CH4 partial oxidation mechanism of LaFeO3 oxygen carrier in chemical looping reforming
-
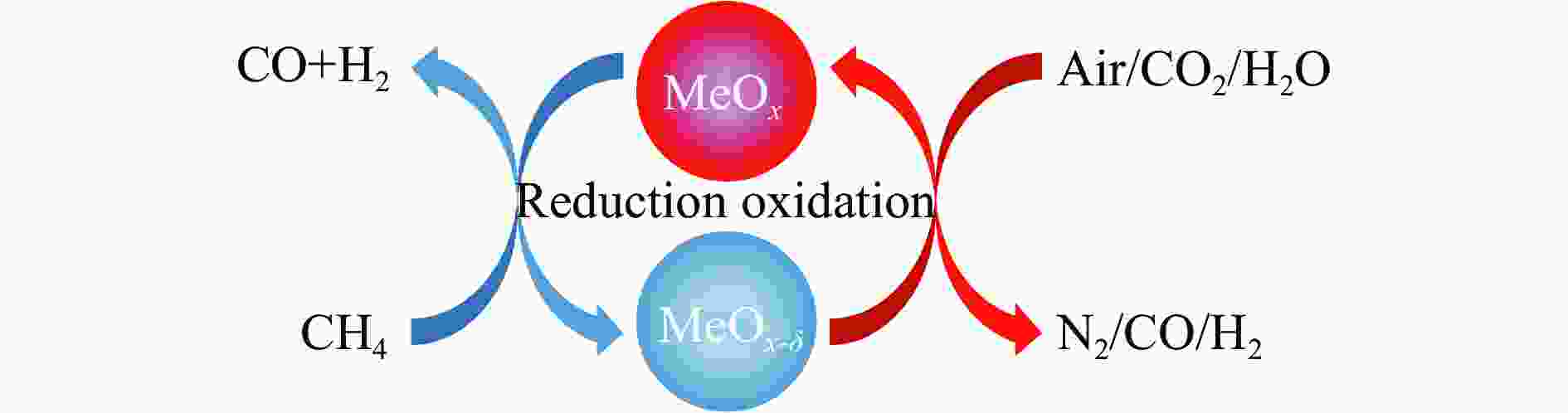
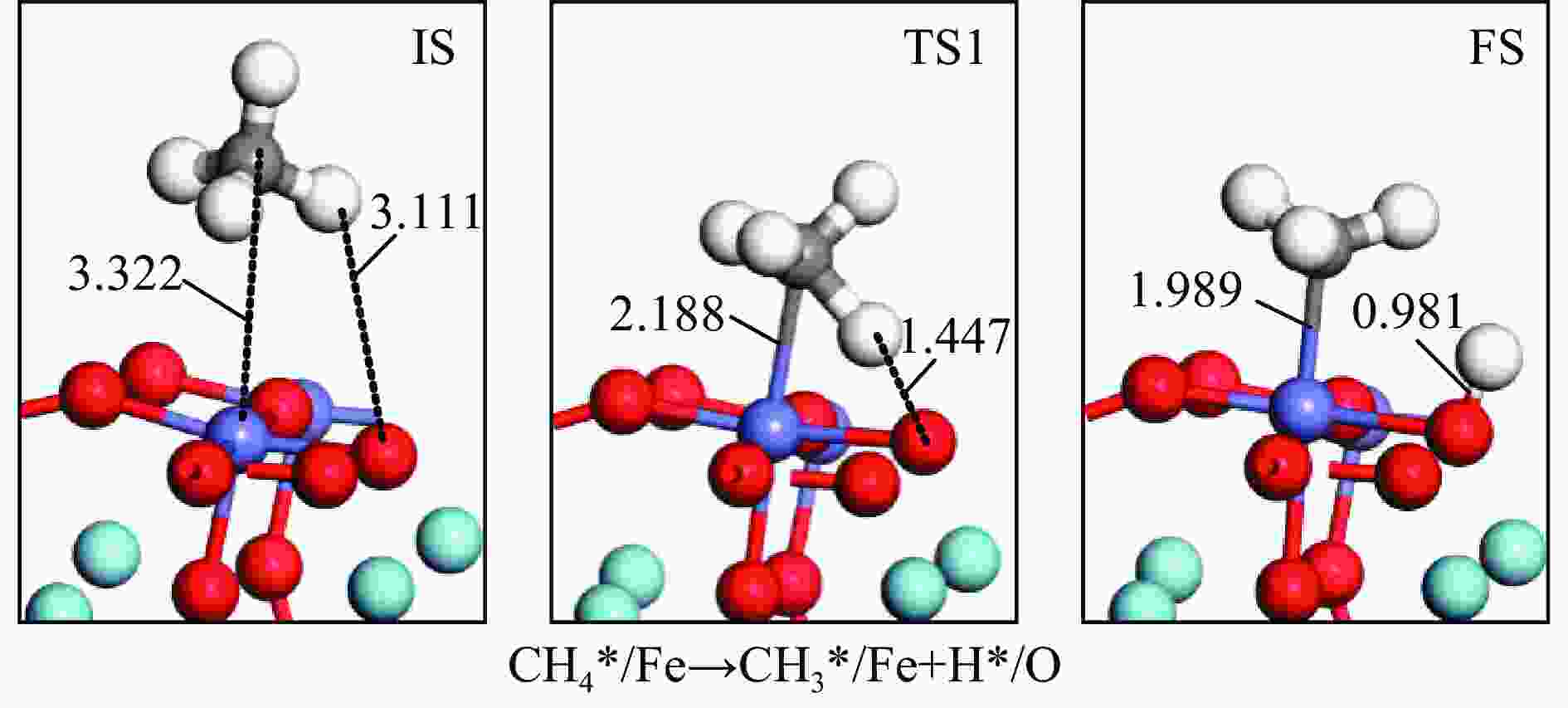
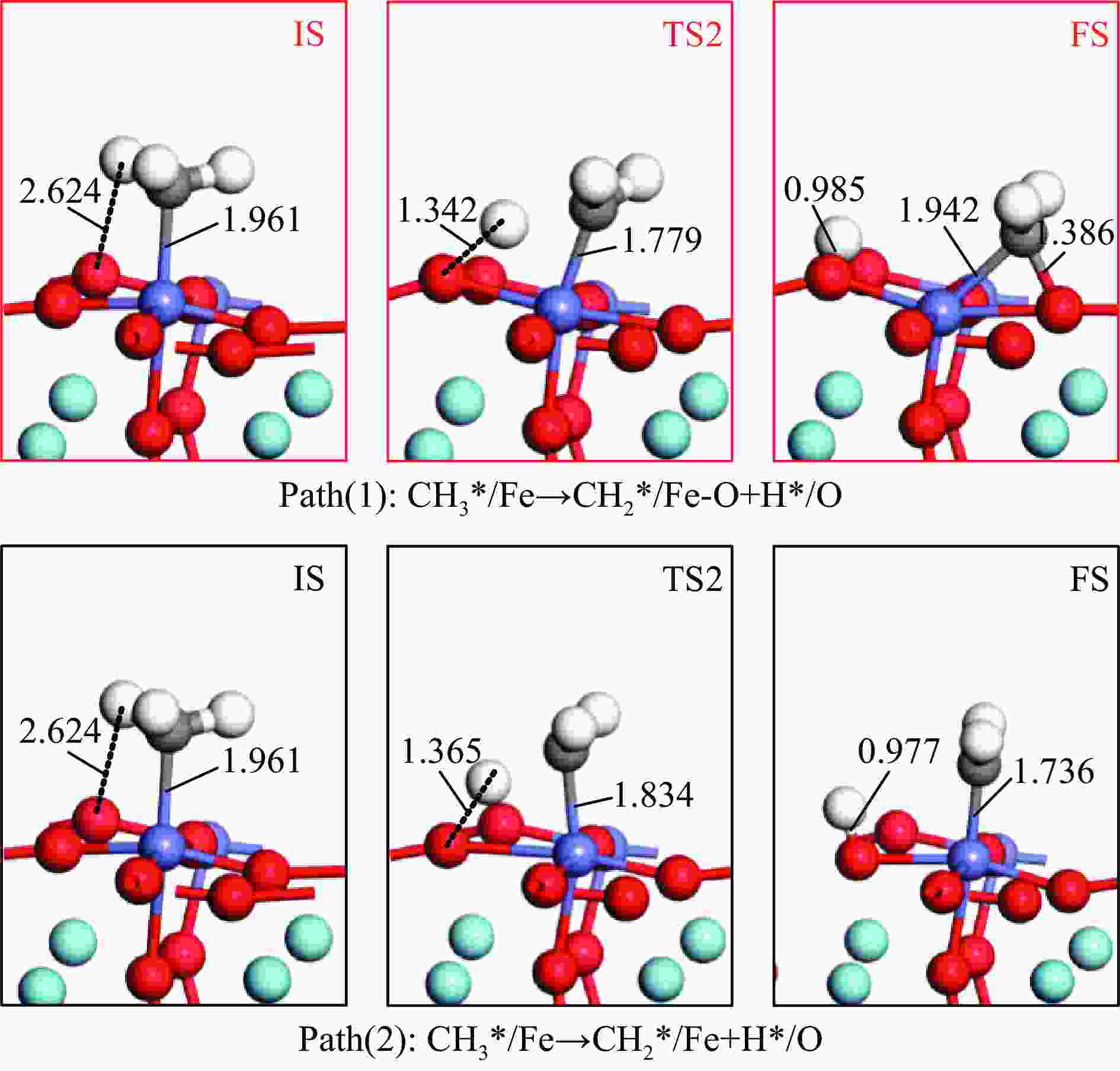
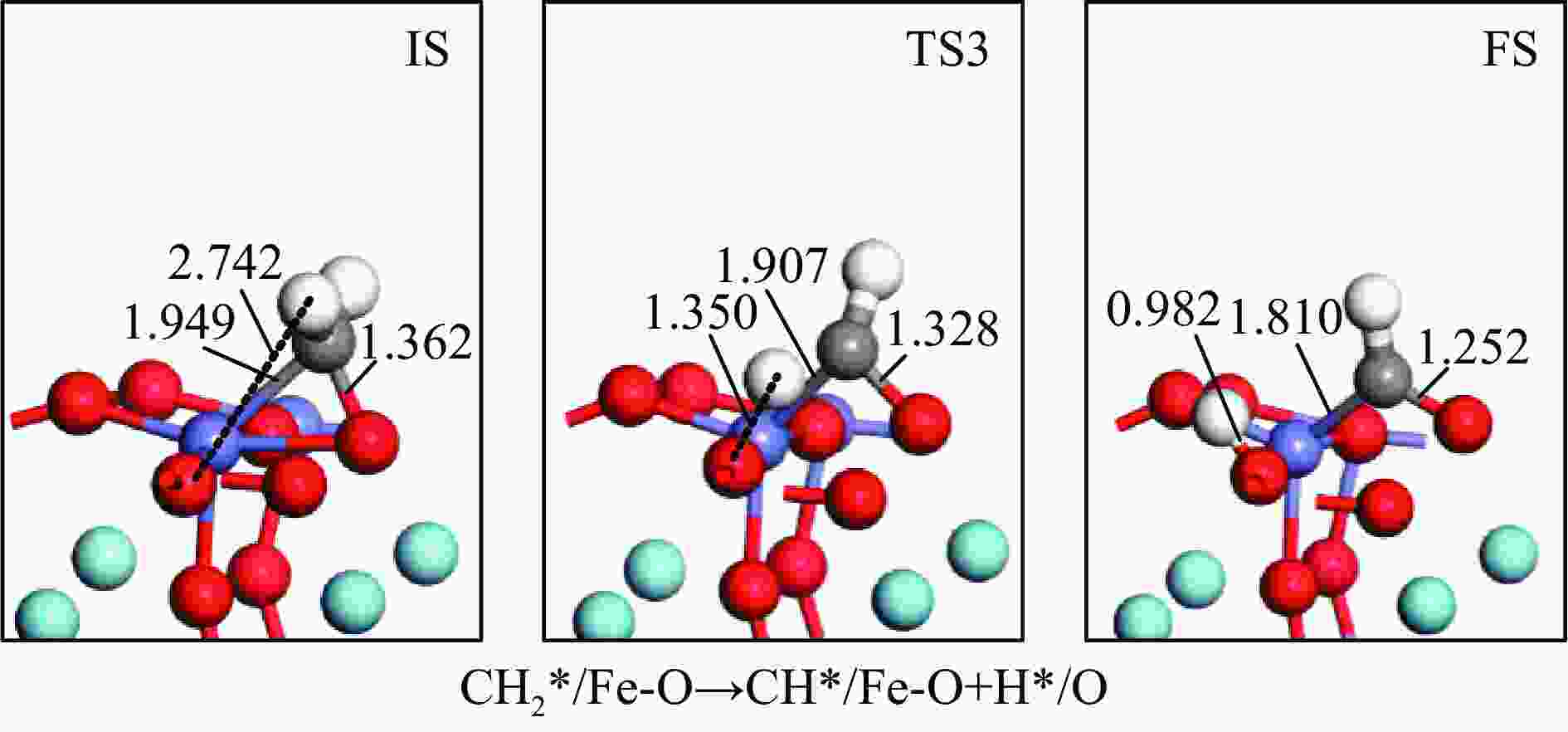
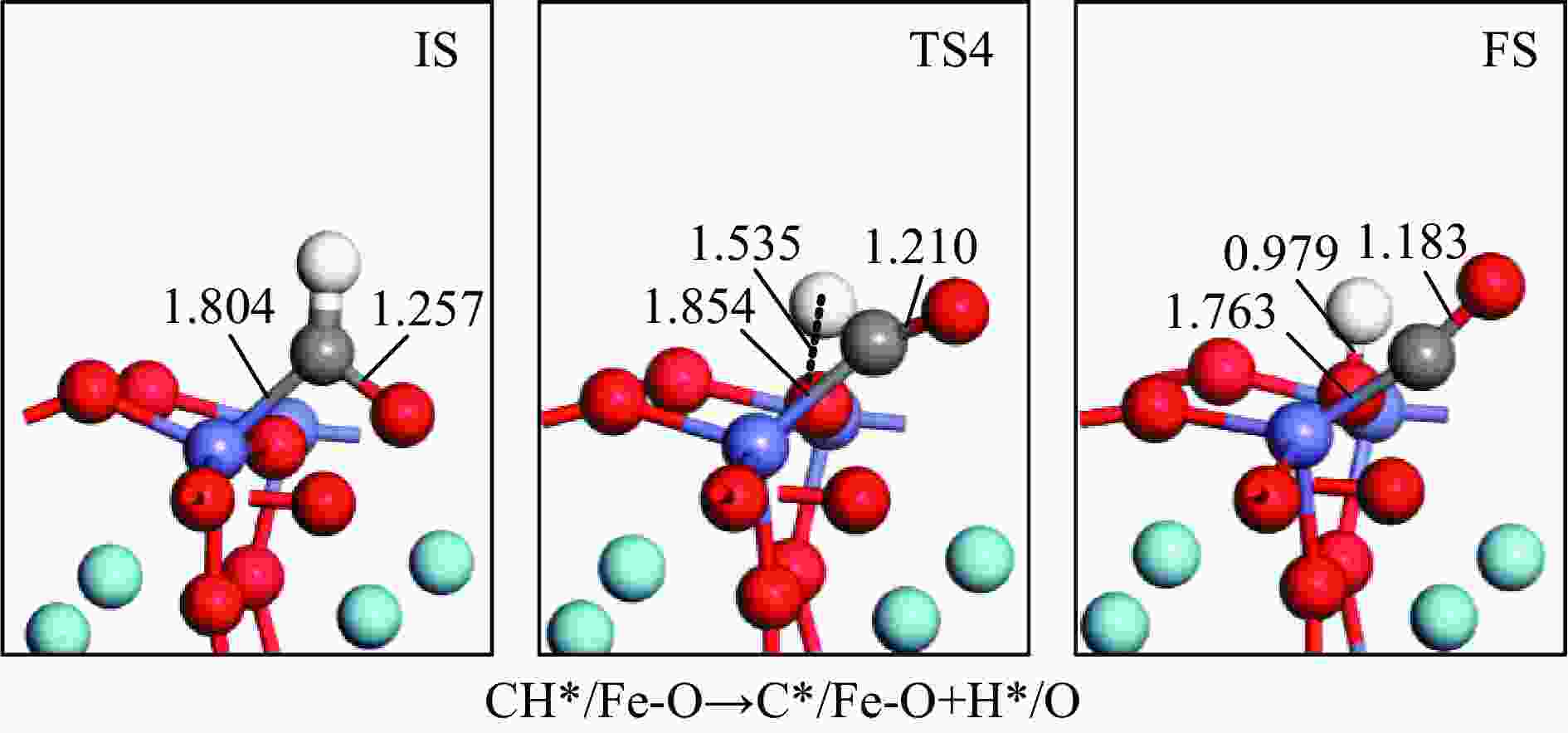
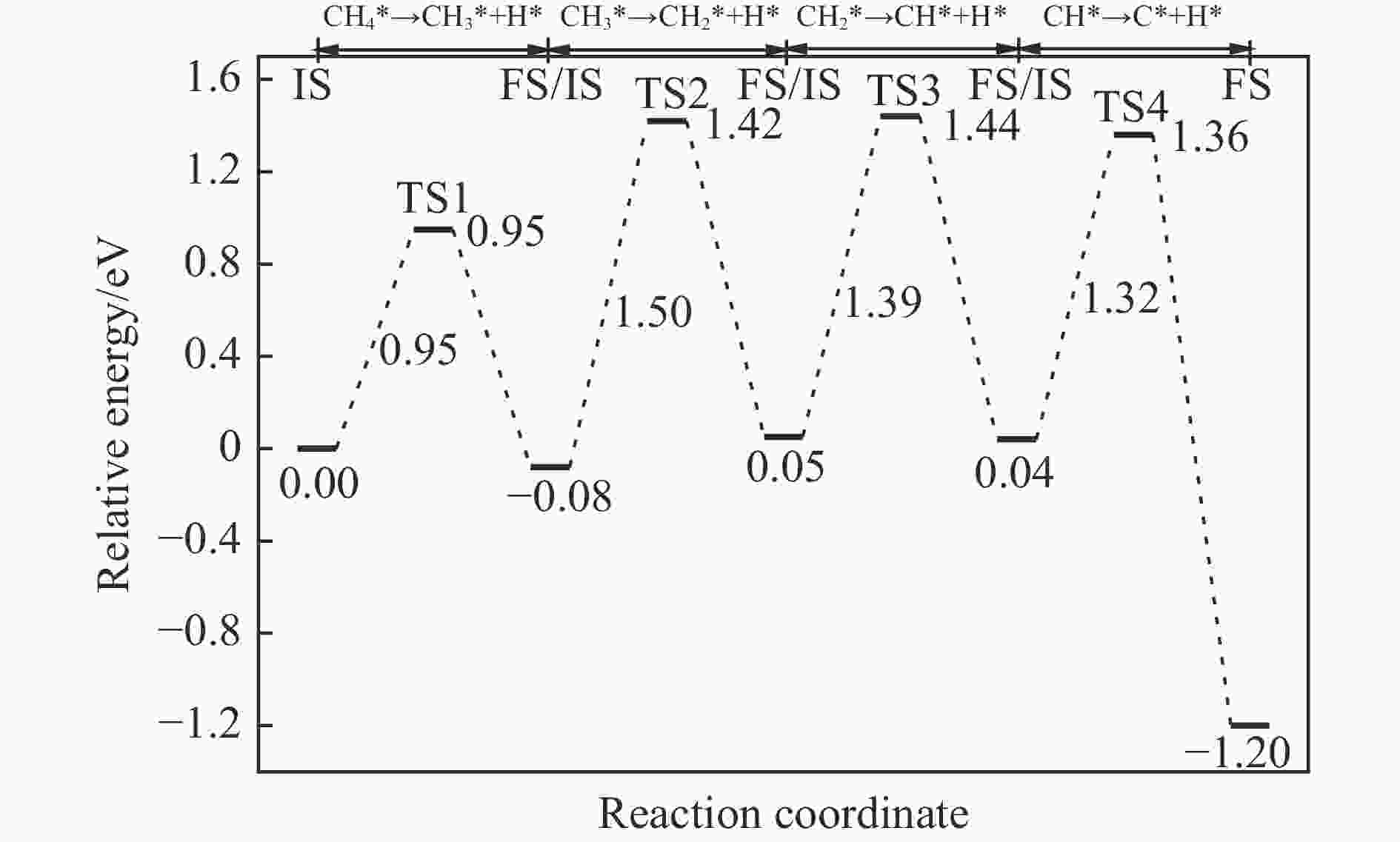
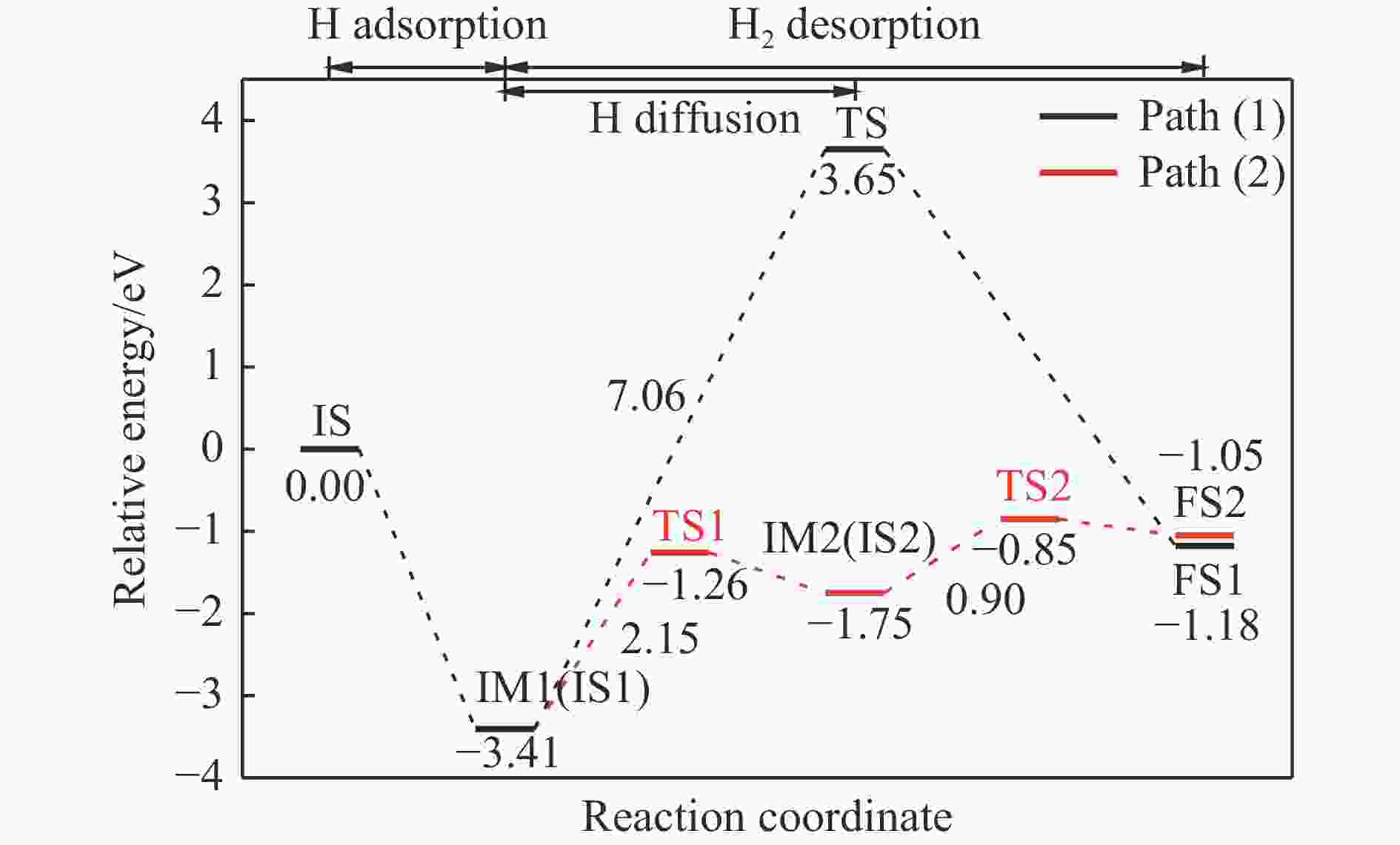
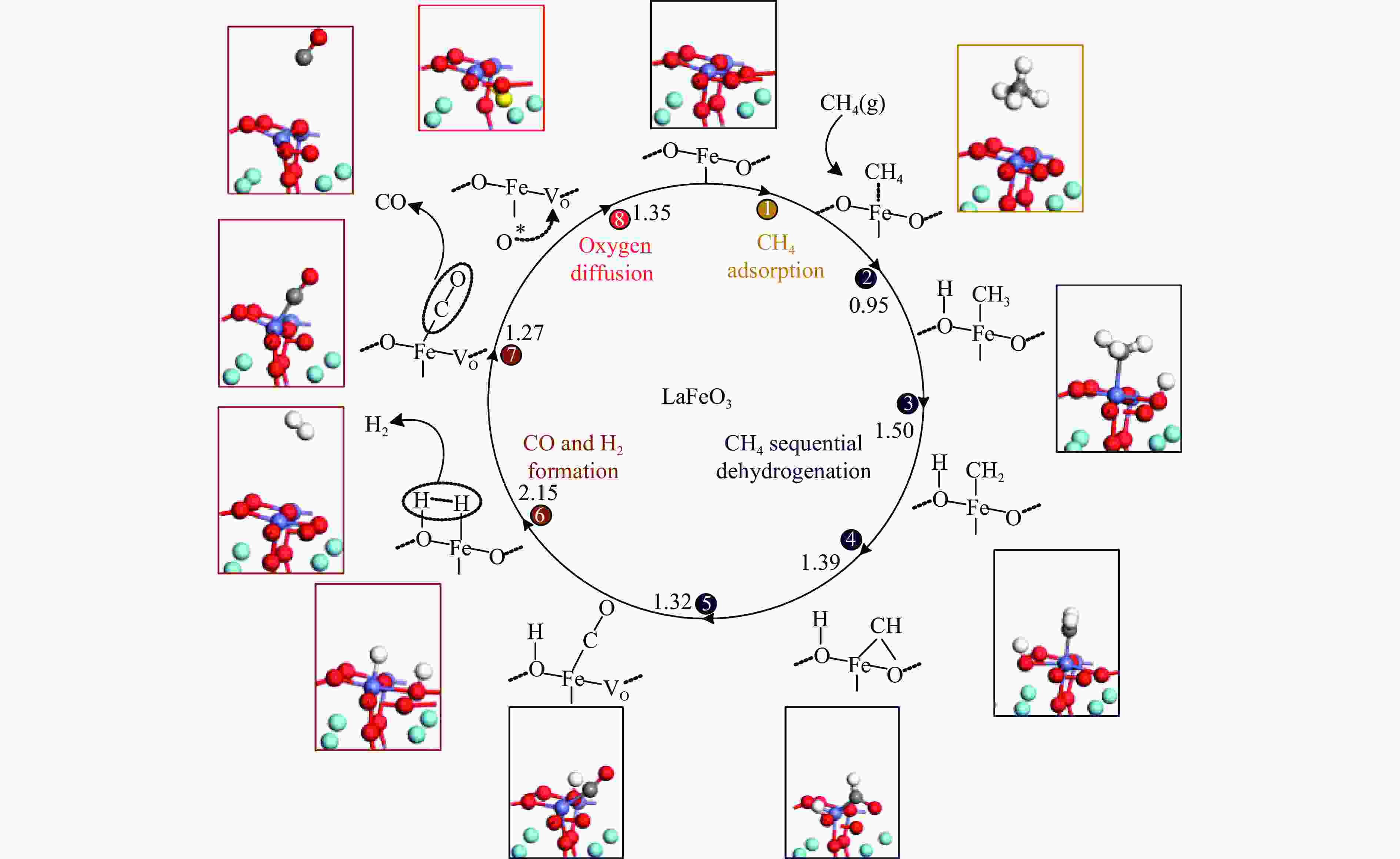
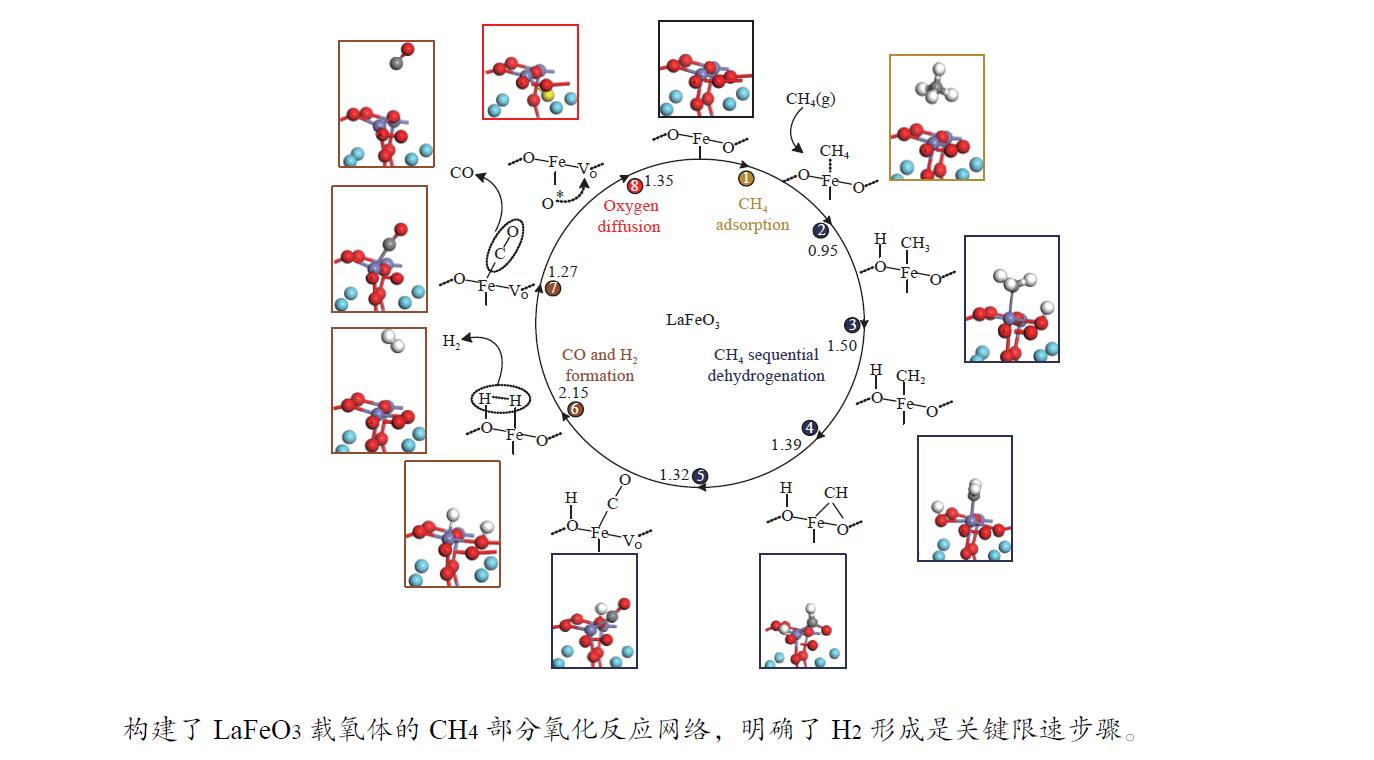
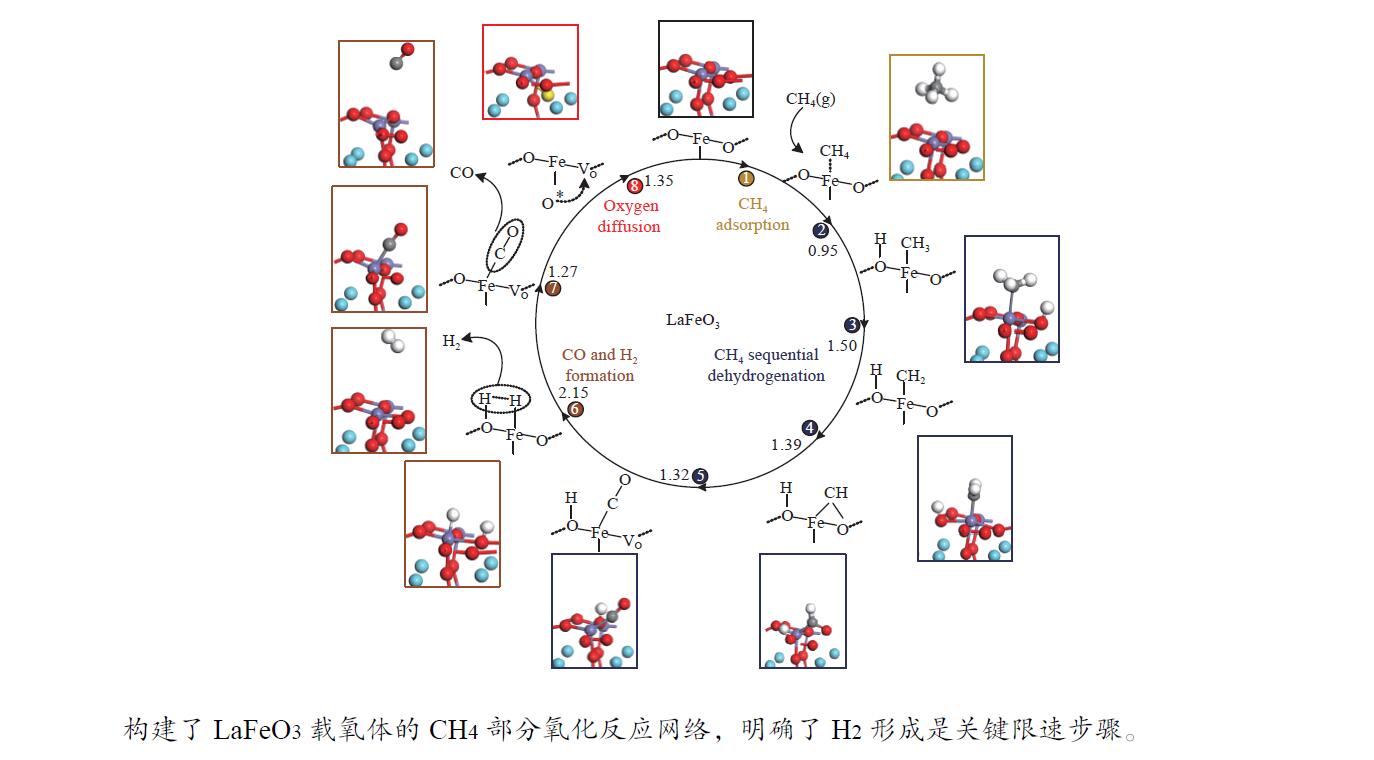
摘要: 本研究基于密度泛函理论(DFT)计算揭示了化学链重整过程中LaFeO3载氧体的CH4部分氧化反应机理,通过系统研究CH4吸附活化、H2和CO形成以及氧扩散等基元反应步骤,构建了CH4部分氧化反应网络。研究发现,CH4发生逐步脱氢反应形成H原子,其中,CH3脱氢反应所需要克服的能垒(1.50 eV)最高,是CH4逐步脱氢反应的限速步骤。载氧体表面H2形成有两种路径,其中,H原子从O顶位迁移到Fe顶位,然后与另外O顶位的H原子成键形成H2分子是主要途径。由于其相对较低的能垒(1.27 eV),CO的形成过程较易发生。氧扩散需要克服1.35 eV的能垒,表明氧扩散过程需要在高温下进行且扩散速率较低。通过比较各基元反应能垒,发现H2形成是LaFeO3载氧体CH4部分氧化反应动力学的限速步骤,而H迁移是限制H2形成的关键,加快H迁移是增强LaFeO3载氧体性能的主要途径。基于DFT计算研究系列A/B位点掺杂LaFeO3载氧体的H迁移过程,有望实现潜在A/B位点有效掺杂剂的快速筛选,指导高性能LaFeO3载氧体的设计开发。Abstract: Density functional theory (DFT) calculations were employed to reveal the CH4 partial oxidation mechanism of LaFeO3 oxygen carrier during chemical looping reforming. The CH4 partial oxidation reaction network was constructed by systematically studying the elementary reaction steps, including CH4 adsorption activation, H2 and CO formation, and oxygen diffusion. It was found that CH4 undergoes a gradual dehydrogenation reaction to form H atoms, and the energy barrier (1.50 eV) of CH3 dehydrogenation is the highest, which is the rate-limiting step. There are two possible paths for H2 formation on the surface of oxygen carrier. It is the main route that the H atom from O-top site to Fe-top site bonds with another H atom on O-top site to form H2 molecule. Due to its relatively low energy barrier (1.27 eV), the CO formation process is easier to occur. Oxygen diffusion needs to overcome an energy barrier of 1.35 eV, indicating that it occurs at high temperatures and the diffusion rate is low. By comparing the energy barrier of each elementary reaction, it was found that the H2 formation is the rate-limiting step of CH4 partial oxidation kinetics for LaFeO3 oxygen carrier. The H migration is the key to limiting H2 formation, and accelerating the H migration is the main approach to improve the performance of LaFeO3 oxygen carrier. Based on DFT calculations, the H migration of A/B site doped LaFeO3 oxygen carriers could be studied, which is expected to achieve the rapid screening of potential A/B site effective dopants and guide the design and development of high-performance LaFeO3 oxygen carriers.
-
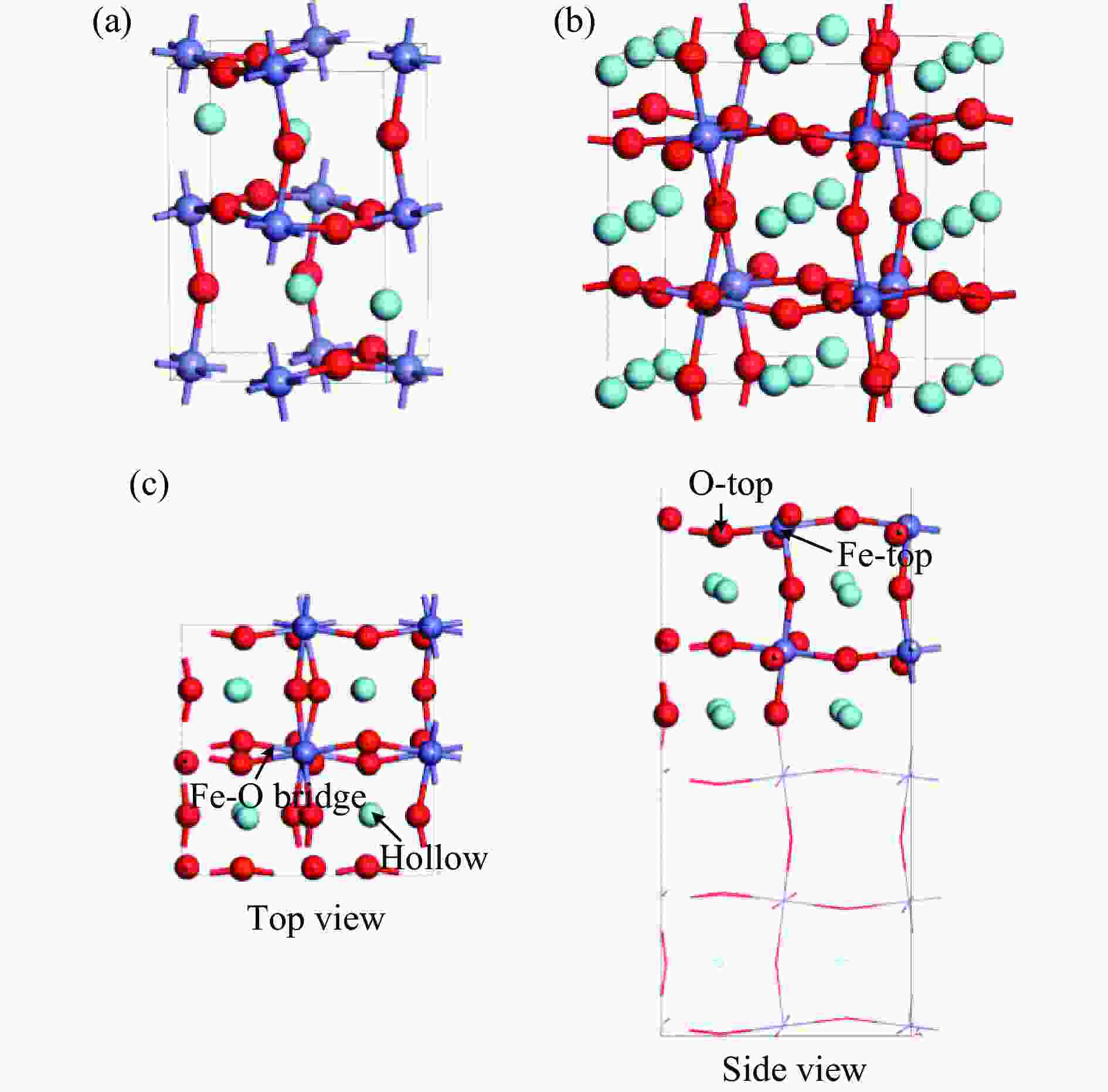
表 1 LaFeO3载氧体CH4部分氧化过程涉及的基元反应
Table 1 The elementary steps for CH4 partial oxidation over LaFeO3 oxygen carrier
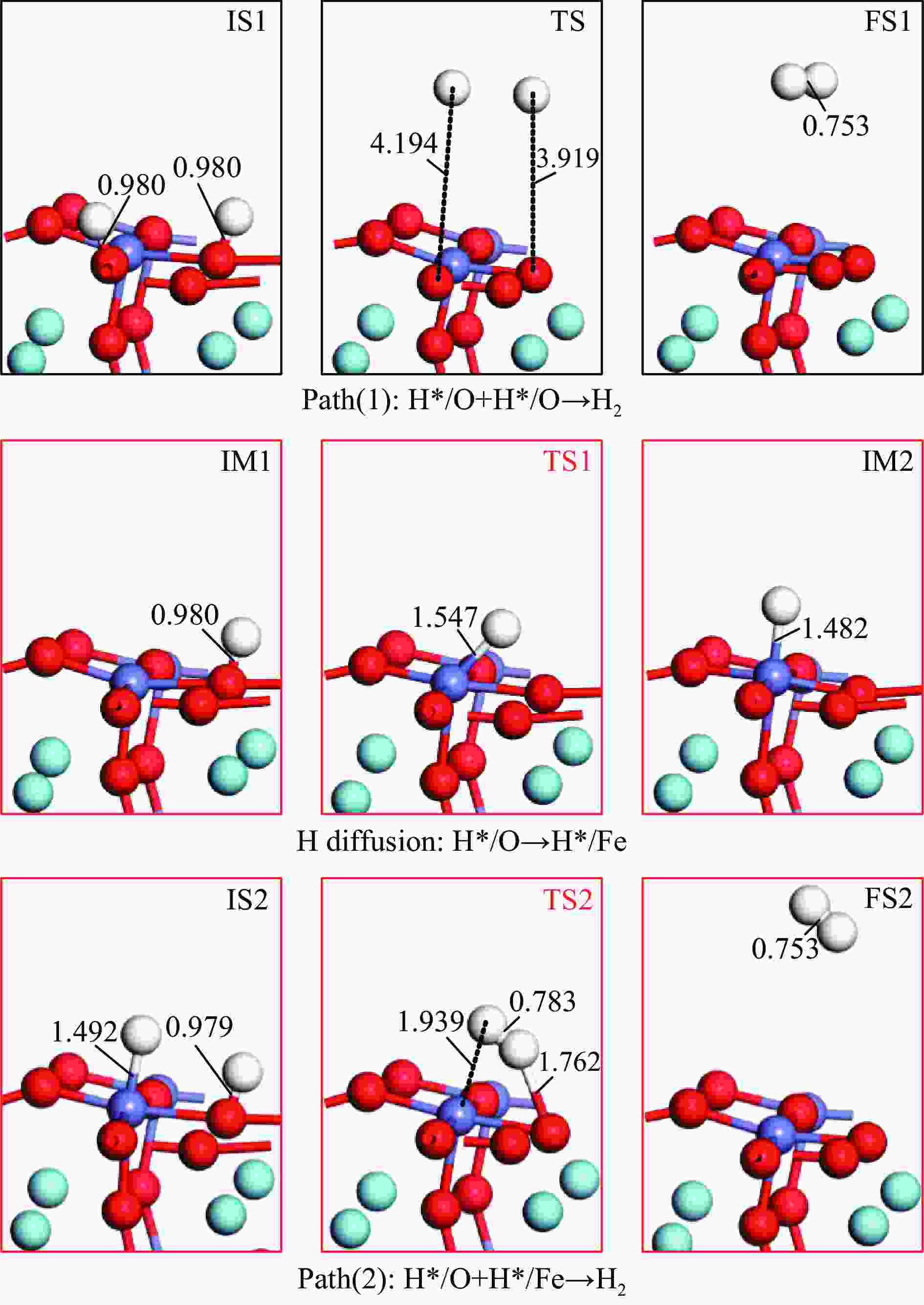
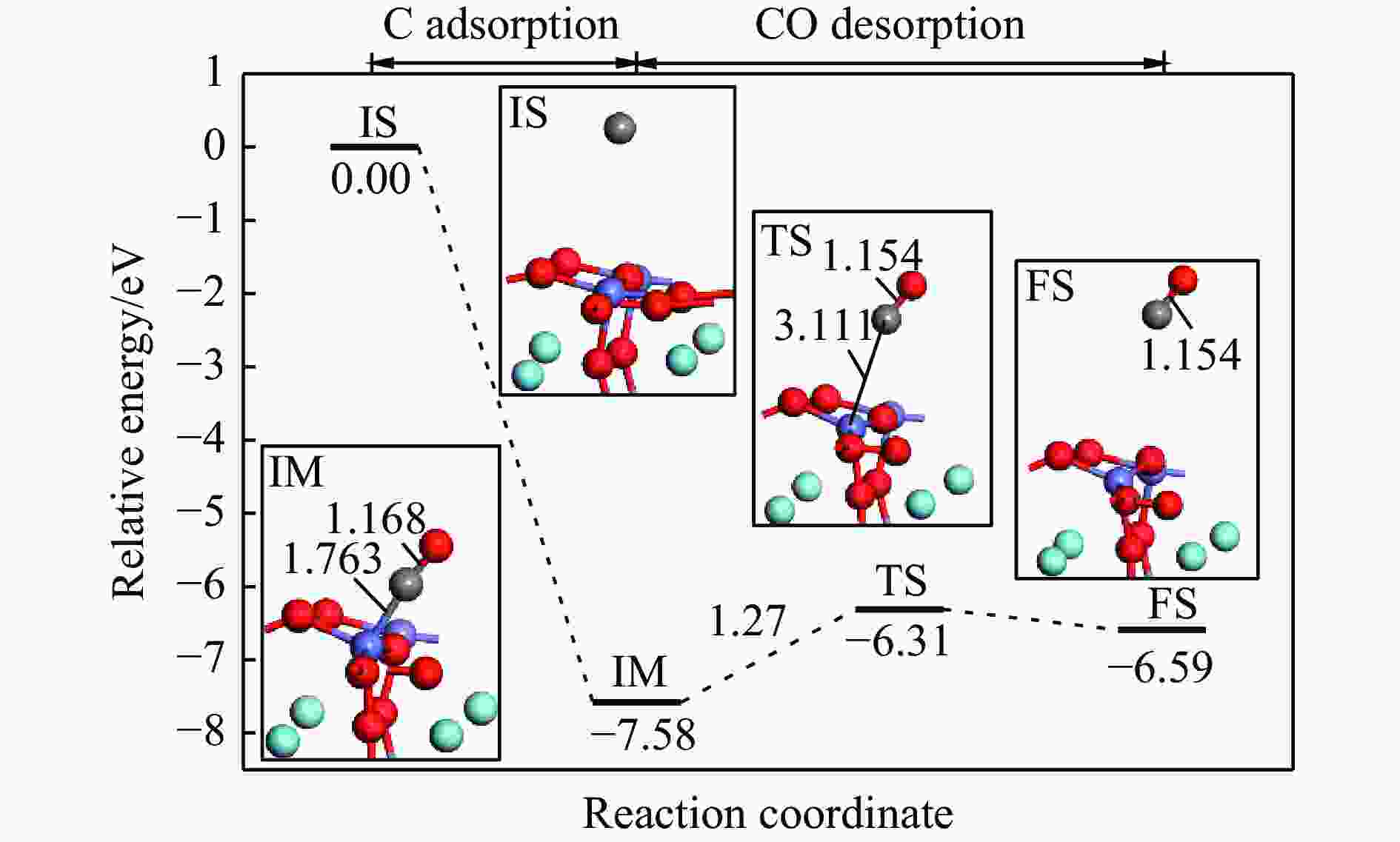
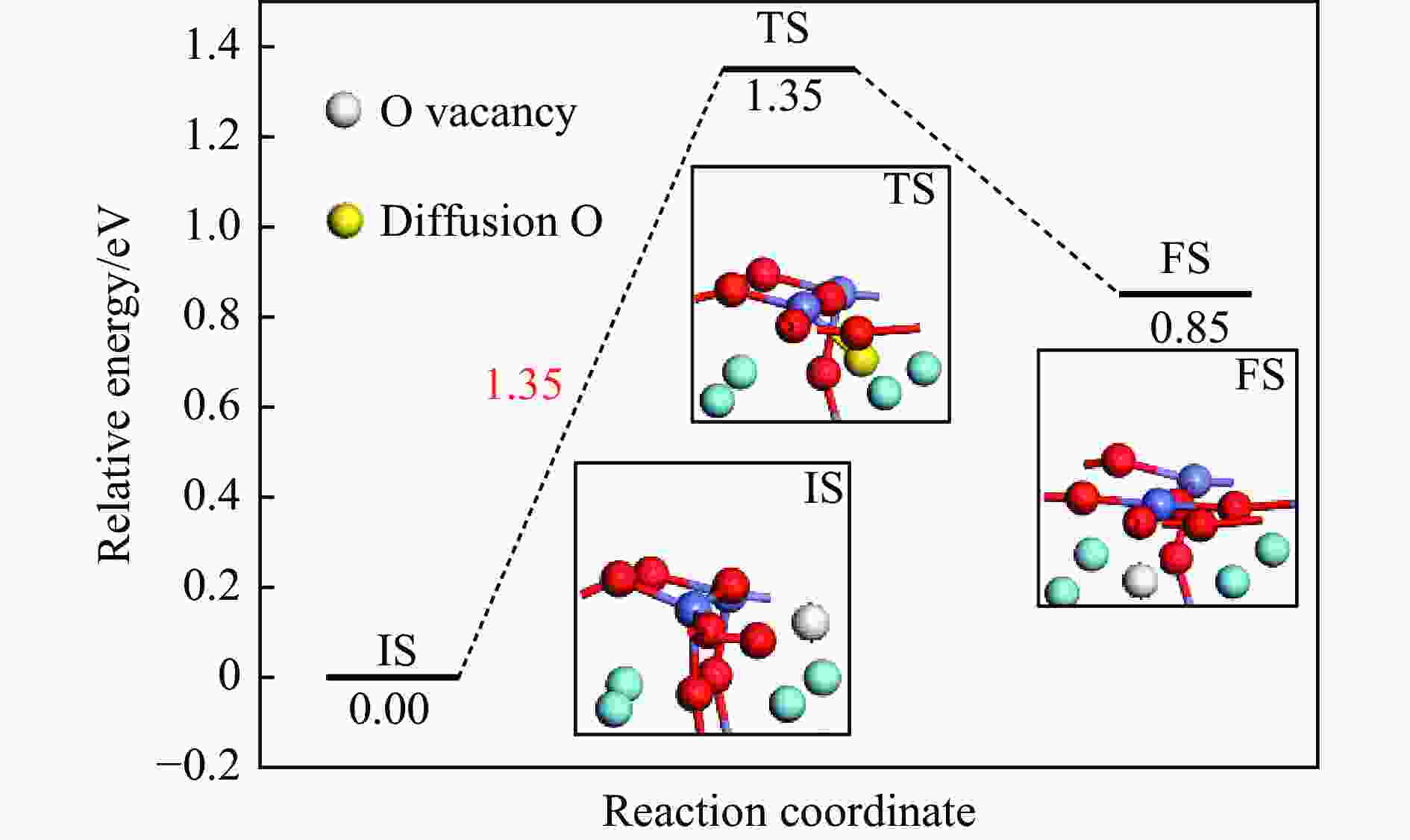
No. Elementary steps CH4 adsorption activation 1 CH4+* → CH4* 2 CH4*+* → CH3*+H* 3 CH3*+* → CH2*+H* 4 CH2*+* → CH*+H* 5 CH*+* → C*+H* Surface reaction 6 H+* → H* 7 2H* → H2(g) 8 C+* → C* 9 C*+OS (Ca2Fe2O5) → CO(g) Oxygen diffusion 10 OB (Ca2Fe2O5) → OS (Ca2Fe2O5) * and X* represent an unoccupied active site and the adsorbed species, respectively. OS and OB denote the surface oxygen and bulk oxygen of LaFeO3(010) surface, respectively. -
[1] BOYANO A, BLANCO-MARIGORTA A M, MOROSUK T, et al. Exergoenvironmental analysis of a steam methane reforming process for hydrogen production[J]. Energy,2011,36:2202−2214. doi: 10.1016/j.energy.2010.05.020 [2] ADÁNEZ J, ABAD A. Chemical-looping combustion: Status and research needs[J]. Proc Combust Inst,2019,37:4303−4317. doi: 10.1016/j.proci.2018.09.002 [3] 金红光, 王宝群. 化学能梯级利用机理探讨[J]. 工程热物理学报,2004,25(2):181−184. doi: 10.3321/j.issn:0253-231X.2004.02.001JIN Hongguang, WANG Baoqun. Principle of cascading utilization of chemical energy[J]. J Eng Thermophys,2004,25(2):181−184. doi: 10.3321/j.issn:0253-231X.2004.02.001 [4] 李振山, 韩海锦, 蔡宁生. 化学链燃烧的研究现状及进展[J]. 动力工程,2006,26(4):538−543.LIN Zhenshan, HAN Haijin, CAI Ningsheng. Research status and progress of chemical looping combustion[J]. J Power Eng,2006,26(4):538−543. [5] TANG M, XU L, FAN M. Progress in oxygen carrier development of methane-based chemical-looping reforming: A review[J]. Appl Energy,2015,151:143−156. doi: 10.1016/j.apenergy.2015.04.017 [6] 靳南南, 张立, 朱燕燕, 等. 甲烷化学链重整制合成气用氧载体的研究进展[J]. 天然气化工(C1化学与化工),2019,44(3):106−116.JIN Nannan, ZHANG Li, ZHU Yanyan, et al. Research progress of oxygen carrier for methane chemical looping reforming to synthesis gas[J]. Nat Gas Chem Ind,2019,44(3):106−116. [7] 黄振, 何方, 赵坤, 等. 基于晶格氧的甲烷化学链重整制合成气[J]. 化学进展,2012,24(8):1599−1609.HUANG Zhen, HE Fang, ZHAO Kun, et al. Synthesis gas production by chemical looping reforming of methane to using lattice oxygen[J]. Prog Chem,2012,24(8):1599−1609. [8] PEÑA M A, FIERRO J L G. Chemical structures and performance of perovskite oxides[J]. Chem Rev,2001,101:1981−2018. doi: 10.1021/cr980129f [9] MIHAI O, CHEN D, HOLMEN A. Chemical looping methane partial oxidation: The effect of the crystal size and O content of LaFeO3[J]. J Catal,2012,293:175−185. doi: 10.1016/j.jcat.2012.06.022 [10] ZHAO K, HE F, HUANG Z, et al. Three-dimensionally ordered macroporous LaFeO3 perovskites for chemical-looping steam reforming of methane[J]. Int J Hydrog Energy,2014,39:3243−3252. doi: 10.1016/j.ijhydene.2013.12.046 [11] ZHENG Y, LI K, WANG H, et al. Designed oxygen carriers from macroporous LaFeO3 supported CeO2 for chemical-looping reforming of methane[J]. Appl Catal B: Environ,2017,202:51−63. doi: 10.1016/j.apcatb.2016.08.024 [12] NEAL L, SHAFIEFARHOOD A, LI F. Effect of core and shell compositions on MeOx@LaySr1−yFeO3 core-shell redox catalysts for chemical looping reforming of methane[J]. Appl Energy,2015,157:391−398. doi: 10.1016/j.apenergy.2015.06.028 [13] SHAFIEFARHOOD A, GALINSKY N, HUANG Y, et al. Fe2O3@LaxSr1−xFeO3 core-shell redox catalyst for methane partial oxidation[J]. ChemCatChem,2014,6:790−799. doi: 10.1002/cctc.201301104 [14] HE F, CHEN J, LIU S, et al. La1−xSrxFeO3 perovskite-type oxides for chemical-looping steam methane reforming: Identification of the surface elements and redox cyclic performance[J]. Int J Hydrog Energy,2019,44:10265−10276. doi: 10.1016/j.ijhydene.2019.03.002 [15] CHANG H, BJØRGUM E, MIHAI O, et al. Effects of oxygen mobility in La-Fe-based perovskites on the catalytic activity and selectivity of methane oxidation[J]. ACS Catal,2020,10:3707−3719. doi: 10.1021/acscatal.9b05154 [16] ZHANG X, PEI C, CHANG X, et al. FeO6 octahedral distortion activates lattice oxygen in perovskite ferrite for methane partial oxidation coupled with CO2 splitting[J]. J Am Chem Soc,2020,142:11540−11549. doi: 10.1021/jacs.0c04643 [17] ZHANG X, SU Y, PEI C, et al. Chemical looping steam reforming of methane over Ce-doped perovskites[J]. Chem Eng Sci,2020,223:115707. doi: 10.1016/j.ces.2020.115707 [18] ZHAO K, HE F, HUANG Z, et al. Perovskite-type oxides LaFe1−xCoxO3 for chemical looping steam methane reforming to syngas and hydrogen co-production[J]. Appl Energy,2016,168:193−203. doi: 10.1016/j.apenergy.2016.01.052 [19] ZHANG L, XU W, WU J, et al. Identifying the role of A-site cations in modulating oxygen capacity of iron-based perovskite for enhanced chemical looping methane-to-syngas conversion[J]. ACS Catal,2020,10:9420−9430. doi: 10.1021/acscatal.0c01811 [20] LI F, LUO S, SUN Z, et al. Role of metal oxide support in redox reactions of iron oxide for chemical looping applications: experiments and density functional theory calculations[J]. Energy Environ Sci,2011,4:3661−3667. doi: 10.1039/c1ee01325d [21] QIN L, CHENG Z, GUO M, et al. Impact of 1% Lanthanum dopant on carbonaceous fuel redox reactions with an iron-based oxygen carrier in chemical looping processes[J]. ACS Energy Lett,2017,2:70−74. doi: 10.1021/acsenergylett.6b00511 [22] ZENG L, CHENG Z, FAN J A, et al. Metal oxide redox chemistry for chemical looping processes[J]. Nat Rev Chem,2018,2:349−364. doi: 10.1038/s41570-018-0046-2 [23] 梁志永, 覃吴, 王建业, 等. 化学链燃烧中基于密度泛函理论的铁基载氧体研究进展[J]. 热能动力工程,2018,33(1):1−5.LIANG Zhi-yong, QIN Wu, WANG Jian-ye, et al. Latest advances in the study of the Fe-based oxygen carrier in chemical looping combustion based on the density functional theory[J]. J Eng Therm,2018,33(1):1−5. [24] LIU Y, QIN L, CHENG Z, et al. Near 100% CO selectivity in nanoscaled iron-based oxygen carriers for chemical looping methane partial oxidation[J]. Nat Commun,2019,10:5503. doi: 10.1038/s41467-019-13560-0 [25] YUAN Y, YOU H, RICARDEZ-SANDOVAL L. Recent advances on first-principles modeling for the design of materials in CO2 capture technologies[J]. Chin J Chem Eng,2019,27:1554−1565. doi: 10.1016/j.cjche.2018.10.017 [26] FENG Y C, WANG N N, GUO X, et al. Reaction mechanism of Ca2Fe2O5 oxygen carrier with CO in chemical looping hydrogen production[J]. Appl Surf Sci,2020,534:147583. doi: 10.1016/j.apsusc.2020.147583 [27] FENG Y C, WANG N N, GUO X. Reaction mechanism of methane conversion over Ca2Fe2O5 oxygen carrier in chemical looping hydrogen production[J]. Fuel,2021,290:120094. doi: 10.1016/j.fuel.2020.120094 [28] FENG Y C, WANG N N, GUO X. Density functional theory study on improved reactivity of alkali-doped Fe2O3 oxygen carriers for chemical looping hydrogen production[J]. Fuel,2019,236:1057−1064. doi: 10.1016/j.fuel.2018.09.079 [29] FENG Y C, CAI X, GUO X, et al. Influence mechanism of H2S on the reactivity of Ni-based oxygen carriers for chemical-looping combustion[J]. Chem Eng J,2016,295:461−467. doi: 10.1016/j.cej.2016.03.058 [30] SEGALL M D, LINDAN P J D, PROBERT M J, et al. First-principles simulation: Ideas, illustrations and the CASTEP code[J]. J Phys Condens Matter,2002,14(11):2717−2744. doi: 10.1088/0953-8984/14/11/301 [31] PAYNE M C, TETER M P, ALLAN D C, et al. Iterative minimization techniques for ab initio total-energy calculations: molecular dynamics and conjugate gradients[J]. Rev Mod Phys,1992,64(4):1045−1097. doi: 10.1103/RevModPhys.64.1045 [32] PERDEW J P, CHEVARY J A, VOSKO S H, et al. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation[J]. Phys Rev B,1992,46(11):6671−6687. doi: 10.1103/PhysRevB.46.6671 [33] WHITE J A, BIRD D M. Implementation of gradient-corrected exchange-correlation potentials in Car-Parrinello total-energy calculations[J]. Phys Rev B,1994,50(7):4954−4957. doi: 10.1103/PhysRevB.50.4954 [34] VANDERBILT D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism[J]. Phys Rev B,1990,41(11):7892−7895. doi: 10.1103/PhysRevB.41.7892 [35] MONKHORST H J, PACK J D. Special points for Brillouin-zone integrations[J]. Phys Rev B,1976,13(12):5188−5192. doi: 10.1103/PhysRevB.13.5188 [36] RITZMANN A M, MUÑOZ-GARCÍA A B, PAVONE M, et al. Ab initio DFT+U analysis of oxygen vacancy formation and migration in La1−xSrxFeO3−δ (x=0, 0.25, 0.50)[J]. Chem Mater,2013,25(15):3011−3019. doi: 10.1021/cm401052w [37] BOATENG I W, TIA R, ADEI E, et al. A DFT+U investigation of hydrogen adsorption on the LaFeO3(010) surface[J]. Phys Chem Chem Phys,2017,19(10):7399−7409. doi: 10.1039/C6CP08698E [38] TAGUCHI H, MASUNAGA Y, HIROTA K, et al. Synthesis of perovskite-type (La1−xCax)FeO3 (0≤x≤0.2) at low temperature[J]. Mater Res Bull,2005,40(5):773−780. doi: 10.1016/j.materresbull.2005.02.009 [39] GLAZER A. Simple ways of determining perovskite structures[J]. Acta Crystallogr A,1975,31(6):756−762. doi: 10.1107/S0567739475001635 [40] CHEN Y, FAN J, LIU T, et al. Theoretical study on the effect of an O vacancy on the hydrogen storage properties of the LaFeO3 (010) surface[J]. Int J Hydrog Energy,2019,44(11):5374−5381. doi: 10.1016/j.ijhydene.2018.09.097 [41] AU C T, NG C F, LIAO M S. Methane dissociation and syngas formation on Ru, Os, Rh, Ir, Pd, Pt, Cu, Ag, and Au: A theoretical study[J]. J Catal,1999,185:12−22. doi: 10.1006/jcat.1999.2498 [42] FENG Y C, HU X D, GUO X, WANG N N. Exploration of the reaction mechanism of the LaFeO3 oxygen carrier for chemical-looping steam methane reforming: a DFT study[J]. Phys Chem Chem Phys,2023,25:13033−13040. doi: 10.1039/D2CP05795F [43] HUANG L, TANG M, FAN M, et al. Density functional theory study on the reaction between hematite and methane during chemical looping process[J]. Appl Energy,2015,159:132−144. doi: 10.1016/j.apenergy.2015.08.118 [44] DAI X, CHENG J, LI Z, et al. Reduction kinetics of lanthanum ferrite perovskite for the production of synthesis gas by chemical-looping methane reforming[J]. Chem Eng Sci,2016,153:236−245. doi: 10.1016/j.ces.2016.07.011 [45] 徐圆圆, 付迁, 闫永波, 等. 钙钛矿型LaFeO3载氧体生物质化学链气化热力学分析及实验研究[J]. 可再生能源,2023,41(6):731−737. doi: 10.3969/j.issn.1671-5292.2023.06.004XU Yuan-yuan, FU Qian, YAN Yong-bo, et al. Thermodynamic analysis and experimental study on biomass chemical looping gasification of perovskite LaFeO3 oxygen carrier[J]. Renewable Energy Res,2023,41(6):731−737. doi: 10.3969/j.issn.1671-5292.2023.06.004 -




 下载:
下载: