Preparation and properties of MnCu/Ce catalyst for CO preferential oxidation reaction
-
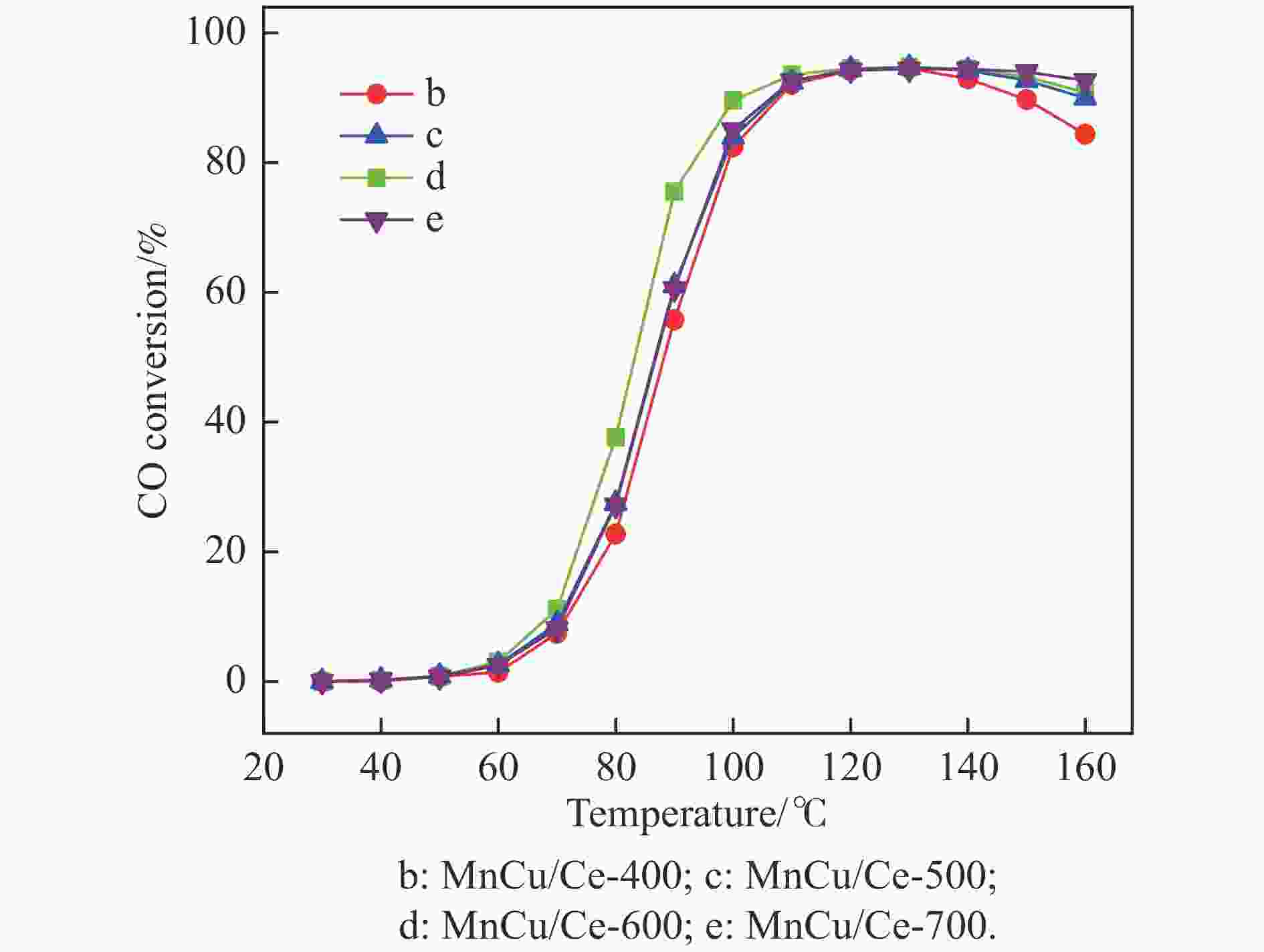
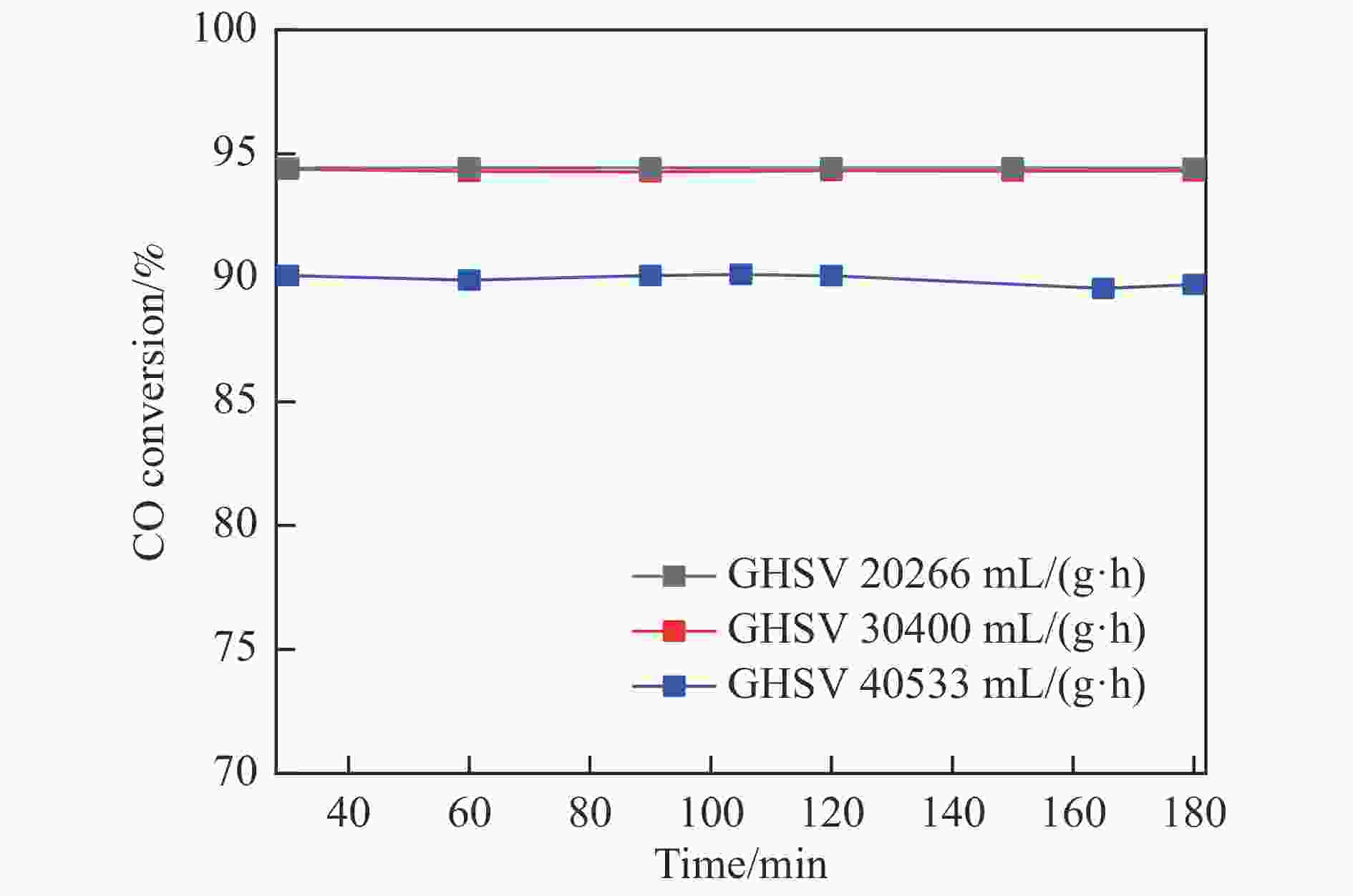
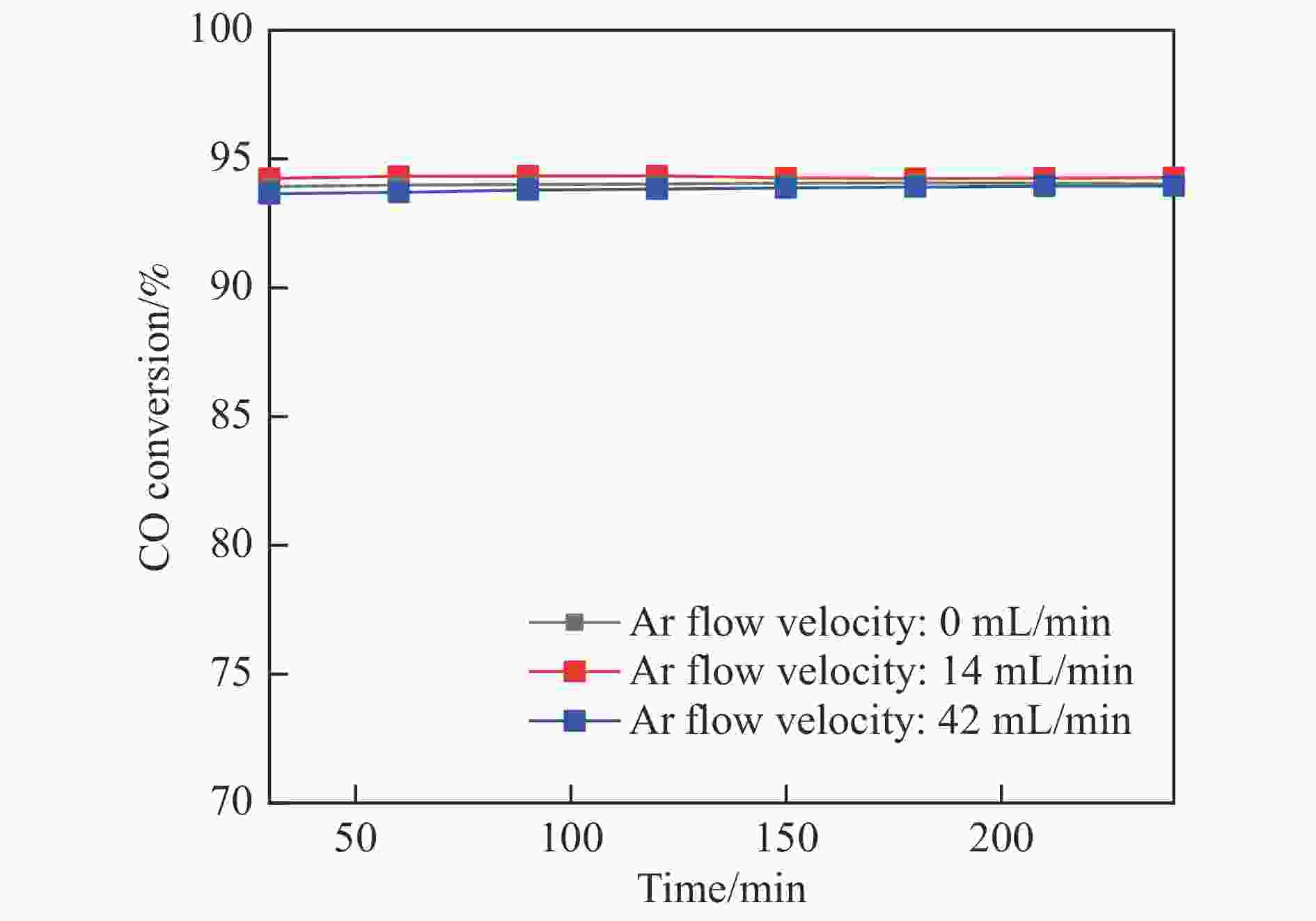
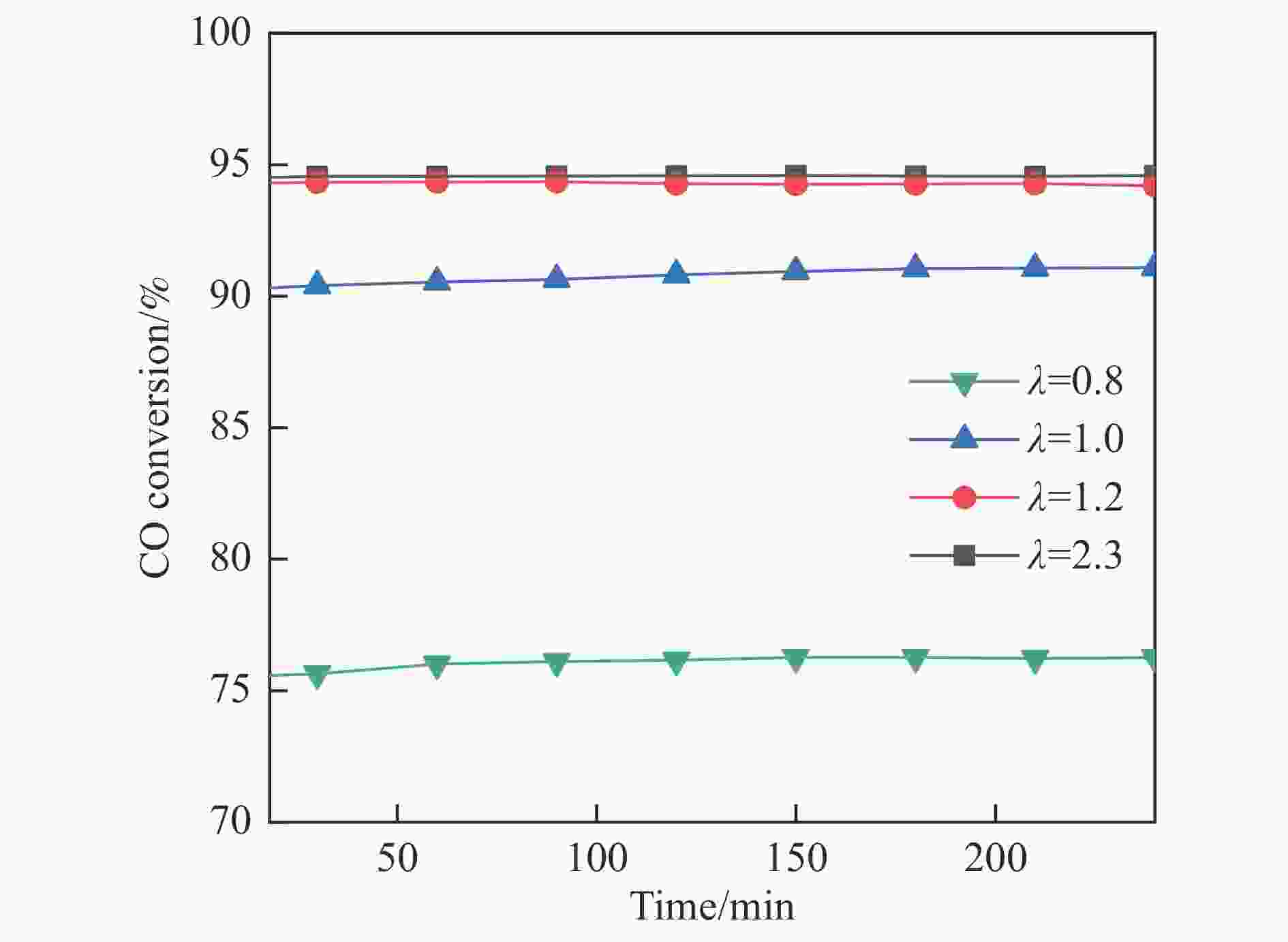
摘要: 采用共浸渍法制备较低Cu含量的MnCu/Ce催化剂,通过XRD、BET、H2-TPR、XPS和CO2-TPD等表征手段对催化剂进行表征,考察催化剂焙烧温度对催化剂结构、性质及其在含有CO2的富氢气氛下对CO优先氧化性能的影响。结果表明,MnCu/Ce催化剂均有Cu/Mn-O-Ce固溶体形成,其中,在焙烧温度600 ℃制备的催化剂中,Mn与Cu、Ce之间相互作用较强,形成较多三元氧化物固溶体,氧空位/Ce3+含量高,具备良好的CO-Prox活性。此外,对反应条件的考察发现,添加不同分压Ar对催化剂的CO-Prox活性影响较小,气体空速和氧过量系数对催化剂活性影响较大,且反应原料气中CO2的存在对CO-Prox反应有负面影响。氧过量系数为1.2、空速范围为20266−30400 mL/(g·h)时,CO转化率最高,达到94.7%。Abstract: The MnCu/Ce catalyst with a lower Cu content was prepared by co-impregnation method, and then was characterized by XRD, BET, H2-TPR, XPS and CO2-TPD. The effects of calcination temperature on the structure and properties of the catalyst and the preferential oxidation of CO in a hydrogen-rich atmosphere containing CO2 were investigated. The results indicated that Cu/Mn-O-Ce solid solution was formed in all MnCu/Ce catalysts. Of theses sample, the one calcined at 600 ℃ had strong interaction among Mn, Cu and Ce, formed more ternary oxide solid solution with more oxygen vacancies/Ce3+, and revealed good CO-Prox activity. In addition, it was found that the addition of different percentage of Ar had little effects on the CO-Prox activity of the catalyst, while the space velocity and oxygen excess coefficient had great effects on the catalytic performance, and the presence of CO2 in the reaction feedstock gas had a negative effect on the CO-Prox reaction. At an oxygen excess coefficient of 1.2 and the space velocity of 20266−30400 mL/(g·h), the highest CO conversion rate reached 94.7%.
-
Key words:
- low content copper /
- roasting temperature /
- cerium oxide /
- CO2/H2 atmosphere /
- reaction condition
-
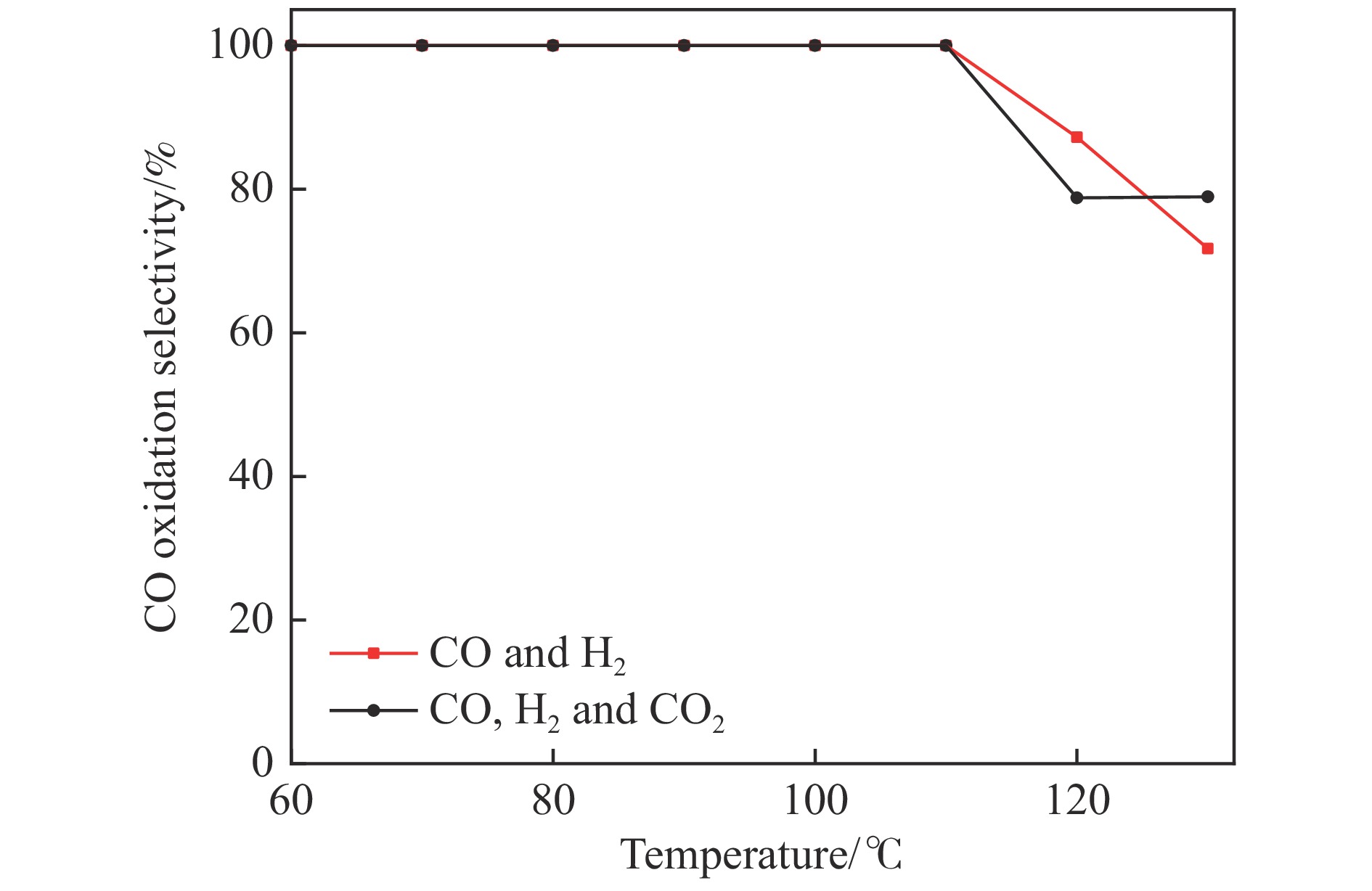
图 11 不同反应原料气下MnCu/Ce-600催化剂一氧化碳转化率
Figure 11 CO conversion of MnCu/Ce-600 catalyst at different reaction feed gas
Reaction conditions: λ=1.2, GHSV: 20266 mL/(g·h), reaction temperature: 130 ℃, raw gas composition: CO: (0.90% CO, 0.54% O2, 98.56% Ar); CO/H2: (0.90% CO, 39.47% H2, 0.99% O2, 58.64% Ar); CO/H2/CO2: (0.90% CO, 11.28% CO2, 66.77% H2, 0.54% O2, 20.51% Ar).
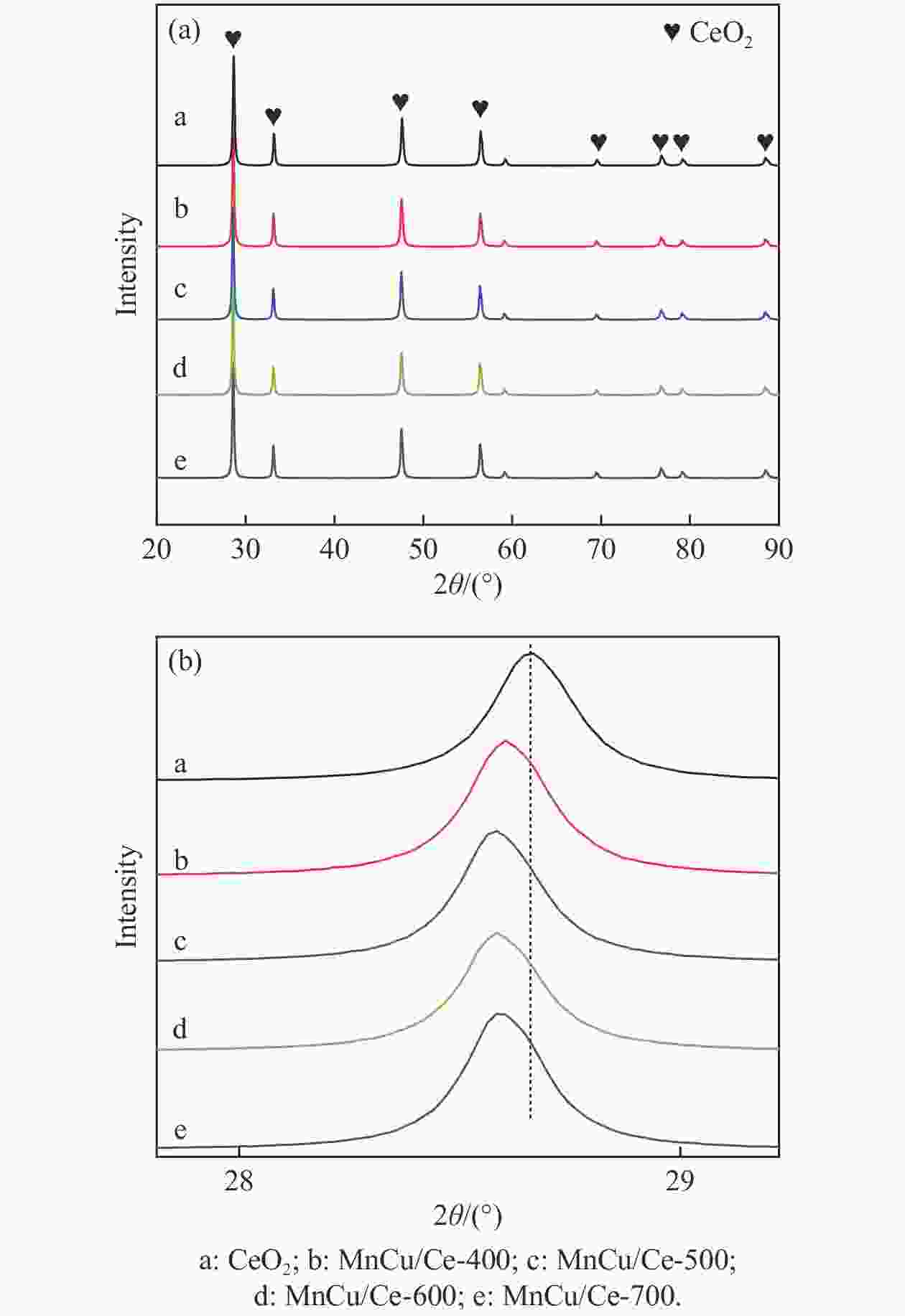
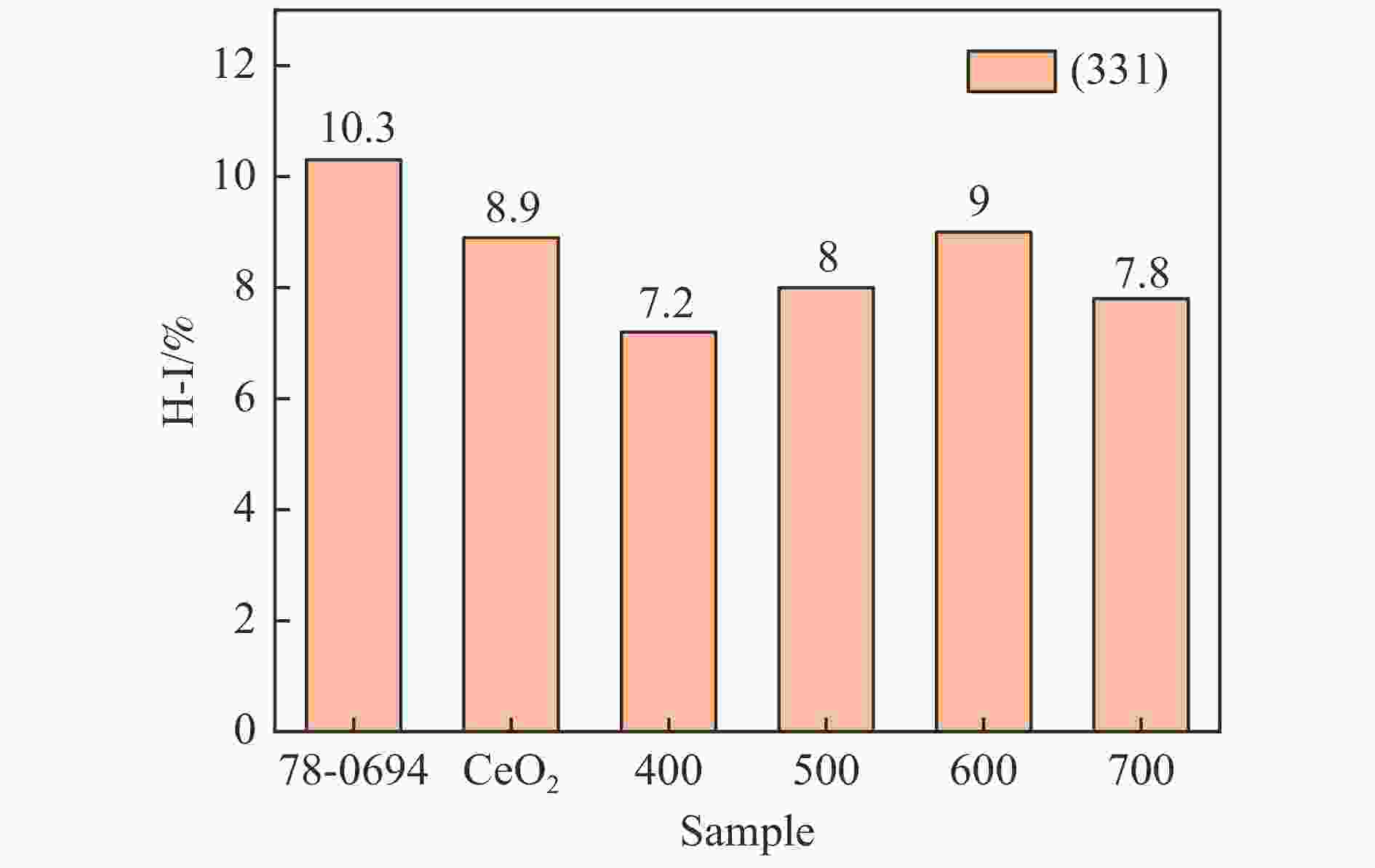
表 1 XRD的最强峰位置、晶胞参数和平均晶粒尺寸
Table 1 Location of the strongest peak, cell parameters and average grain size of XRD
Catalyst Peak position of (111) plane/(°) CeO2 cell parameter/Å Average size of grains/nm CeO2 28.67 5.3969 28.3 MnCu/Ce-400 28.61 5.4032 28.2 MnCu/Ce-500 28.59 5.4045 28.5 MnCu/Ce-600 28.59 5.4039 27.5 MnCu/Ce-700 28.61 5.4032 28.6 表 2 CeO2和MnCu/Ce-t催化剂的物理化学性质
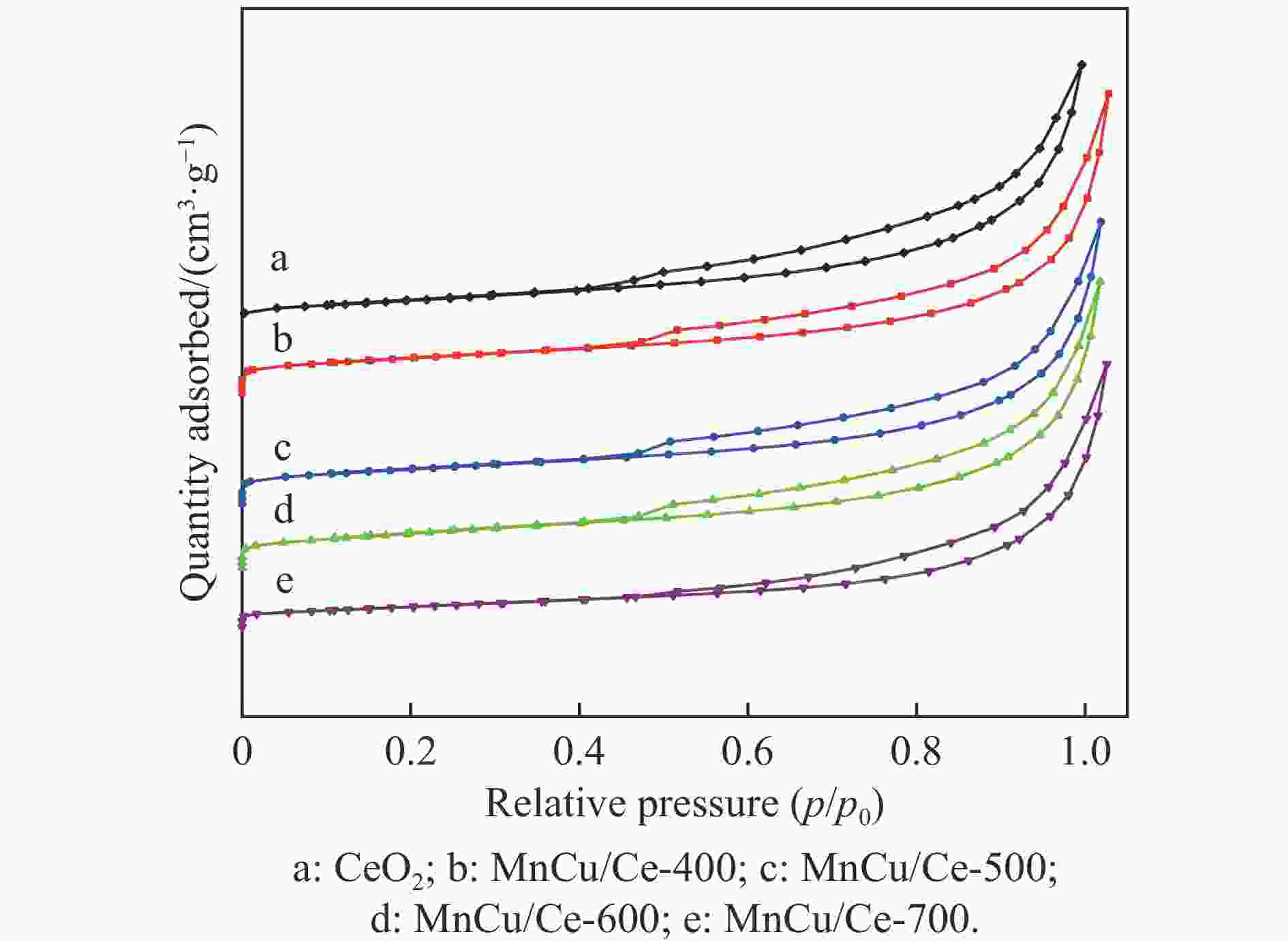
Table 2 Physical chemical properties of CeO2 and MnCu/Ce-t catalysts
Catalyst Specific surface
area
/(m2·g−1)Pore volume
/(cm3·g−1)Average pore
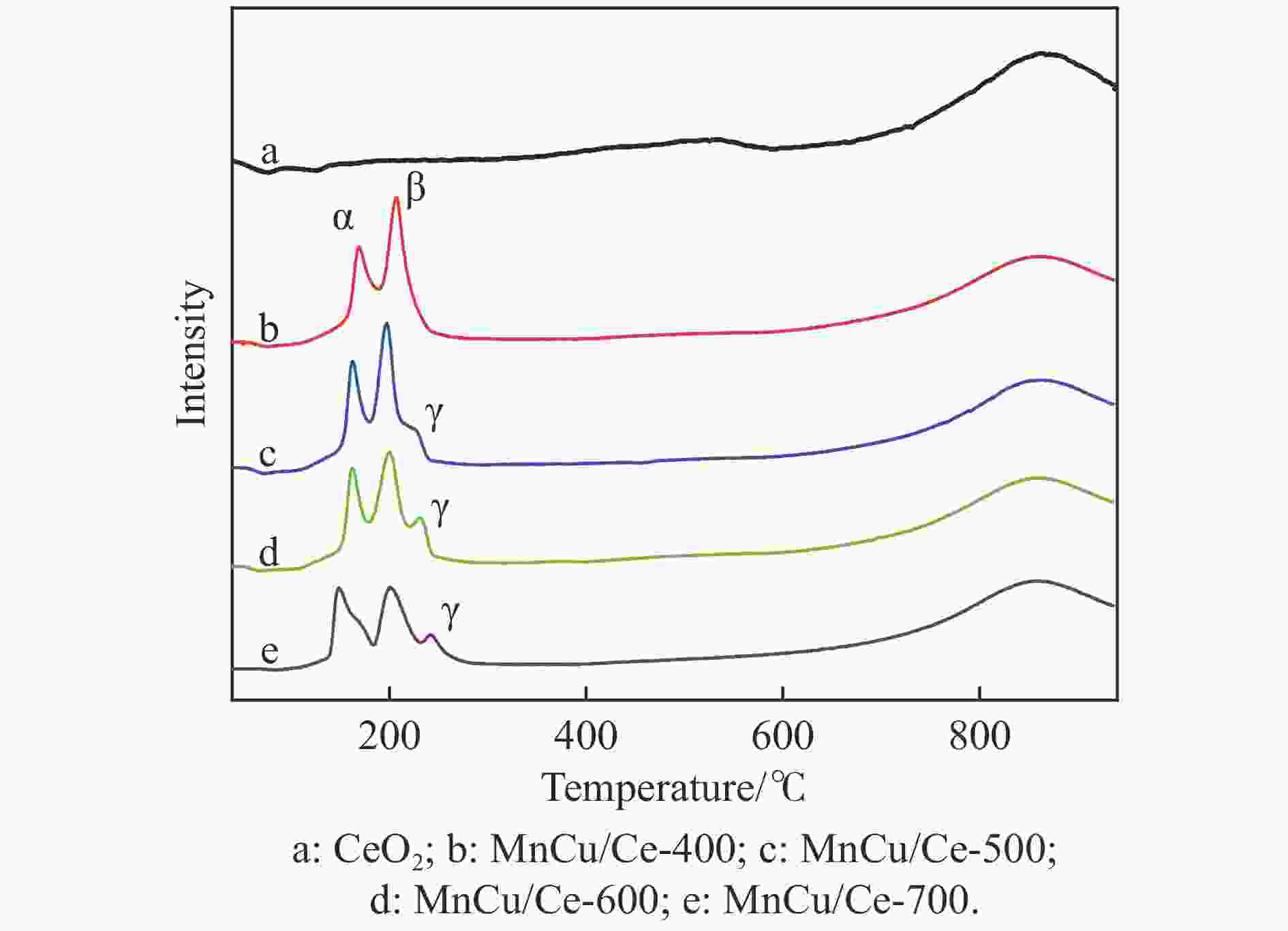
size/nmCeO2 24.3 0.0577 9.49 MnCu/Ce-400 16.9 0.0568 13.48 MnCu/Ce-500 16.7 0.0497 11.87 MnCu/Ce-600 17.0 0.0514 12.10 MnCu/Ce-700 10.5 0.0524 19.87 表 3 CeO2和MnCu/Ce-t催化剂的氢气程序升温还原测试结果
Table 3 Hydrogen temperature programmed reduction test results of CeO2 and MnCu/Ce-t catalysts
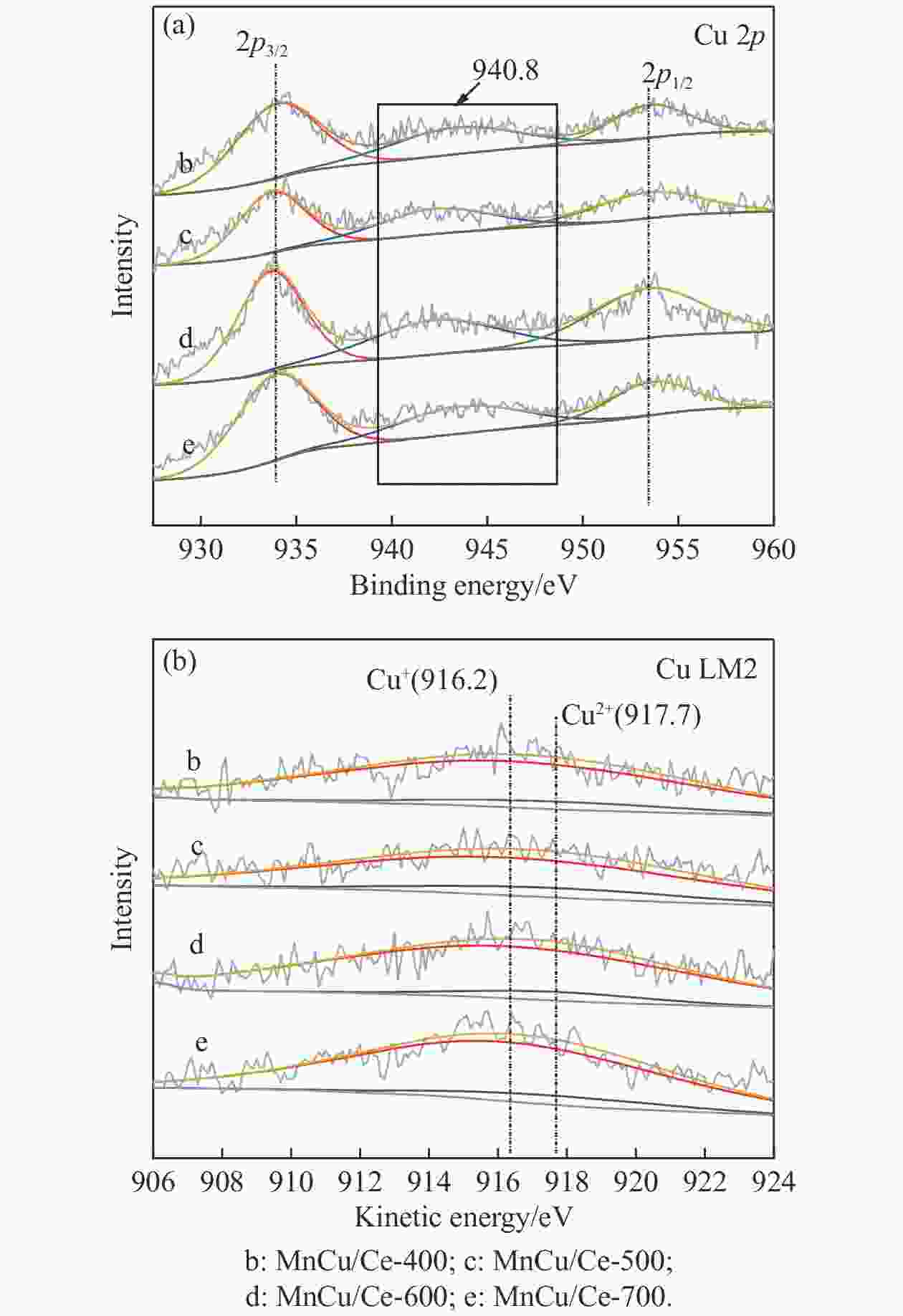
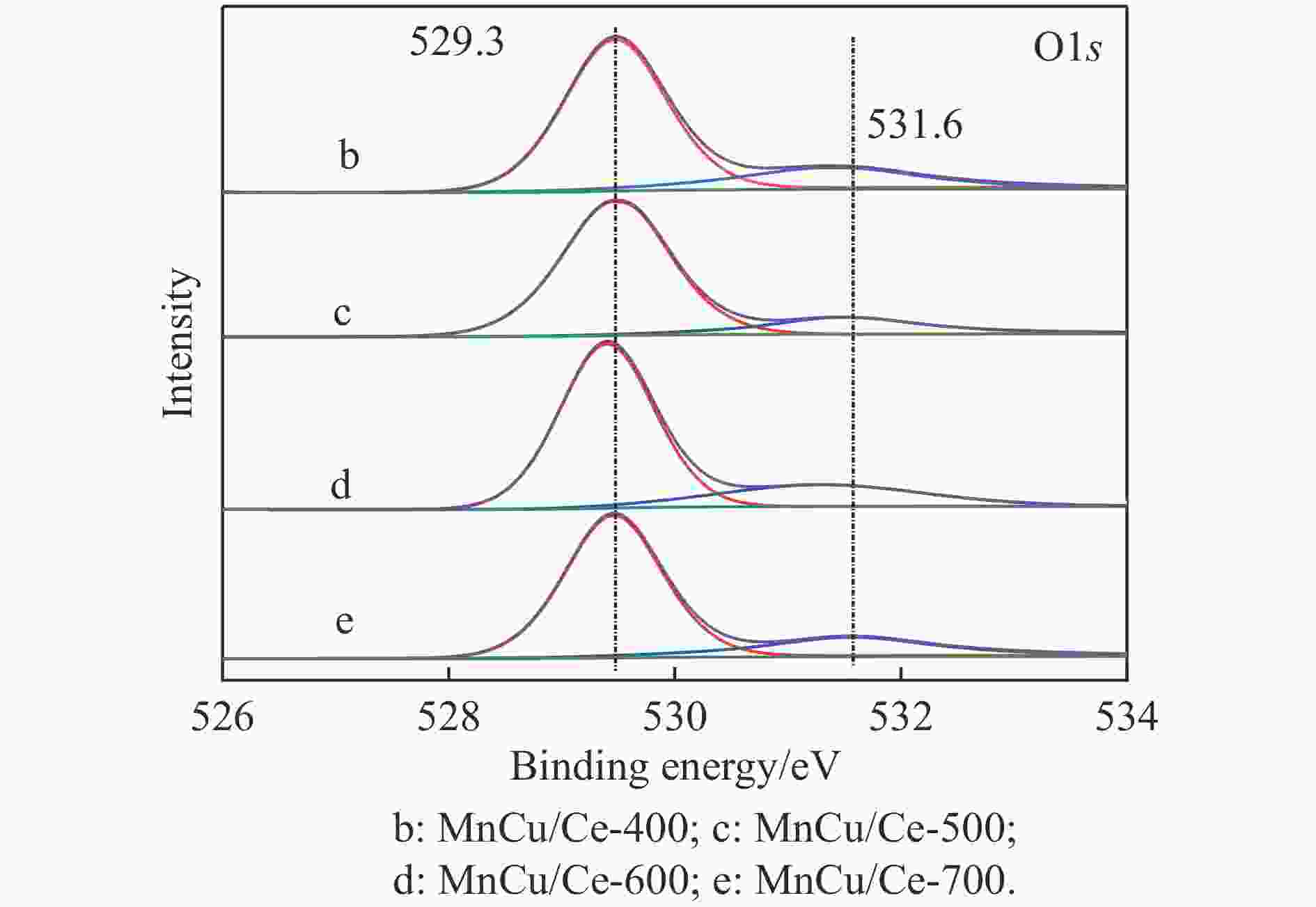
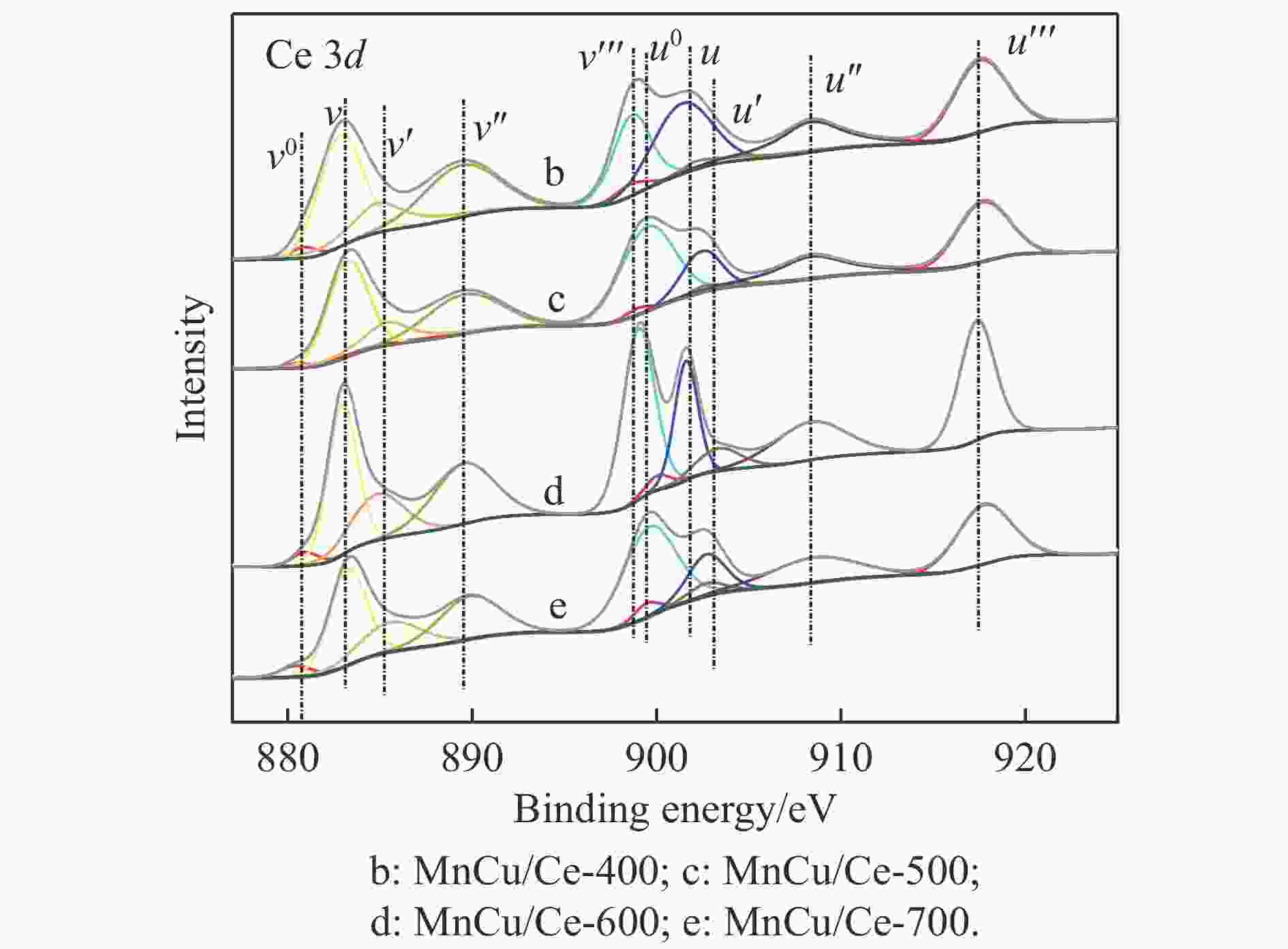
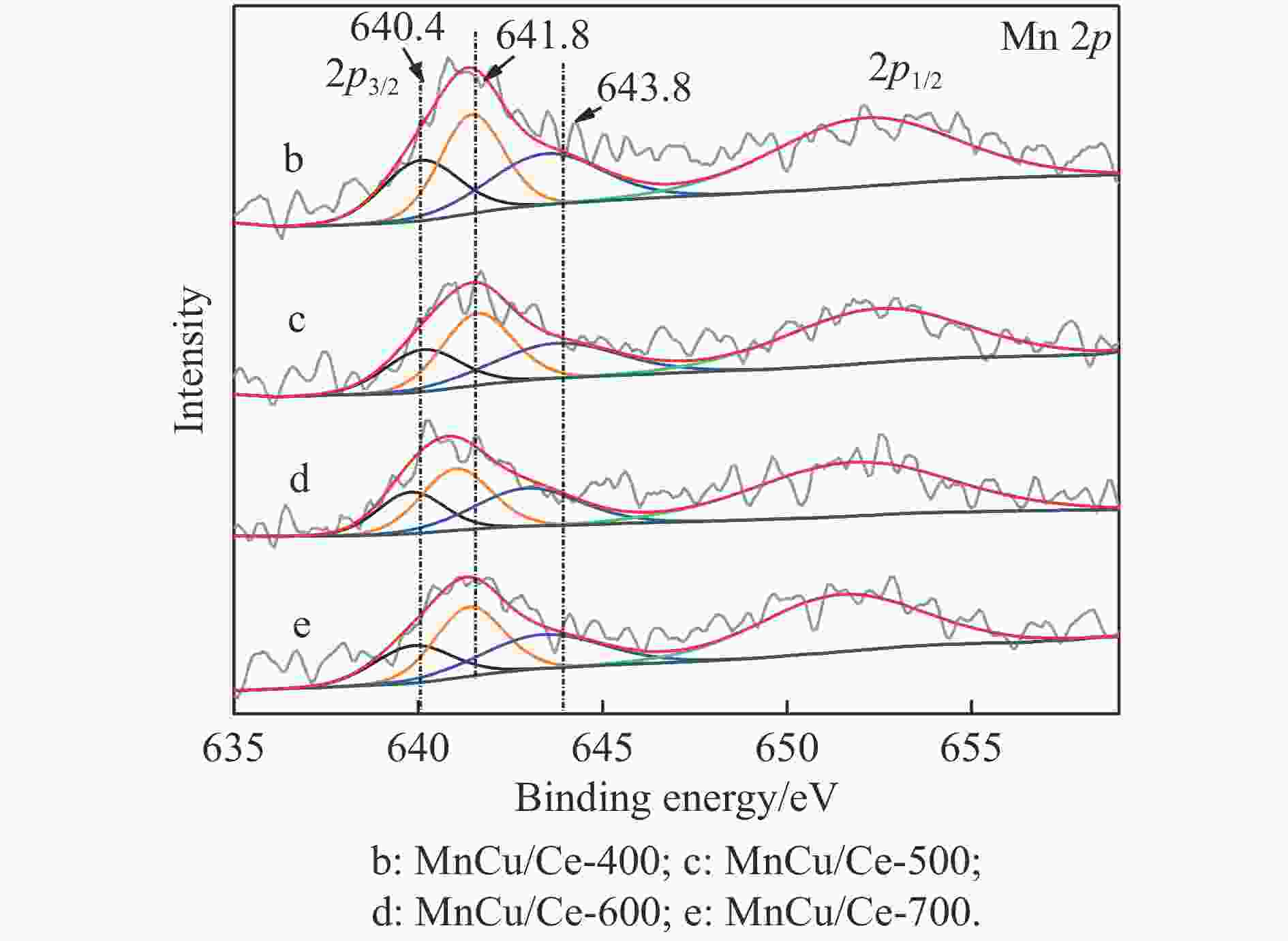
Catalyst H2 consumption/(μmol·g−1) α β γ MnCu/Ce-400 129.6 201.1 — MnCu/Ce-500 111.6 152.7 62.3 MnCu/Ce-600 108.1 143.8 67.3 MnCu/Ce-700 120.0 113.2 48.3 表 4 MnCu/Ce-t催化剂的XPS曲线拟合结果
Table 4 XPS curve fitting results of MnCu/Ce-t catalysts
Catalyst /% Ce3+/
(Ce3++Ce4+)Oads/
(Oads+Olat)Mnb/(Mn2++Mn3++Mn4+) Mn2+ Mn3+ Mn4+ MnCu/Ce-400 12.8 19.9 27.9 37.7 34.4 MnCu/Ce-500 14.0 21.1 25.2 39.9 34.9 MnCu/Ce-600 14.9 22.5 24.9 38.9 36.2 MnCu/Ce-700 13.9 20.9 24.8 40.4 34.8 表 5 MnCu/Ce-t催化剂的CO2程序升温脱附测试结果
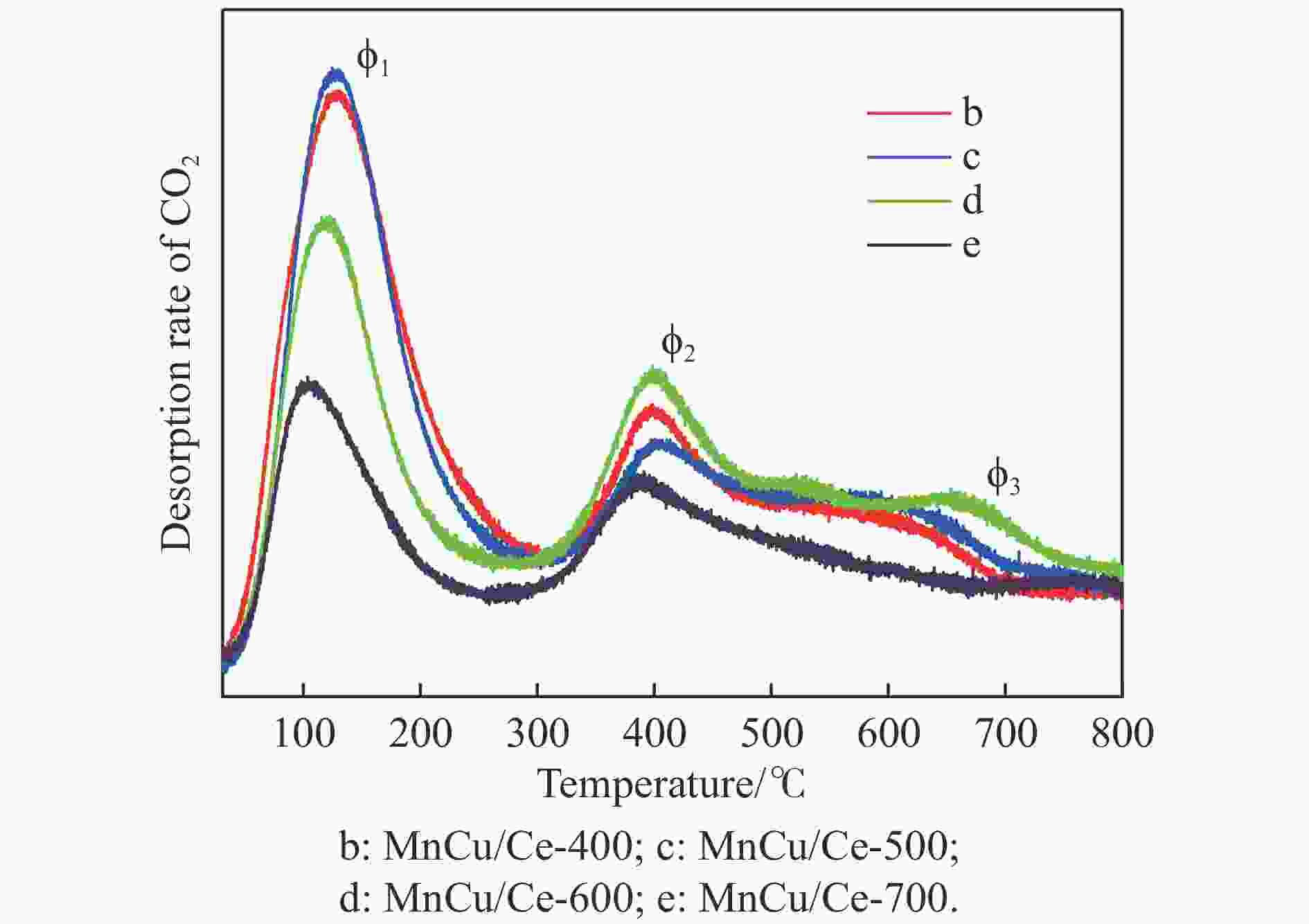
Table 5 CO2 temperature programmed desorption test results of MnCu/Ce-t catalysts
Catalyst Total desorption/(μmol·g−1) ϕ1 ϕ2 ϕ3 temp./℃ A/% temp./℃ A/% temp./℃ A/% MnCu/Ce-400 202.73 127.6 61.8 398.3 28.2 586.9 10.0 MnCu/Ce-500 193.23 125.5 58.0 404.8 27.3 578.5 14.7 MnCu/Ce-600 172.77 118.6 43.7 399.3 38.5 657.9 17.8 MnCu/Ce-700 99.53 103.1 47.8 383.1 38.8 747.6 13.4 Notes: temp.: peak temperature; A: peak area percentage. -
[1] 杨淑倩, 张娜, 贺建平, 等. Ce的浸渍顺序对Cu/Zn-Al水滑石衍生催化剂用于甲醇水蒸气重整制氢性能的影响[J]. 燃料化学学报,2018,46(4):479−488.YANG Shuqian, ZHANG Na, HE Jianping, et al. Effect of Ce impregnation sequence on hydrogen production performance of Cu/Zn-Al hydrotalcite derived catalyst for methanol steam reforming[J]. J Fuel Chem Technol,2018,46(4):479−488. [2] 乔韦军, 张楷文, 张娜, 等. 甲醇水蒸气重整制氢CuAl2O4催化材料的研究[J]. 燃料化学学报,2020,48(8):980−985.QIAO Weijun, ZHANG Kaiwen, ZHANG Na, et al. Study on CuAl2O4 catalytic material for hydrogen production from methanol steam reforming[J]. J Fuel Chem Technol,2020,48(8):980−985. [3] 王丽宝, 王东哲, 张磊, 等. 铈源对甲醇水蒸气重整制氢CuO/CeO2催化剂的影响[J]. 燃料化学学报,2020,48(7):852−859.WANG Libao, WANG Dongzhe, ZHANG Lei, et al. Effect of cerium source on CuO/CeO2 catalyst for hydrogen production from methanol Steam reforming[J]. J Fuel Chem Technol,2020,48(7):852−859. [4] 焦桐, 许雪莲, 张磊, 等. 甲醇水蒸气重整制氢 CuO/CeO2-ZrO2/SiC 整体催化剂的研究[J]. 燃料化学学报,2020,48(9):1122−1130.JIAO Tong, XU Xuelian, ZHANG Lei, et al. Research on CuO/CeO2-ZrO2/SiC monolithic catalysts for hydrogen production from steam reforming of methanol[J]. J Fuel Chem Technol,2020,48(9):1122−1130. [5] YANG S Q, ZHOU F, LIU Y J, et al. Morphology effect of ceria on the performance of CuO/CeO2 catalysts for hydrogen production by methanol steam reforming[J]. Int J Hydrogen Energy,2019,44(14):7252−7261. doi: 10.1016/j.ijhydene.2019.01.254 [6] CHAKIK F E, KADDAMI M, MIKOU M. Effect of operating parameters on hydrogen production by electrolysis of water[J]. Int J Hydrogen Energy,2017,42(40):25550−2557. doi: 10.1016/j.ijhydene.2017.07.015 [7] ERCOLINO G, ASHRAF M A, SPECCHIA V, et al. Performance evaluation and comparison of fuel processors integrated with PEM fuel cell based on steam or autothermal reforming and on CO preferential oxidation or selective methanation[J]. Appl Energy,2015,143:138−153. doi: 10.1016/j.apenergy.2014.12.088 [8] 王丽宝, 王宏浩, 张磊, 等. 柠檬酸量对水热合成CuO/Ce0.8Zr0.2O2催化水气变换制氢性能的影响[J]. 燃料化学学报,2022,50(3):337−345.WANG Libao, WANG Honghao, ZHANG Lei, et al. Effect of citric acid content on the hydrothermal synthesis of CuO/Ce0.8Zr0.2O2 catalytic water gas shift hydrogen production performance[J]. J Fuel Chem Technol,2022,50(3):337−345. [9] 张楷文, 刘鑫尧, 张磊, 等. 甲醇水蒸气重整制Cu-Zn-Al尖晶石催化剂的研究[J]. 燃料化学学报,2022,50(4):494−502.ZHAGNG Kaiwen, LIU Xinyao, ZHANG Lei, et al. Cu-Zn-Al spinel catalyst for hydrogen production from methanol steam reforming[J]. J Fuel Chem Technol,2022,50(4):494−502. [10] 乔韦军, 肖国鹏, 张磊, 等. 甲醇水蒸气重整制氢CuO/La1−xCexCrO3催化剂[J]. 燃料化学学报,2021,49(2):205−210.QIAO Weijun, XIAO Guopeng, ZHANG Lei, et al. CuO/La1−xCexCrO3 catalyst for methanol steam reforming to produce hydrogen[J]. J Fuel Chem Technol,2021,49(2):205−210. [11] QIAO W J, YANG S Q, ZHANG L, et al. Performance of Cu-Ce/M-Al (M= Mg, Ni, Co, Zn) hydrotalcite derived catalysts for hydrogen production from methanol steam reforming[J]. Int J Hydrogen Energy,2021,45(9):12773−12783. [12] QIAO W J, ZHANG L, ZHANG K W, et al. Low temperature synthesis of Cu1−xAl2.5 spinel solid solution as sustained release catalyst for methanol to hydrogen[J]. Int J Hydrogen Energy,2022,47(75):32133−32144. doi: 10.1016/j.ijhydene.2022.07.121 [13] HE J, YANG Z X, ZHANG L, et al. Cu supported on ZnAl-LDHs precursor prepared by in-situ synthesis method on γ-Al2O3 as catalytic material with high catalytic activity for methanol steam reforming[J]. Int J Hydrogen Energy,2017,42(15):9930−9937. doi: 10.1016/j.ijhydene.2017.01.229 [14] XU M G, SUN H N, WANG W, et al. Scandium and phosphorus co-doped perovskite oxides as high-performance electrocatalysts for the oxygen reduction reaction in an alkaline solution[J]. J Mater Sci Technol,2020,39:22−27. doi: 10.1016/j.jmst.2019.09.007 [15] LU J C, LI X F, HE S F, et al. Hydrogen production via methanol steam reforming over Ni-based catalysts: Influences of Lanthanum (La) addition and supports[J]. Int J Hydrogen Energy,2017,42(6):3647−3657. doi: 10.1016/j.ijhydene.2016.08.165 [16] HORNÉS A, HUNGRÍA A B, BERA P, et al. Inverse CeO2/CuO catalyst as an alternative to classical direct configurations for preferential oxidation of CO in hydrogen-rich stream[J]. J Am Chem Soc,2010,132(1):34−35. doi: 10.1021/ja9089846 [17] DING J Y, FANG Q, HUO G D, et al. A novel Zn-Al spinel-alumina composite supported gold catalyst for efficient CO oxidation[J]. Chem Commun,2021,57(80):10335−10338. doi: 10.1039/D1CC02614C [18] CHEN C, WANG R, SHEN P, et al. Inverse CeO2/CuO catalysts prepared from heterobimetallic metal-organic framework precursor for preferential CO oxidation in H2-rich stream[J]. Int J Hydrogen Energy,2015,40(14):4830−4839. doi: 10.1016/j.ijhydene.2015.02.066 [19] 金石山, 张大山, 冯旭浩, 等. Ni含量对NiO/CeO2催化剂催化CO氧化性能的影响[J]. 燃料化学学报,2022,50(8):1034−1040.JIN Shishan, ZHANG Dashan, FENG Xuhao, et al. Effect of Ni content on catalytic oxidation of CO over NiO/CeO2 catalyst[J]. J Fuel Chem Technol,2022,50(8):1034−1040. [20] 余强, 高飞, 董林. 铜基催化剂用于一氧化碳催化消除研究进展[J]. 催化学报,2012,33(8):1245−1256.(YU Qiang, GAO Fei, DONG Lin. Research progress of copper based catalysts for carbon monoxide catalytic elimination[J]. Chin J Catal,2012,33(8):1245−1256. [21] MARBÁN G, FUERTES A B. Highly active and selective CuOx/CeO2 catalyst prepared by a single-step citrate method for preferential oxidation of carbon monoxide[J]. Appl Catal B: Environ,2005,57:43−53. doi: 10.1016/j.apcatb.2004.10.011 [22] JAIN S K, CRABB E M, SMART L E, et al. Controlled modification of Pt/Al2O3 for the preferential oxidation of CO in hydrogen: A comparative study of modifying element[J]. Appl Catal B: Environ,2009,89(3-4):349−355. doi: 10.1016/j.apcatb.2008.12.013 [23] TU Y B, MENG M, SUN Z S, et al. CO preferential oxidation over Au/MnOx–CeO2 catalysts prepared with ultrasonic assistance: Effect of calcination temperature[J]. Fuel Process Technol,2012,93(1):78−84. doi: 10.1016/j.fuproc.2011.10.001 [24] LI J, ZHU P F, ZHOU R X. Effect of the preparation method on the performance of CuO-MnOx-CeO2 catalysts for selective oxidation of CO in H2-rich streams[J]. J Power Sources,2011,196(22):9590−9598. doi: 10.1016/j.jpowsour.2011.07.052 [25] 陈业娜, 孟明. 焙烧温度对CuO/Co3O4-CeO2催化剂CO优先氧化性能的影响[J]. 化学工业与工程,2015,32(2):1−5.CHEN Yena, MENG Ming. Effect of calcination temperature on CO preferential oxidation performance of CuO/Co3O4-CeO2 catalyst[J]. Chemical Industry and Engineering,2015,32(2):1−5. [26] CHEN Y N, LIU D S, YANG L J, et al. Ternary composite oxide catalysts CuO/Co3O4-CeO2 with wide temperature-window for the preferential oxidation of CO in H2-rich stream[J]. Chem Eng J,2013,234:88−98. doi: 10.1016/j.cej.2013.08.063 [27] XING Y, WU J X, ZHANG C S, et al. Mn-induced Cu/Ce catalysts with improved performance for CO preferential oxidation in H2/CO2-rich streams[J]. Int J Hydrogen Energy,2023,48:20667−20679. doi: 10.1016/j.ijhydene.2023.03.043 [28] 刘玉娟, 许骥, 佟宇飞, 等. 氧化铈纳米材料合成方法的研究进展[J]. 辽宁石油化工大学学报,2017,37(5):8−12. doi: 10.3969/j.issn.1672-6952.2017.05.002LIU Yujuan, XU Ji, TONG Yufei, et al. Research progress in the synthesis methods of cerium oxide nanomaterials[J]. J Liaoning Petrochem Univ,2017,37(5):8−12. (in Chinese) doi: 10.3969/j.issn.1672-6952.2017.05.002 [29] GAMARRA D, MARTÍNEZ-ARIAS A. Preferential oxidation of CO in rich H2 over CuO/CeO2: Operando-DRIFTS analysis of deactivating effect of CO2 and H2O[J]. J Catal,2009,263(1):189−195. doi: 10.1016/j.jcat.2009.02.012 [30] MORETTI E, STORARO L, TALON A, et al. Effect of thermal treatments on the catalytic behaviour in the CO preferential oxidation of a CuO-CeO2-ZrO2 catalyst with a flower-like morphology[J]. Appl Catal B: Environ,2011,102(3-4):627−637. doi: 10.1016/j.apcatb.2011.01.004 [31] ZHANG X, SU L F, KONG Y L, et al. CeO2 nanoparticles modified by CuO nanoparticles for low-temperature CO oxidation with high catalytic activity[J]. J Phys Chem Solids,2020,147:109651. doi: 10.1016/j.jpcs.2020.109651 [32] 魏雪梅, 唐浩东, 李鑫, 等. 不同工艺条件下 CO 甲烷化催化剂的稳定性[J]. 化学反应工程与工艺,2015,(5):459−468.WEI Xuemei, TANG Haodong, LI Xin, et al. Stability of CO methanation catalysts under different process conditions[J]. Chem React Eng Technol,2015,(5):459−468. [33] ZOU Q, ZHAO Y H, JIN X, et al. Ceria-nano supported copper oxide catalysts for CO preferential oxidation: Importance of oxygen species and metal-support interaction[J]. Appl Surf Sci,2019,494(15):1166−1176. [34] 焦桐, 许雪莲, 张磊, 等. CuO/CeO2-ZrO2/SiC整体催化剂催化甲醇水蒸气重整制氢的研究[J]. 化学学报,2021,79(4):513−519. doi: 10.6023/A20120562JIAO Tong, XU Xuelian, ZHANG Lei, et al. Study on hydrogen production from methanol steam reforming catalyzed by CuO/CeO2-ZrO2/SiC monolithic catalyst[J]. Acta Chim Sinica,2021,79(4):513−519. doi: 10.6023/A20120562 [35] 肖国鹏, 乔韦军, 王丽宝, 等. LaNiO3的焙烧温度对甲醇水蒸气重整制氢CuO/LaNiO3催化剂的影响[J]. 燃料化学学报,2020,48(2):213−220.XIAO Guopeng, QIAO Weijun, WANG Libao, et al. Effect of calcination temperature of LaNiO3 on CuO/LaNiO3 catalyst for hydrogen production by steam reforming of methanol[J]. J Fuel Chem Technol,2020,48(2):213−220. [36] 卢畅, 张财顺, 刘道胜, 等. CuO-SiO2-CeO2催化剂的设计、制备及性能研究[J]. 石油化工高等学校学报,2022,35(1):29−34. doi: 10.3969/j.issn.1006-396X.2022.01.005LU Chang, ZHANG Caishun, LIU Daosheng, et al. Design preparation and properties of CuO-SiO2-CeO2 catalyst[J]. J Petrochem Univ,2022,35(1):29−34. doi: 10.3969/j.issn.1006-396X.2022.01.005 [37] 张宣娇, 孙羽, 刘明, 等. CeO2形貌结构对催化湿式空气氧化苯酚性能的影响[J]. 中国环境科学,2020,40(10):4330−4334.ZHANG Xuanjiao, SUN Yu, LIU Ming, et al. Effect of morphology on the performance of CeO2 for catalytic wet air oxidation of phenol[J]. Chin Environ Sci,2020,40(10):4330−4334. [38] 肖国鹏, 乔韦军, 张磊, 等. 钙钛矿型甲醇水蒸气重整制氢催化材料的研究[J]. 化学学报,2021,79(1):100−107. doi: 10.6023/A20080374XIAO Guopeng, QIAO Weijun, ZHANG Lei, et al. Study on hydrogen production catalytic materials for perovskite methanol steam reforming[J]. Acta Chim Sin,2021,79(1):100−107. doi: 10.6023/A20080374 [39] 龚磊. 金属氧化物对铜铈催化剂富氢条件下CO优先氧化性能的影响[D]. 南昌: 南昌大学, 2012.GONG Lei. Effect of metal oxides on the preferential oxidation of CO in copper-cerium catalysts with hydrogen enrichment[D]. Nanchang: Nanchang University, 2012. [40] CHEN Y Z, LIAW B J, CHEN H C. Selective oxidation of CO in excess hydrogen over CuO/CexZr1−xO2CuO/CexZr1−xO2 catalysts[J]. Int J Hydrogen Energy,2006,31(3):427−435. doi: 10.1016/j.ijhydene.2005.11.004 [41] STROHMEIER B R, LEVDEN D E, FIELD R S, et al. Surface spectroscopic characterization of Cu/Al2O3 catalysts[J]. J Catal,1985,94(2):514−530. doi: 10.1016/0021-9517(85)90216-7 [42] LIU Y J, KANG H F, HOU X N, et al. Sustained release catalysis: Dynamic copper releasing from stoichiometric spinel CuAl2O4 during methanol steam reforming[J]. Appl Catal B: Environ,2023,323:122043. doi: 10.1016/j.apcatb.2022.122043 [43] YANG K, LIU J F, SI R R, et al. Comparative study of Au/TiO2 and Au/Al2O3 for oxidizing CO in the presence of H2 under visible light irradiation[J]. J Catal,2014,317:229−239. doi: 10.1016/j.jcat.2014.06.005 [44] MA W J, MASHIMO T, TAMURA S, et al. Cerium oxide (CeO2−x) nanoparticles with high Ce3+ proportion synthesized by pulsed plasma in liquid[J]. Ceram Int,2020,46(17):26502−26510. doi: 10.1016/j.ceramint.2020.07.093 [45] 康玉姝, 王丽宝, 李永志, 等. 水热合成时间对Cu/Ce-Zr催化水气变换反应性能的影响[J]. 燃料化学学报(中英文),2023,51(6):776−782. doi: 10.19906/j.cnki.jfct.2022074KANG Yushu, WANG Libao, LI Yongzhi, et al. Effect of hydrothermal synthesis time on the performance of Cu/Ce-Zr catalyzed water-vapor conversion reaction[J]. J Fuel Chem Technol,2023,51(6):776−782. doi: 10.19906/j.cnki.jfct.2022074 [46] ZHANG H L, WANG J L, CAO Y, et al. Effect of Y on improving the thermal stability of MnOx-CeO2 catalysts for diesel soot oxidation[J]. Chin J Catal,2015,36:1333−1341. doi: 10.1016/S1872-2067(15)60867-1 [47] SUBHASHISH D, CHANDRA D G, DEVENDRA M, et al. Effect of various metal oxides phases present in CuMnOx catalyst for selective CO oxidation[J]. Mater Discovery,2018,12:63−71. doi: 10.1016/j.md.2018.11.002 [48] SIRICHAIPRASERT K, LUENGNARUEMITCHAI A, PONGSTABODEE S. Selective oxidation of CO to CO2 over Cu-Ce-Fe-O composite-oxide catalyst in hydrogen feed stream[J]. Int J Hydrogen Energy,2007,32(7):915−926. doi: 10.1016/j.ijhydene.2006.10.060 -




 下载:
下载: