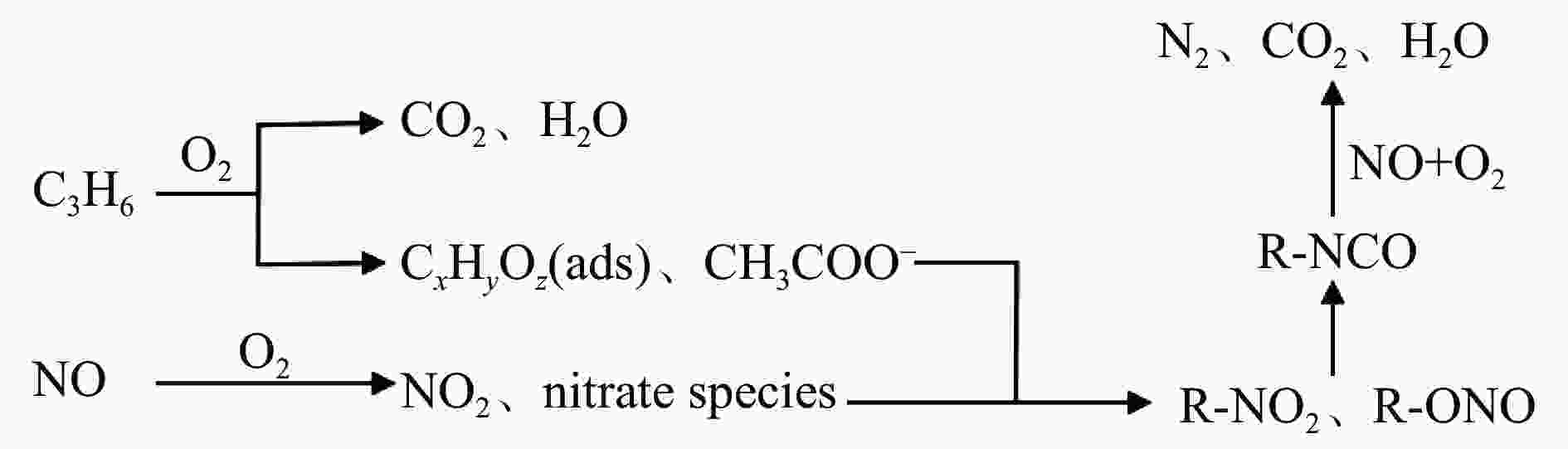
C3H6-SCR denitration characteristics of CuCoCe-LDH catalysts
-
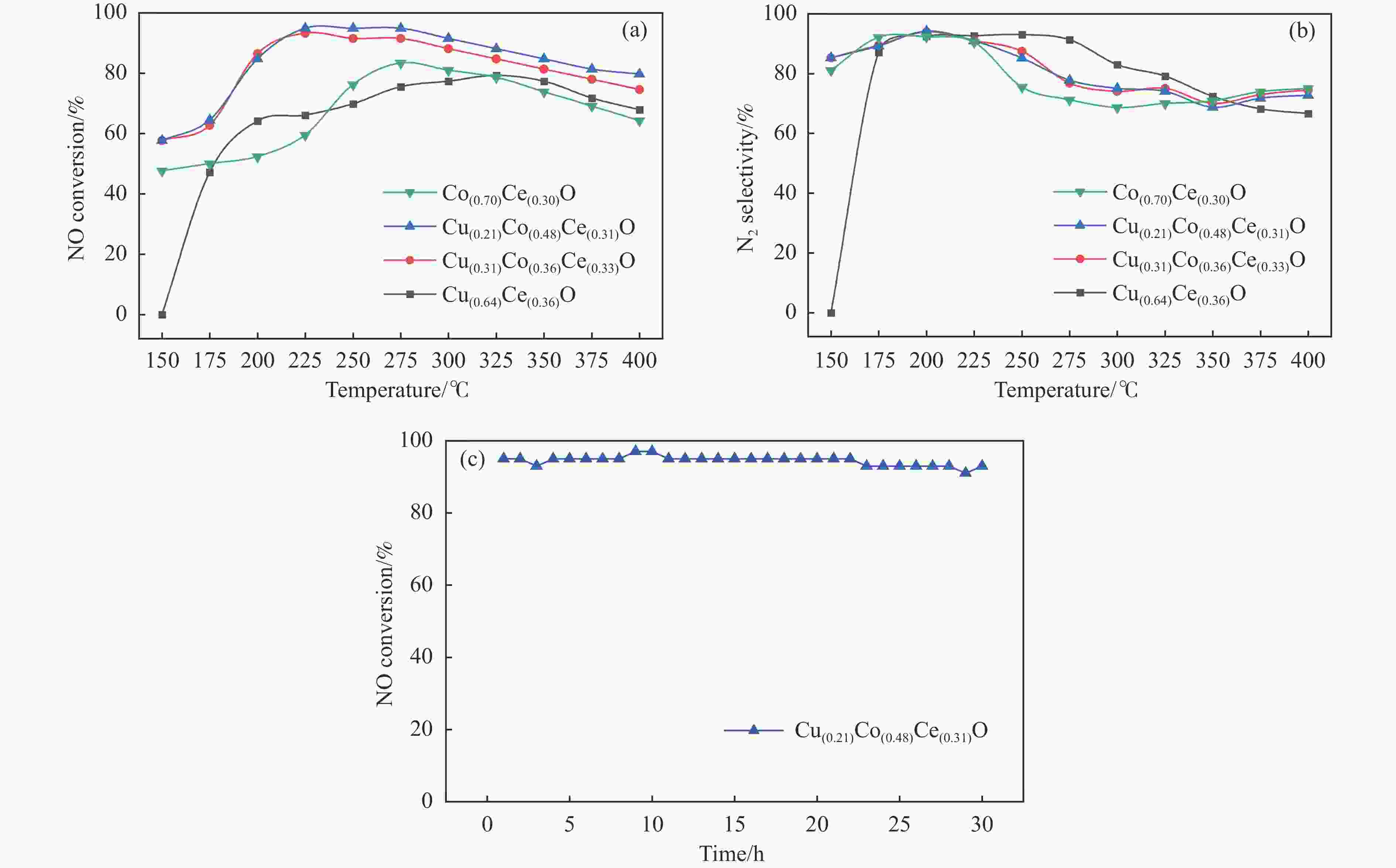
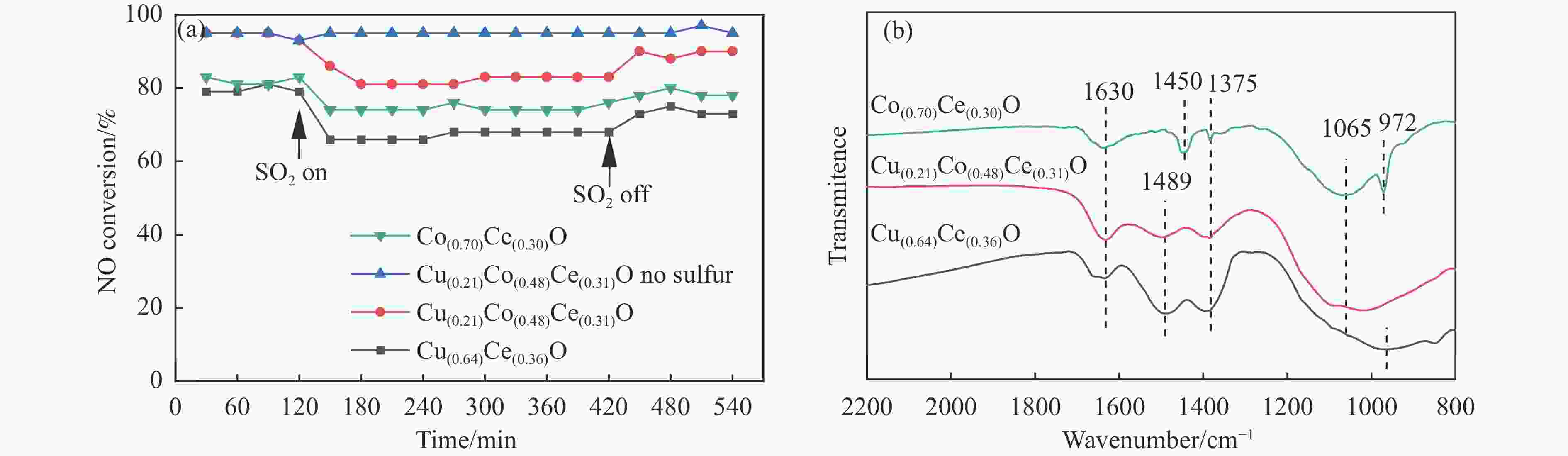
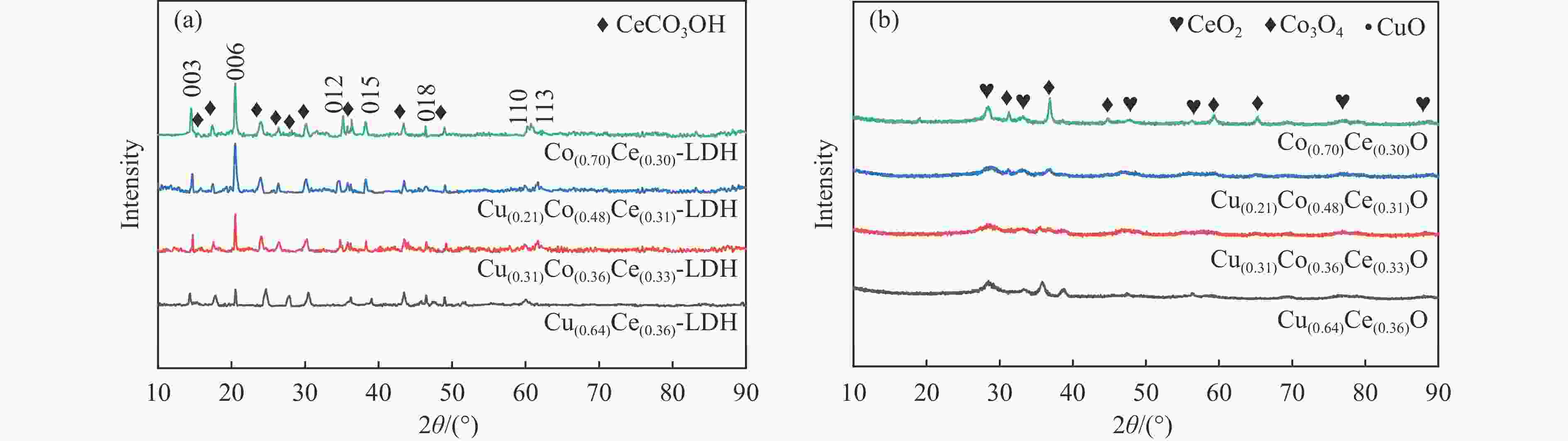
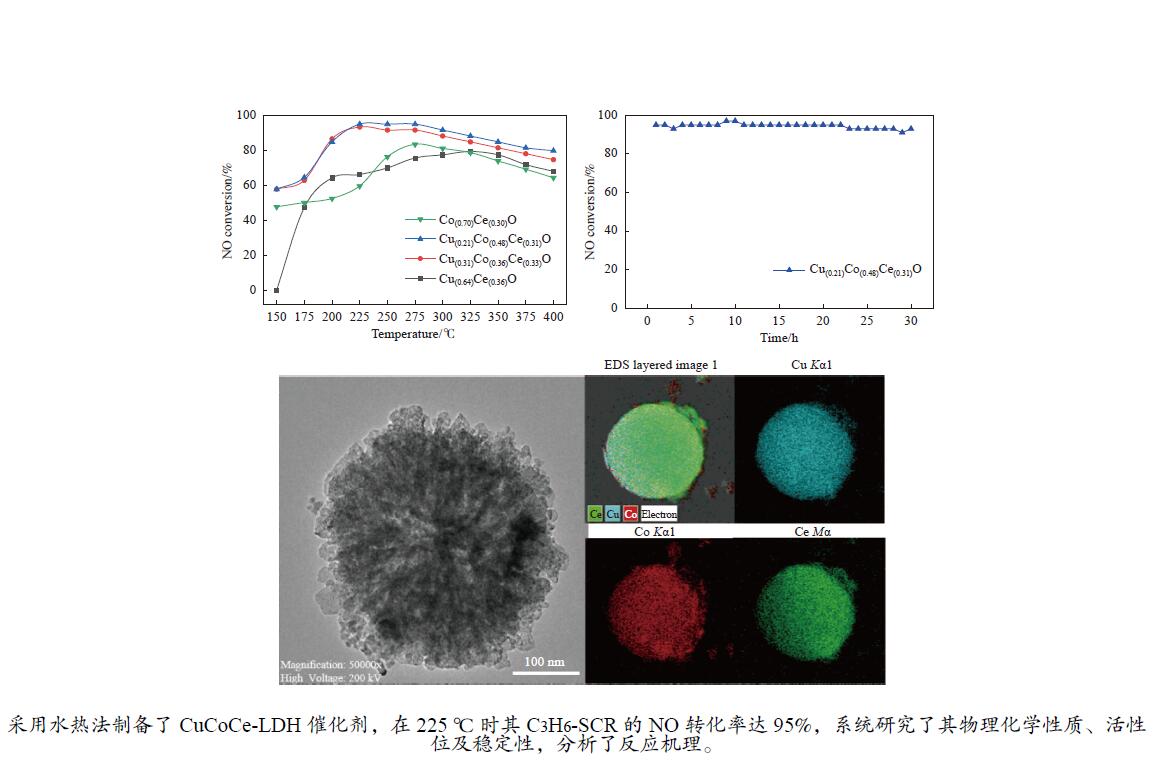
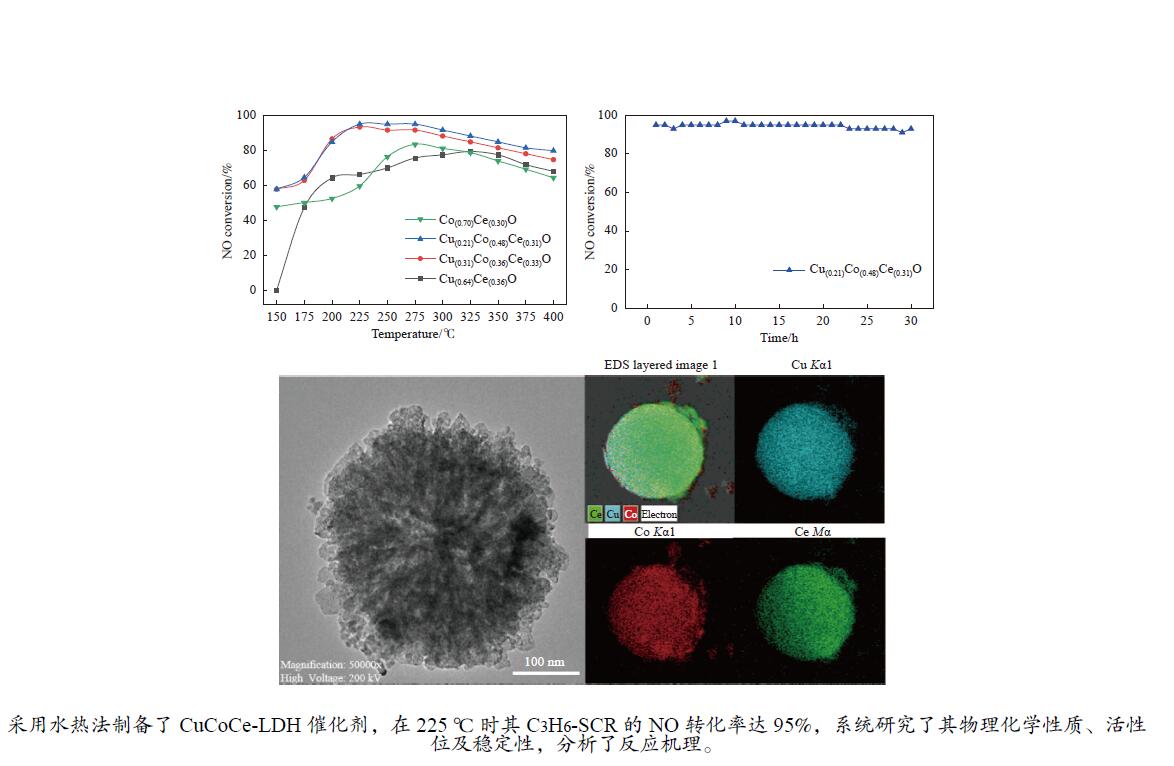
摘要: 采用水热法一步合成一系列的Cu(x)Co(y)Ce(z)-LDH前驱体,煅烧后形成Cu(x)Co(y)Ce(z)O混合金属氧化物催化剂,在固定床微反应器上实验研究了其C3H6选择性催化还原NO的性能(C3H6-SCR)。得益于Cu、Co、Ce之间强大的协同作用,Cu(0.21)Co(0.48)Ce(0.31)O在225 ℃时达到95%的NO转化率和90%的N2选择性。此外,运用ICP、XRD、TEM、XPS、H2-TPR等表征来研究其物理化学性质和催化还原能力之间的关系。XRD结果表明,Cu、Co、Ce之间形成了固溶体,促进了活性金属的分散。XPS和H2-TPR进一步证明Cu和Co之间发生了氧化还原反应,促进氧空位的形成,从而提升其催化还原性能。
-
关键词:
- 氮氧化物 /
- 选择性催化还原 /
- C3H6 /
- CuCoCe-LDH催化剂 /
- 水热法
Abstract: A series of Cu(x)Co(y)Ce(z)-LDH precursors were synthesized by one-step hydrothermal method, and Cu(x)Co(y)Ce(z)O mixed metal oxide catalysts were prepared after calcination and used to study the selective catalytic reduction of NO by C3H6 (C3H6-SCR) with a fixed bed micro-reactor. Due to the strong synergy between Cu, Co and Ce, Cu(0.21)Co(0.48)Ce(0.31)O achieves 95% NO conversion and 90% N2 selectivity at 225 ℃. In addition, ICP, XRD, TEM, XPS, H2-TPR were used to characterize the basic physical-chemical properties of the catalysts to investigate the relationship between physicochemical properties and catalytic reduction abilities. XRD results show that solid solutions are formed between Cu, Co and Ce, which promotes the dispersion of active metals. XPS and H2-TPR further demonstrate that redox reactions occur between Cu and Co, promoting the formation of oxygen vacancies, thereby improving their catalytic reduction capacity.-
Key words:
- nitrogen oxides /
- selective catalytic reduction /
- C3H6 /
- CuCoCe-LDH /
- hydrothermal method
-
表 1 Cu(x)Co(y)Ce(z)O催化剂的表面原子比
Table 1 Atomic concentration on Cu(x)Co(y)Ce(z)O catalyst surface
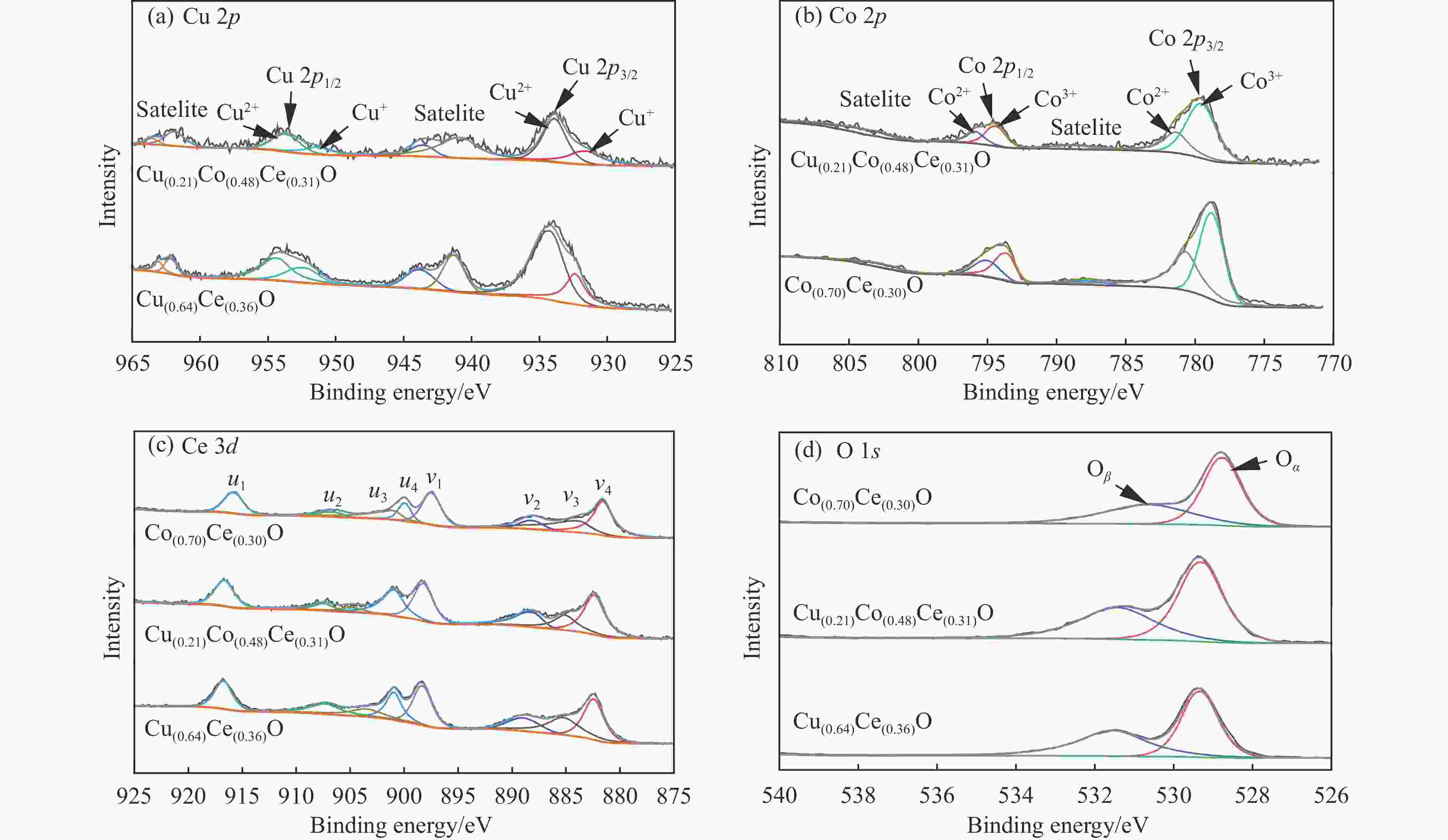
Catalyst Binding energy/eV Surface atomic ratio/% Cu+ Cu2+ Co2+ Co3+ Cu+/Cu2+ Co2+/Co3+ Ce4+/Ce3+ Oβ/Oα Cu(0.64)Ce(0.36)O 932.4 934.3 − − 0.45 − 3.34 0.72 952.3 954.3 Cu(0.21)Co(0.48)Ce(0.31)O 931.6 933.9 795.8 794.4 0.55 0.46 4.90 0.81 951.3 953.6 781.3 779.6 Co(0.70)Ce(0.30)O − − 794.9 793.6 − 0.77 2.55 0.73 780.7 778.8 表 2 Cu(0.21)Co(0.48)Ce(0.31)O催化剂表面原子比
Table 2 Atomic concentration on Cu(x)Co(y)Ce(z)O catalyst surface
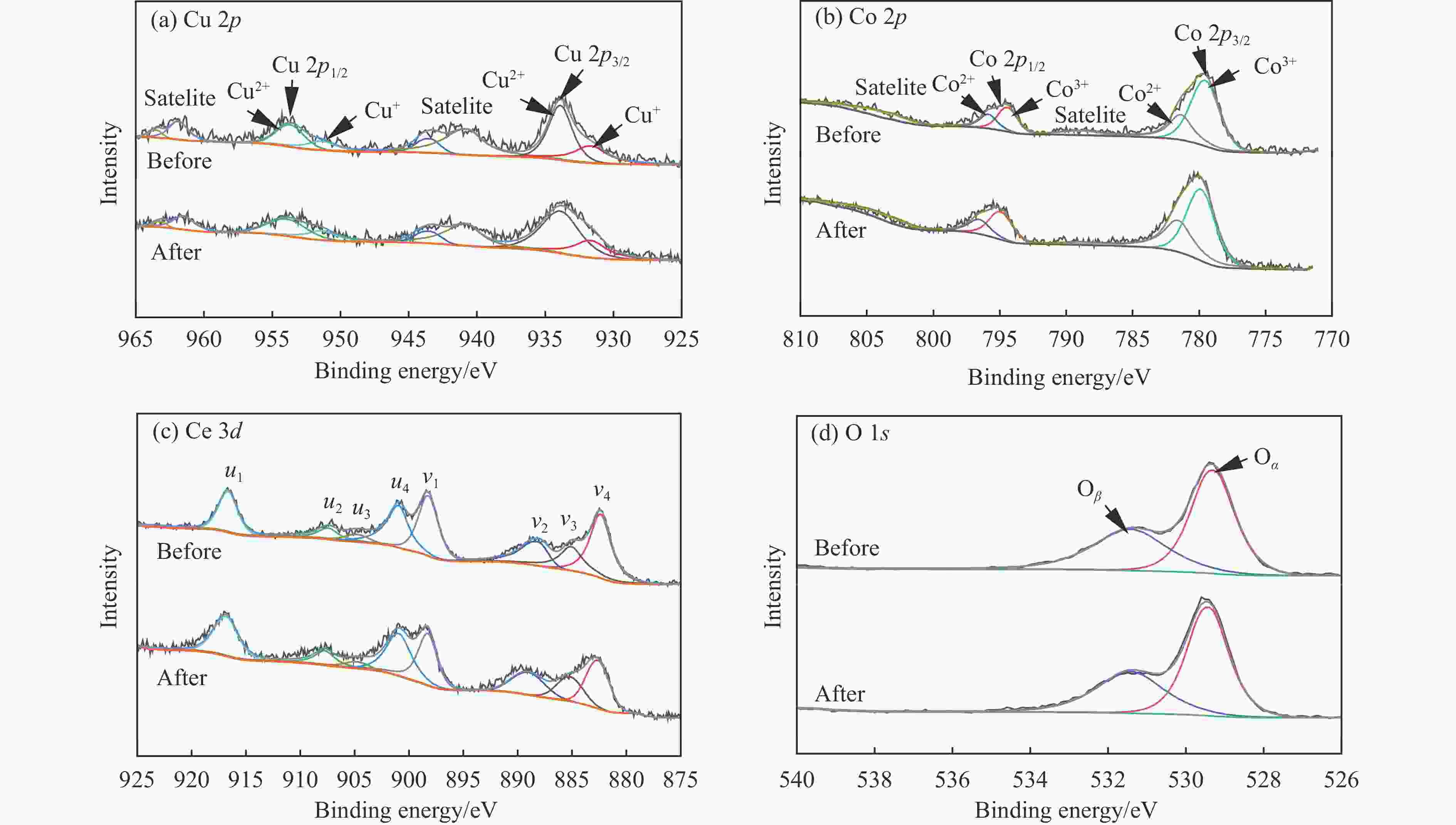
Cu(0.21)Co(0.48)Ce(0.31)O Surface atomic ratio/% Cu+/Cu2+ Co2+/Co3+ Ce4+/Ce3+ Oβ/Oα Before 0.55 0.46 4.90 0.81 After 0.47 0.47 4.27 0.65 表 3 Cu(x)Co(y)Ce(z)O催化剂的比表面积、孔容、孔径及ICP数据
Table 3 Specific surface area, pore volume, pore size and ICP data of Cu(x)Co(y)Ce(z)O catalyst
Catalyst ABET/
(m2·g−1)vP/
(cm3·g−1)dP/
nmElement content/(mg·g−1) Molar ratio Cu Co Ce x y z Cu(0.64)Ce(0.36)O 80 0.060 6.566 287.32 − 353.54 0.64 − 0.36 Cu(0.31)Co(0.36)Ce(0.33)O 59 0.170 10.328 141.93 151.94 330.50 0.31 0.36 0.33 Cu(0.21)Co(0.48)Ce(0.31)O 53 0.125 11.670 100.75 212.29 325.33 0.21 0.48 0.31 Co(0.70)Ce(0.30)O 29 0.123 14.853 − 329.03 329.01 − 0.70 0.30 -
[1] BONINGARI T, SMIRNIOTIS P G. Impact of nitrogen oxides on the environment and human health: Mn-based materials for the NOx abatement[J]. Curr Opin Chem Eng,2016,13:133−141. doi: 10.1016/j.coche.2016.09.004 [2] SHAN W, GENG Y, CHEN X, et al. A highly efficient CeWOx catalyst for the selective catalytic reduction of NOx with NH3[J]. Catal Sci Technol,2016,6(4):1195−200. doi: 10.1039/C5CY01282A [3] ZHANG X, SU Y, CHENG J, et al. Effect of Ag on deNOx performance of SCR-C3H6 over Fe/Al-PILC catalysts[J]. J Fuel Chem Technol,2019,47(11):1368−1378. doi: 10.1016/S1872-5813(19)30055-6 [4] XU J, WANG H, GUO F, et al. Recent advances in supported molecular sieve catalysts with wide temperature range for selective catalytic reduction of NOx with C3H6[J]. RSC Adv,2019,9(2):824−838. doi: 10.1039/C8RA08635D [5] 周皞, 苏亚欣, 邓文义等. 金属氧化物类催化剂上HC-SCR研究进展[J]. 环境科学与技术,2016,39(1):93−100.ZHOU Hao, SU Yaxin, DENG Wenyi, et al. A review of HC-SCR over metal oxides-based catalysts[J]. Environ Sci Technol,2016,39(1):93−100. [6] 宁淑英, 苏亚欣, 杨洪海等. 用于HC-SCR还原NOx的Cu基分子筛催化剂研究进展[J]. 化工进展,2023,42(3):1308−1320.NING Shuying, SU Yaxin, YANG Honghai, et al. Research progress on supported Cu-based zeolite catalysts for the selective catalytic reduction of NOx with hydrocarbons[J]. Chem Ind Eng Prog,2023,42(3):1308−1320. [7] KASHIF M, YUAN M, SU Y, et al. A review on pillared clay-based catalysts for low-temperature selective catalytic reduction of NO with hydrocarbons[J]. Appl Clay Sci,2023,233:106847. doi: 10.1016/j.clay.2023.106847 [8] HUANG F, HU W, CHEN J, et al. Insight into enhancement of NO reduction with methane by multifunctional catalysis over a mixture of Ce/HZSM-5 and CoOx in excess of oxygen[J]. Ind Eng Chem Res,2018,57(40):13312−13317. doi: 10.1021/acs.iecr.8b00773 [9] SERHAN N, TSOLAKIS A, WAHBI A, et al. Modifying catalytically the soot morphology and nanostructure in diesel exhaust: Influence of silver De-NOx catalyst (Ag/Al2O3)[J]. Appl Catal B: Environ,2019,241:471−482. doi: 10.1016/j.apcatb.2018.09.068 [10] YUAN D, LI X, ZHAO Q, et al. A novel CuTi-containing catalyst derived from hydrotalcite-like compounds for selective catalytic reduction of NO with C3H6 under lean-burn conditions[J]. J Catal,2014,309:268−279. doi: 10.1016/j.jcat.2013.09.010 [11] YAN Q, HOU X, LIU G, et al. Recent advances in layered double hydroxides (LDHs) derived catalysts for selective catalytic reduction of NOx with NH3[J]. J Hazard Mater,2020,400:123260. doi: 10.1016/j.jhazmat.2020.123260 [12] LIU X, FAN X, HUANG H, et al. Electronic modulation of oxygen evolution on metal doped NiFe layered double hydroxides[J]. J Colloid Interface Sci,2021,587:385−392. doi: 10.1016/j.jcis.2020.12.023 [13] WU X, LIU J, LIU X, et al. Fabrication of carbon doped Cu-based oxides as superior NH3-SCR catalysts via employing sodium dodecyl sulfonate intercalating CuMgAl-LDH[J]. J Catal,2022,407:265−280. doi: 10.1016/j.jcat.2022.02.004 [14] DU Y, LIU L, FENG Y, et al. Enhancement of NH3-SCR performance of LDH-based MMnAl (M=Cu, Ni, Co) oxide catalyst: influence of dopant M[J]. RSC Adv,2019,9(68):39699−39708. doi: 10.1039/C9RA08391J [15] CHEN S, YAN Q, ZHANG C, et al. A novel highly active and sulfur resistant catalyst from Mn-Fe-Al layered double hydroxide for low temperature NH3-SCR[J]. Catal Today,2019,327:81−89. doi: 10.1016/j.cattod.2018.06.006 [16] YUAN D, LI X, ZHAO Q. Preparation and characterization of Ni-Ti-O mixed oxide for selective catalytic reduction of NO under lean-burn conditions[J]. Chin J Catal,2013,34(7):1449−1455. doi: 10.1016/S1872-2067(12)60614-7 [17] TRET’YAKOV V F, ZAKIROVA A G, SPOZHAKINA A A, et al. Selective reduction of nitrogen oxides by hydrocarbons on hydrotalcite Co and Ni catalysts[J]. Catal Ind,2010,2(1):62−66. doi: 10.1134/S2070050410010101 [18] WEN N, SU Y, DENG W, et al. Synergy of CuNiFe-LDH based catalysts for enhancing low-temperature SCR-C3H6 performance: Surface properties and reaction mechanism[J]. Chem Eng J,2022,438:135570. doi: 10.1016/j.cej.2022.135570 [19] ADAMOWSKA M, KRZTOŃ A, NAJBAR M, et al. DRIFT study of the interaction of NO and O2 with the surface of Ce0.62Zr0.38O2 as deNOx catalyst[J]. Catal Today,2008,137(2/4):288−291. doi: 10.1016/j.cattod.2008.01.013 [20] ZHAO L, ZHANG Y, BI S, et al. Metal-organic framework-derived CeO2-ZnO catalysts for C3H6-SCR of NO: An in situ DRIFTS study[J]. RSC Adv,2019,9(33):19236−19242. doi: 10.1039/C9RA03103K [21] LI X, LU G, QU Z, et al. The role of titania pillar in copper-ion exchanged titania pillared clays for the selective catalytic reduction of NO by propylene[J]. Appl Catal A: Gen,2011,398(1/2):82−87. doi: 10.1016/j.apcata.2011.03.020 [22] AMIN N A S, TAN E F, MANAN Z A. Selective reduction of NOx with C3H6 over Cu and Cr promoted CeO2 catalysts[J]. Appl Catal B: Environ,2003,43(1):57−69. doi: 10.1016/S0926-3373(02)00275-8 [23] WEN N, SU Y, DENG W, et al. Selective catalytic reduction of NO with C3H6 over CuFe-containing catalysts derived from layered double hydroxides[J]. Fuel,2021,283:119296. doi: 10.1016/j.fuel.2020.119296 [24] WANG X, WEN W, MI J, et al. The ordered mesoporous transition metal oxides for selective catalytic reduction of NOx at low temperature[J]. Appl Catal B: Environ,2015,176:454−463. [25] PAN K, YU F, LIU Z, et al. Enhanced low-temperature CO-SCR denitration performance and mechanism of two-dimensional CuCoAl layered double oxide[J]. J Environ Chem Eng,2022,10(3):108030. doi: 10.1016/j.jece.2022.108030 [26] ZHANG X P, CUI Y Z, TAN B J, et al. The adsorption and catalytic oxidation of the element mercury over cobalt modified Ce–ZrO2 catalyst[J]. RSC Adv,2016,6(91):88332−88339. doi: 10.1039/C6RA19450H [27] WANG Z, LAN J, HANEDA M, et al. Selective catalytic reduction of NOx with NH3 over a novel Co-Ce-Ti catalyst[J]. Catal Today,2021,376:222−228. doi: 10.1016/j.cattod.2020.05.040 [28] LI Z, CHEN J, JIANG M, Et al. Study on SO2 poisoning mechanism of CO catalytic oxidation reaction on copper-cerium catalyst[J]. Catal Lett,2021,152:2729−2737. [29] SHI M, YE S, QU H, et al. Synergistic effect of Cu2+ doping and sulfation in Cu-Ce-S, tolerance to H2O and SO2 and decomposition behaviors of ammonia salts[J]. Mol Catal,2018,459:135−140. doi: 10.1016/j.mcat.2018.08.023 [30] YAN Q, CHEN S, QIU L, et al. The synthesis of CuyMnzAl1-zOx mixed oxide as a low-temperature NH3-SCR catalyst with enhanced catalytic performance[J]. Dalton Trans,2018,47(9):2992−3004. doi: 10.1039/C7DT02000G [31] ZHANG Y, ZHAO L, KANG M, et al. Insights into high CO-SCR performance of CuCoAlO catalysts derived from LDH/MOFs composites and study of H2O/SO2 and alkali metal resistance[J]. Chem Eng J,2021,426:131873. doi: 10.1016/j.cej.2021.131873 [32] WANG L, YANG Z, LIAO Y, et al. Synthesis of reusable and anti-fouling Co-Al-Ce LDHs coated stainless steel mesh for ultrafast oil/water separation and photocatalytic degradation[J]. Appl Clay Sci,2023,232:106769. doi: 10.1016/j.clay.2022.106769 [33] DING J, HAN Y, HONG G. Tailoring the activity of NiFe layered double hydroxide with CeCO3OH as highly efficient water oxidation electrocatalyst[J]. Int J Hydrogen Energy,2021,46(2):2018−2025. doi: 10.1016/j.ijhydene.2020.10.075 [34] JING F, LIU S, WANG R, et al. Hydrogen production through glycerol steam reforming over the NiCexAl catalysts[J]. Renewable Energy,2020,158:192−201. doi: 10.1016/j.renene.2020.05.044 [35] ZHANG Q, XU R, LIU N, et al. In situ Ce-doped catalyst derived from NiCeAl-LDHs with enhanced low-temperature performance for CO2 methanation[J]. Appl Surf Sci,2022,579:152204. doi: 10.1016/j.apsusc.2021.152204 [36] HE C, YU Y, YUE L, et al. Low-temperature removal of toluene and propanal over highly active mesoporous CuCeOx catalysts synthesized via a simple self-precipitation protocol[J]. Appl Catal B: Environ,2014,147:156−166. doi: 10.1016/j.apcatb.2013.08.039 [37] YAN Q, GAO Y, LI Y, et al. Promotional effect of Ce doping in Cu4Al1Ox – LDO catalyst for low-T practical NH3-SCR: Steady-state and transient kinetics studies[J]. Appl Catal B: Environ,2019,255:117749. doi: 10.1016/j.apcatb.2019.117749 [38] YAO X, KANG K, CAO J, et al. Enhancing the denitration performance and anti-K poisoning ability of CeO2-TiO2/P25 catalyst by H2SO4 pretreatment: Structure-activity relationship and mechanism study[J]. Appl Catal B: Environ,2020,269:118808. doi: 10.1016/j.apcatb.2020.118808 [39] LI D, LIU X, ZHANG Q, et al. Cobalt and copper composite oxides as efficient catalysts for preferential oxidation of CO in H2-rich stream[J]. Catal Lett,2008,127(3/4):377−385. [40] VARGHESE S, CUTRUFELLO M G, ROMBI E, et al. CO oxidation and preferential oxidation of CO in the presence of hydrogen over SBA-15-templated CuO-Co3O4 catalysts[J]. Appl Catal A: Gen,2012,443:161−170. [41] IVANOVA S, PETIT C, PITCHON V. A new preparation method for the formation of gold nanoparticles on an oxide support[J]. Appl Catal A: Gen,2004,267(1/2):191−201. doi: 10.1016/j.apcata.2004.03.004 [42] NIVANGUNE N T, RANADE V V, KELKAR A A. MgFeCe ternary layered double hydroxide as highly efficient and recyclable heterogeneous base catalyst for synthesis of dimethyl carbonate by transesterification[J]. Catal Lett,2017,147(10):2558−2569. doi: 10.1007/s10562-017-2146-x [43] YANG H, LIU X, QIN K, et al. Enhancement strategy of photoelectrocatalytic activity of cobalt-copper layer double hydroxide toward methanol oxidation: Cerium doping and modification with porphyrin[J]. Inorg Chem,2022,61(19):7414−7425. doi: 10.1021/acs.inorgchem.2c00438 [44] DU Y, LIU X, LIU J, et al. DeNOx performance enhancement of Cu-based oxides via employing a TiO2 phase to modify LDH precursors[J]. RSC Adv,2022,12(16):10142−10153. doi: 10.1039/D2RA00316C [45] WU X, FENG Y, LIU X, et al. Redox & acidity optimizing of LDHs-based CoMnAl mixed oxides for enhancing NH3-SCR performance[J]. Appl Surf Sci,2019,495:1443513. [46] ZANG P, LIU J, HE Y, et al. LDH-derived preparation of CuMgFe layered double oxides for NH3-SCR and CO oxidation reactions: Performance study and synergistic mechanism[J]. Chem Eng J,2022,446:137414. doi: 10.1016/j.cej.2022.137414 [47] LI J, ZHU P, ZHOU R. Effect of the preparation method on the performance of CuO-MnOx-CeO2 catalysts for selective oxidation of CO in H2-rich streams[J]. J Power Sources,2011,196(22):9590−9598. doi: 10.1016/j.jpowsour.2011.07.052 [48] KONSOLAKIS M, CARABINEIRO S A, TAVARES P B, et al. Redox properties and VOC oxidation activity of Cu catalysts supported on Ce1-xSmxO delta mixed oxides[J]. J Hazard Mater,2013,261:512−521. doi: 10.1016/j.jhazmat.2013.08.016 [49] GUO X, WU H, WANG H, et al. Sulfadiazine advanced oxidizing-degradation: Defects generation by boosting electron transfer at interfaces of Co-Cu LDH catalysts[J]. J Environ Chem Eng,2022,10(6):108411. doi: 10.1016/j.jece.2022.108411 [50] WANG Z, DENG J, LIU Y, et al. Three-dimensionally ordered macroporous CoCr2O4-supported Au-Pd alloy nanoparticles: Highly active catalysts for methane combustion[J]. Catal Today,2017,281:467−476. doi: 10.1016/j.cattod.2016.05.035 [51] HE C, YU Y, CHEN C, et al. Facile preparation of 3D ordered mesoporous CuOx-CeO2 with notably enhanced efficiency for the low temperature oxidation of heteroatom-containing volatile organic compounds[J]. RSC Adv,2013,3(42):19639−19656. doi: 10.1039/c3ra42566e [52] ZHANG Y, ZHAO L, DUAN J, et al. Insights into deNOx processing over Ce-modified Cu-BTC catalysts for the CO-SCR reaction at low temperature by in situ DRIFTS[J]. Sep Purif Technol,2020,234:116081. doi: 10.1016/j.seppur.2019.116081 [53] SUN R, YU F, WAN Y, et al. Reducing N2O formation over CO-SCR systems with CuCe mixed metal oxides[J]. ChemCatChem,2021,13(11):2709−2718. doi: 10.1002/cctc.202100057 [54] CAI S, ZHANG D, SHI L, et al. Porous Ni-Mn oxide nanosheets in situ formed on nickel foam as 3D hierarchical monolith de-NOx catalysts[J]. Nanoscale,2014,6(13):7346−7353. doi: 10.1039/C4NR00475B [55] LI L, HAN W, ZHANG J, et al. Controlled pore size of 3D mesoporous Cu-Ce based catalysts and influence of surface textures on the CO catalytic oxidation[J]. Microporous Mesoporpous Mater,2016,231:9−20. doi: 10.1016/j.micromeso.2016.05.018 [56] LIU Z, LI J, BUETTNER M, et al. Metal-support interactions in CeO2-and SiO2-supported cobalt catalysts: Effect of support morphology, reducibility, and interfacial configuration[J]. ACS Appl Mater Interfaces,2019,11(18):17035−17049. doi: 10.1021/acsami.9b02455 [57] WANG X, ZHANG C, ZHANG Z, et al. Insights into the interfacial effects in Cu-Co/CeOx catalysts on hydrogenolysis of 5-hydroxymethylfurfural to biofuel 2, 5-dimethylfuran[J]. J Colloid Interf Sci,2022,615:19−29. doi: 10.1016/j.jcis.2022.01.168 [58] SHEN H, LI H, YANG Z, et al. Magic of hydrogen spillover: Understanding and application[J]. Green Energy Environ,2022,7(6):1161−1198. doi: 10.1016/j.gee.2022.01.013 [59] XIONG M, GAO Z, QIN Y. Spillover in heterogeneous catalysis: New insights and opportunities[J]. ACS Catal,2021,11(5):3159−3172. doi: 10.1021/acscatal.0c05567 -




 下载:
下载: