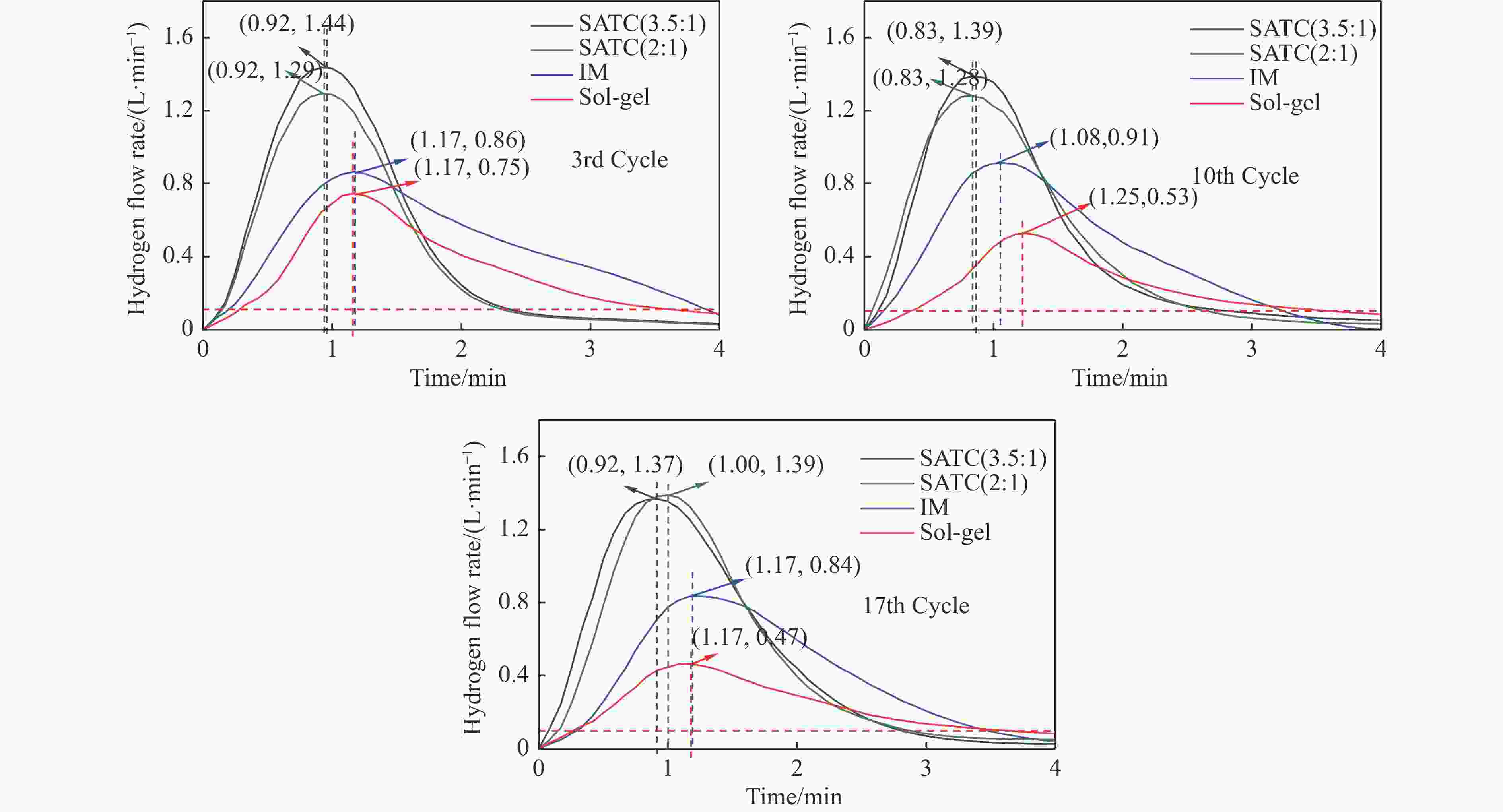
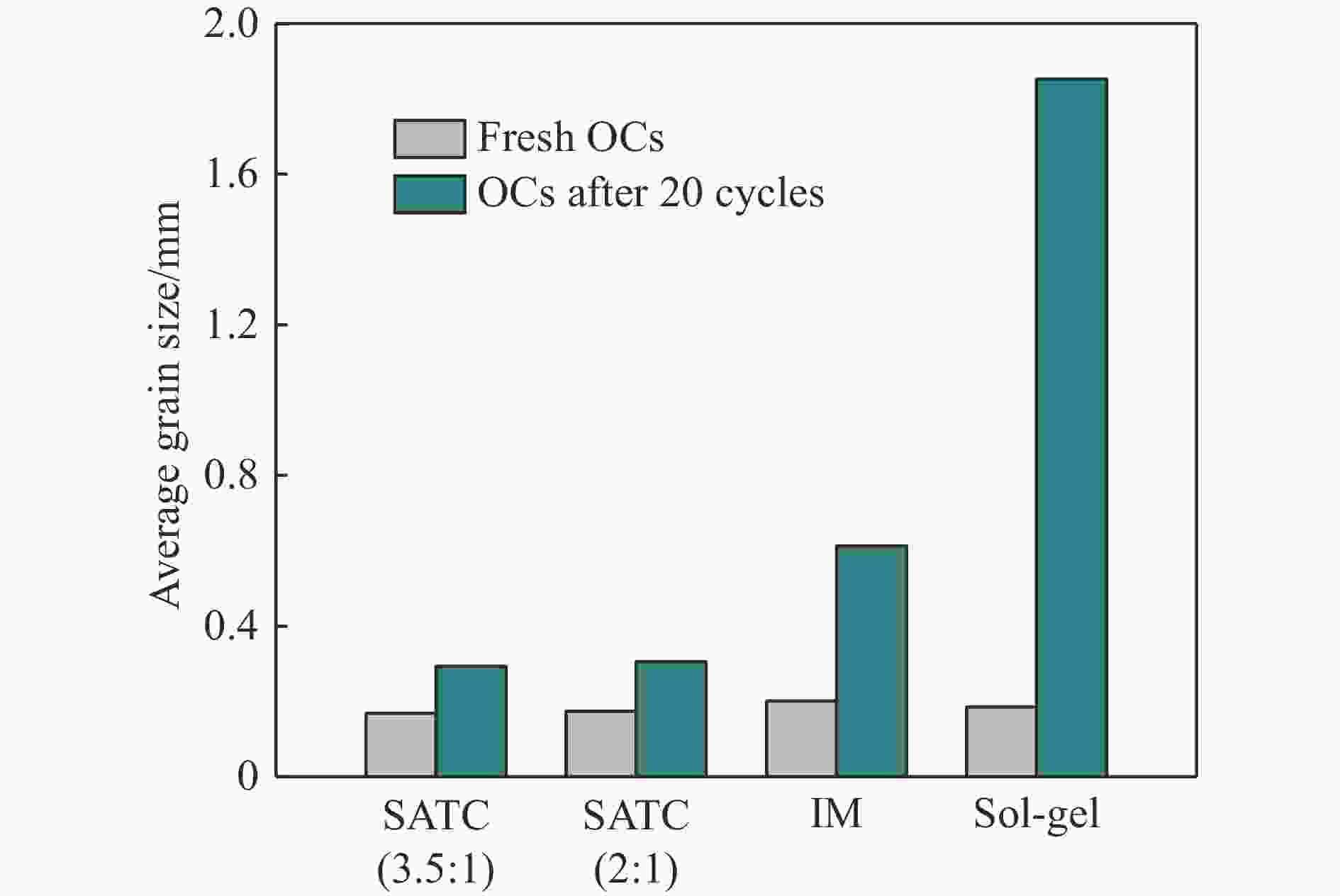
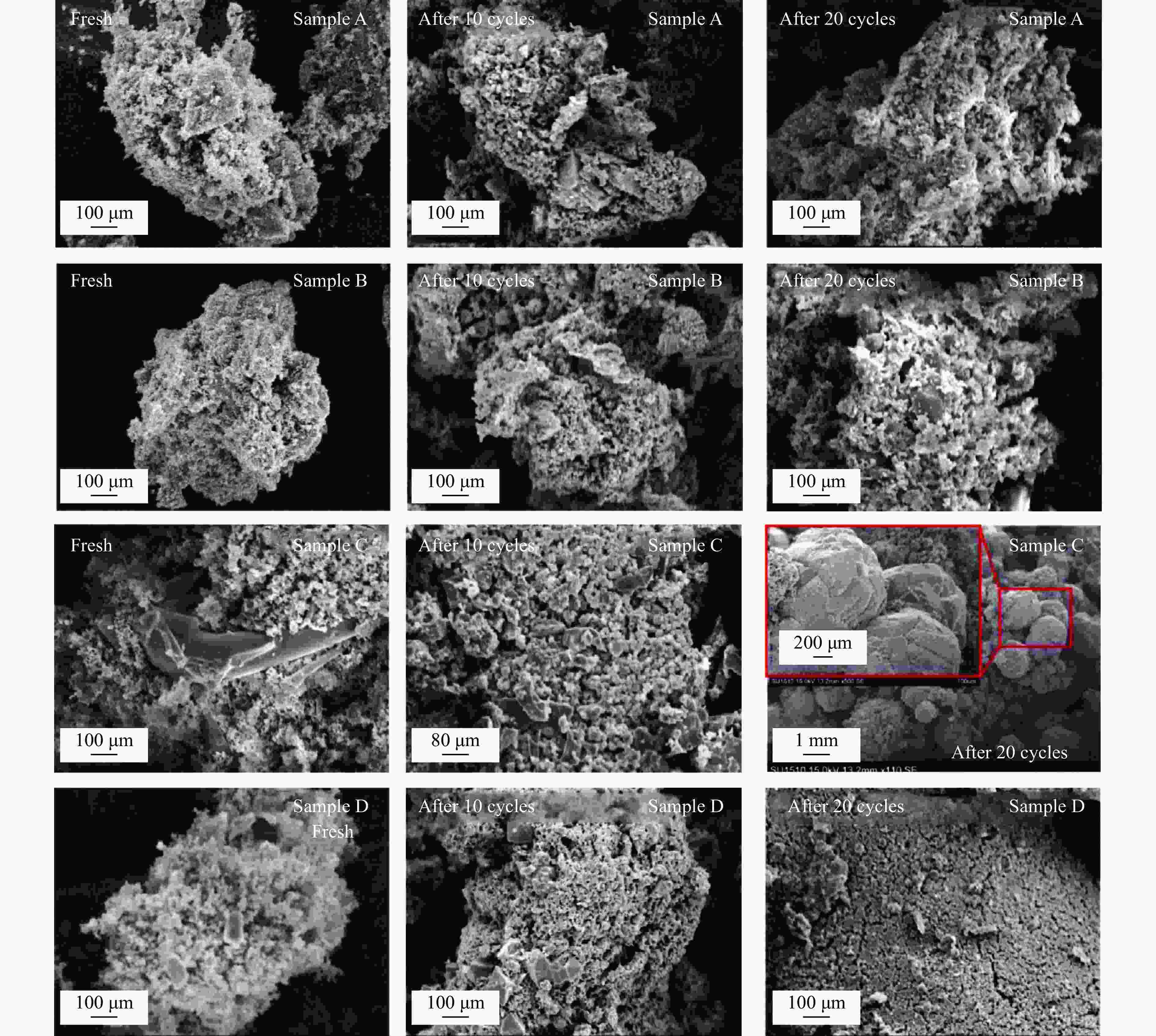
摘要: Fe-Al-Ti载氧体在化学链制氢工艺中具有良好的循环稳定性和抗积炭性能,但反应形成FeAl2O4会降低抗烧结性能和氢气产率。为抑制FeAl2O4的生成并进一步提升载氧体反应性能,本研究采用自组装模板燃烧法制备多层核壳结构载氧体,以TiO2为介层阻隔Fe2O3与Al2O3,形成多层核壳Fe@Al-Ti载氧体,在固定床上进行化学链制氢循环,评价多层核壳结构对反应性能的影响。结果表明,Fe@Al-Ti载氧体的介层有效阻隔Fe2O3与Al2O3的接触,抑制了FeAl2O4形成,抗烧结性能得到进一步提升。Fe@Al-Ti载氧体在化学链制氢循环实验中无明显积炭和团聚现象,制氢能力随循环次数逐渐增加,循环稳定性较好;尤其物质的量比Al∶Ti=3.5∶1的核壳载氧体的碳转化率、制氢率和储氧量最高,分别为57.4%、75.0%和6.01 mmol/g,比非核壳Fe-Al-Ti载氧体分别增加28.4%、30.0%、26.9%。
-
关键词:
- 化学链制氢 /
- Fe2O3/TiO2/Al2O3 /
- 核壳结构 /
- 固定床
Abstract: Fe-Al-Ti oxygen carriers have good cycling stability and good properties of anti-carbon deposition in the chemical looping hydrogen generation (CLHG) process. However, the formation of FeAl2O4 reduces hydrogen yield and increases sintering. To weaken the formation of FeAl2O4 and to promote properties, the core-shell oxygen carriers of Fe@Al-Ti were prepared by self-assembly template combustion method, which took TiO2 as the inter-layer to separate Fe2O3 and Al2O3. The effect of multi-layer core-shell structure on reaction performance was evaluated on a fixed bed. The results indicated that the inter-layer of Fe@Al-Ti oxygen carriers effectively weakened the contact between Fe2O3 and Al2O3, thus reducing the formation of FeAl2O4 and improving properties of anti-sintering. The Fe@Al-Ti oxygen carriers significantly prevented carbon deposition and surface agglomeration, and had great cycling stability during the CLHG cycles. The core-shell oxygen carrier with a molar ratio of Al∶Ti=3.5∶1 got the highest carbon conversion rate and H2 yield, and oxygen storage capacity in a single cycle, with 57.4%, 75.0%, and 6.01 mmol/g, respectively, which were 28.4%, 30.0%, and 26.9% higher than those of non core-shell Fe-Al-Ti oxygen carriers.-
Key words:
- chemical looping hydrogen generation /
- Fe2O3/TiO2/Al2O3 /
- core-shell /
- fixed bed
-
表 1 载氧体制备原料及制备方法
Table 1 Materials and methods of oxygen carriers prepared in this work
Sample Preparation method Materials/g Al2O3 TiO2 Fe(NO3)3·9H2O CO(NH2)2 SATC(3.5∶1) SATC 7.00 2.00 68.18 23.25 SATC(2∶1) SATC 6.00 3.00 68.18 23.25 IM Impregnation 7.00 2.00 68.18 − Sol-gel Sol-gel 9.00 − 68.18 − 表 2 化学链制氢实验循环工况
Table 2 Experimental procedure in a cycle
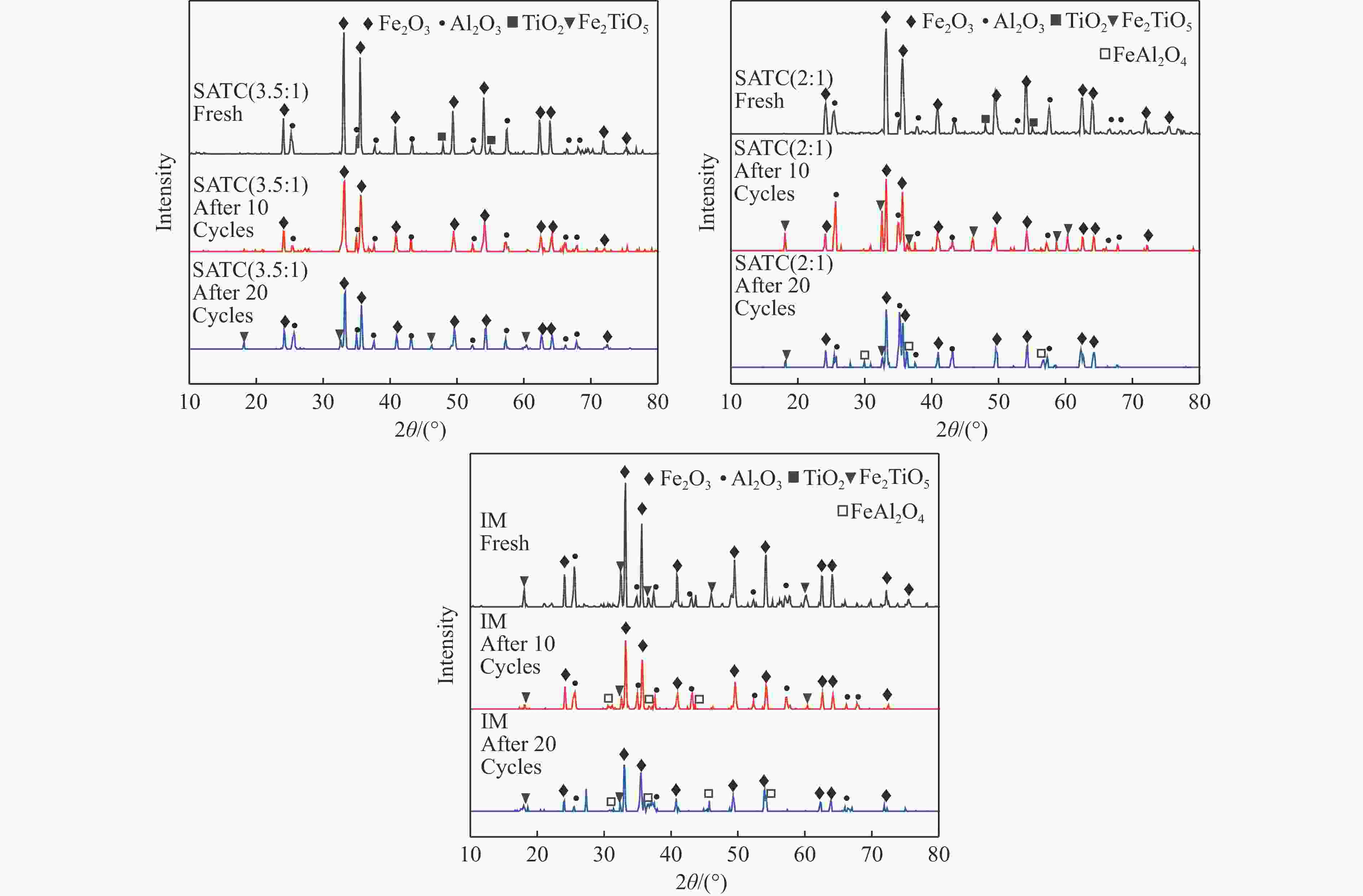
No. Experimental stage Time/min Gas flow rate/(L·min−1) 1 CO reduction 20 CO∶0.30 N2∶1.50 2 nitrogen blowing 8 N2∶1.50 3 steam oxidization 4 N2∶1.50 H2O(l)∶0.45 4 nitrogen blowing 8 N2∶1.50 5 air oxidization 10 O2∶0.30 N2∶1.50 6 nitrogen blowing 8 N2∶1.50 表 3 载氧体XRD物相及晶体尺寸
Table 3 XRD phase and crystallite of oxygen carrier
Crystal form Crystallite/Å fresh after 10 cycles after 20 cycles SATC(3.5∶1) Fe2TiO5 − − 365.5 FeAl2O4 − − − SATC(2∶1) Fe2TiO5 − 364.7 364.7 FeAl2O4 − − 406.5 IM Fe2TiO5 370.0 366.6 363.8 FeAl2O4 − 437.4 496.5 -
[1] JOHN A. Sustainable hydrogen production[J]. Science,2004,305(5686):972−974. doi: 10.1126/science.1103197 [2] MERIEM M, NAJOUA B, NAUSIKA Q, et al. Toward sustainable hydrogen storage and carbon dioxide capture in post-combustion condition[J]. J Environ Chem Eng,2017,5(2):1628−1637. doi: 10.1016/j.jece.2017.03.003 [3] MARTINO M, RUOCCO C, MELONI E, et al. Main hydrogen production processes: An overview[J]. Catalysts,2021,11(5):547. doi: 10.3390/catal11050547 [4] KHAN M, SHAMIM T. Techno-economic assessment of a plant based on a three reactor chemical looping reforming system[J]. Int J Hydrogen Energy,2016,41(48):22677−22688. [5] LUIS F, ORTIZ M, GARCIA-LABIANO F, et al. Hydrogen production by chemical-looping reforming in a circulating fluidized bed reactor using Ni-based oxygen carriers[J]. J Power Sourc,2009,192(1):27−34. doi: 10.1016/j.jpowsour.2008.11.038 [6] PAOLO C, GIOVANNI L, ALBERTO M, et al. Three-reactors chemical looping process for hydrogen production[J]. Int J Hydrogen Energy,2008,33(9):2233−2245. doi: 10.1016/j.ijhydene.2008.02.032 [7] HSIEH T, XU D, ZHANG Y, et al. 250 kWth high pressure pilot demonstration of the syngas chemical looping system for high purity H2 production with CO2 capture[J]. Appl Energy,2018,230:1660−1672. doi: 10.1016/j.apenergy.2018.09.104 [8] CHO W C, LEE D Y, SEO M W, et al. Continuous operation characteristics of chemical looping hydrogen production system[J]. Appl Energy,2014,113:1667−1674. doi: 10.1016/j.apenergy.2013.08.078 [9] LIU T, YU Z, JIAO W, et al. Interaction of coal ashes with potassium-decorated Fe2O3/Al2O3 oxygen carrier in coal-direct chemical looping hydrogen generation (CLHG)[J]. J Energy Inst,2020,93(5):1790−1797. doi: 10.1016/j.joei.2020.03.010 [10] WANG T, GAO Y, LIU Y, et al. Core-shell Na2WO4/CuMn2O4 oxygen carrier with high oxygen capacity for chemical looping oxidative dehydrogenation of ethane[J]. Fuel,2021,303:121286. doi: 10.1016/j.fuel.2021.121286 [11] CHEN Y, GALINSKY N, WANG Z, et al. Investigation of perovskite supported composite oxides for chemical looping conversion of syngas[J]. Fuel,2014,134:521−530. doi: 10.1016/j.fuel.2014.06.017 [12] KHAN M, SHAMIM T. Thermodynamic screening of suitable oxygen carriers for a three reactor chemical looping reforming system[J]. Int J Hydrogen Energy,2017,42(24):15745−15760. doi: 10.1016/j.ijhydene.2017.05.037 [13] LUO M, YI Y, WANG S Z, WANG Z L, et al. Review of hydrogen production using chemical-looping technology[J]. Renewable Sustainable Energy Rev,2018,81(2):3186−3214. [14] GUO Q, CHENG Y, LIU Y, et al. Coal chemical looping gasification for syngas generation using an iron-based oxygen carrier[J]. Ind Eng Chem Res.,2014,53:78−86. doi: 10.1021/ie401568x [15] WANG J, LI K, WANG H, et al. Sandwich Ni-phyllosilicate@doped-ceria for moderate-temperature chemical looping dry reforming of methane[J]. Fuel Process Technol,2022,232:107268. doi: 10.1016/j.fuproc.2022.107268 [16] CHEN S, SHI Q, XUE Z, SUN X, et al. Experimental investigation of chemical-looping hydrogen generation using Al2O3 or TiO2-supported iron oxides in a batch fluidized bed[J]. Int J Hydrogen Energy,2011,36(15):8915−8926. doi: 10.1016/j.ijhydene.2011.04.204 [17] WU H, KU Y, HUANG Y, et al. Fabrication of iron-based oxygen carriers on various supports for chemical looping hydrogen generation[J]. Aerosol Air Qual,2021,21:200322. doi: 10.4209/aaqr.2020.06.0322 [18] TOMON C, SARAWUTANUKUL S, PHATTHARASUPAKUN N, et al. Core-shell structure of LiMn2O4 cathode material reduces phase transition and Mn dissolution in Li-ion batteries[J]. Commun Chem,2022,5(1):54. doi: 10.1038/s42004-022-00670-y [19] HAO J, LIU B, MAENOSONO S, et al. One-pot synthesis of Au-M@SiO2 (M = Rh, Pd, Ir, Pt) core-shell nanoparticles as highly efficient catalysts for the reduction of 4-nitrophenol[J]. Scic Rep,2022,12(1):7615. doi: 10.1038/s41598-022-11756-x [20] RAMI M, FOROUZANDEHDEL S, SALAMI M, et al. Synthesis of the core/shell structure of polycaprolactone@curcumin-gluten for drug delivery applications[J]. Bio Res Appl Chem,2023,13(1):87. [21] XU K, LIU D, FENG L, et al. Mercury removal by Co3O4@TiO2@Fe2O3 magnetic core-shell oxygen carrier in chemical-looping combustion[J]. Fuel,2021,306:121604. doi: 10.1016/j.fuel.2021.121604 [22] YIN X, WANG S, SUN R, et al. A Ce-Fe oxygen carrier with a core-shell structure for chemical looping steam methane reforming[J]. Ind Eng Chem Res,2020,59(21):9775−9786. doi: 10.1021/acs.iecr.0c00055 [23] SUN Y, LI J, LI H, et al. Core-shell-like Fe2O3/MgO oxygen carriers matched with fluidized bed reactor for chemical looping reforming[J]. Chem Eng J,2022,431(2):134−143. [24] XU Z, ZHAO H, WEI Y, et al. Self-assembly template combustion synthesis of a core-shell CuO@TiO2-Al2O3 hierarchical structure as an oxygen carrier for the chemical-looping processes[J]. Combust Flame,2015,162(8):3030−3045. doi: 10.1016/j.combustflame.2015.05.006 [25] LIANG Z, QIN W, DONG C, et al. Experimental and theoretical study of the interactions between Fe2O3/Al2O3 and CO[J]. Energies,2017,10(5):598. [26] ZHU M, CHEN S, SOOMRO A, et al. Effects of supports on reduction activity and carbon deposition of iron oxide for methane chemical looping hydrogen generation[J]. Appl Energy,2018,225:912−921. doi: 10.1016/j.apenergy.2018.05.082 [27] POOYA L, ZAINAL A Z, MAEDEH M, et al. Conversion of the greenhouse gas CO2 to the fuel gas CO via the Boudouard reaction: A review[J]. Renewable Sustainable Energy Rev,2015,41:615−632. doi: 10.1016/j.rser.2014.08.034 [28] 马士伟. 基于改性Fe2O3/CeO2载氧体制氢特性研究[D]. 江苏: 东南大学, 2019.MA Shiwei. Research on hydrogen production characteristics based on modified Fe2O3/CeO2 oxygen carriers[D]. Jiangsu: Southeast University, 2019. -




 下载:
下载: