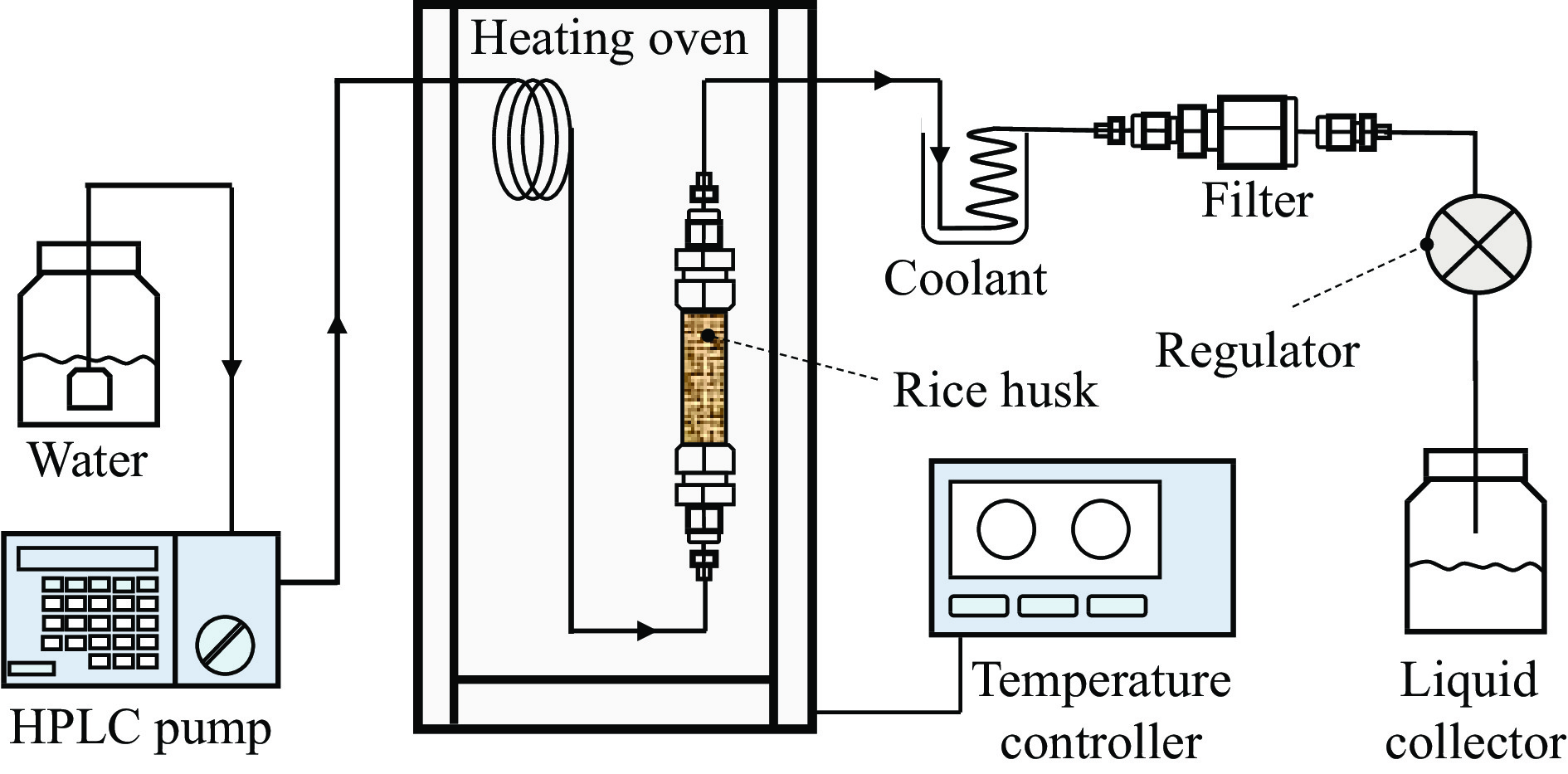
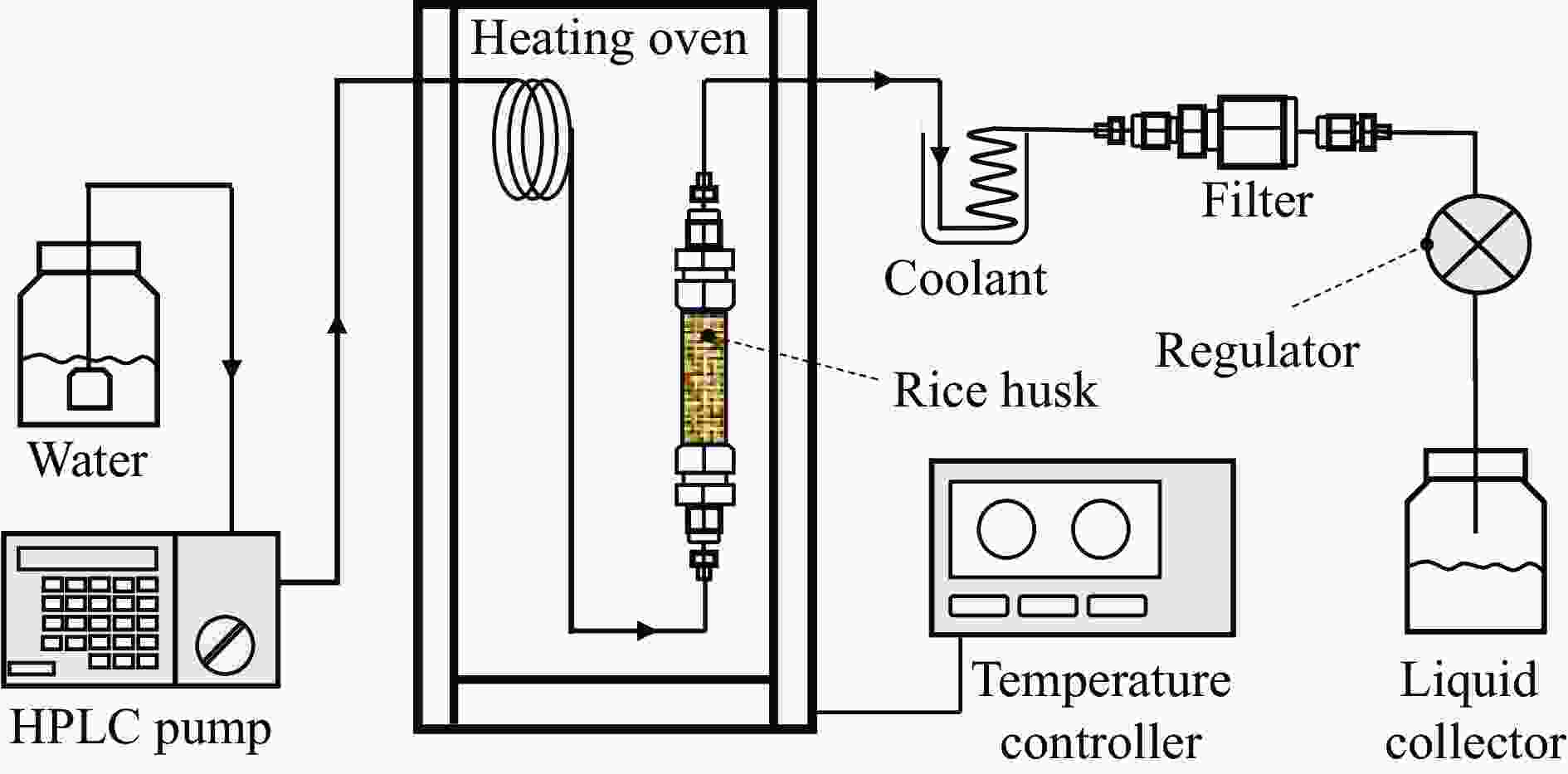
Hydrothermal flowthrough pretreatment of biomass and pyrolysis characteristics of residual solid
-
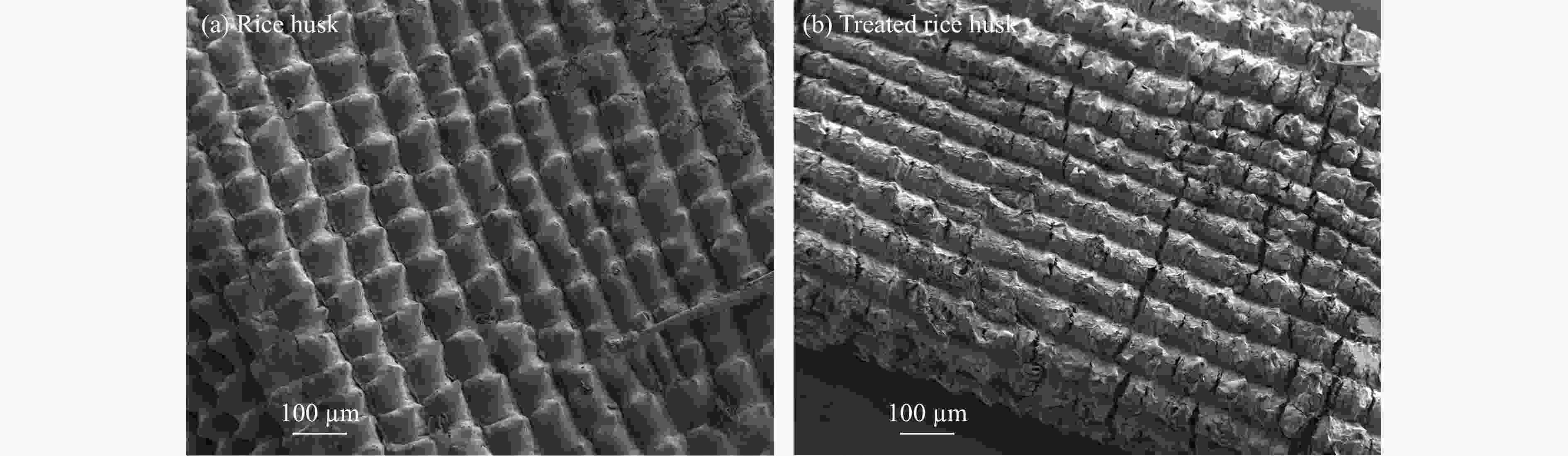
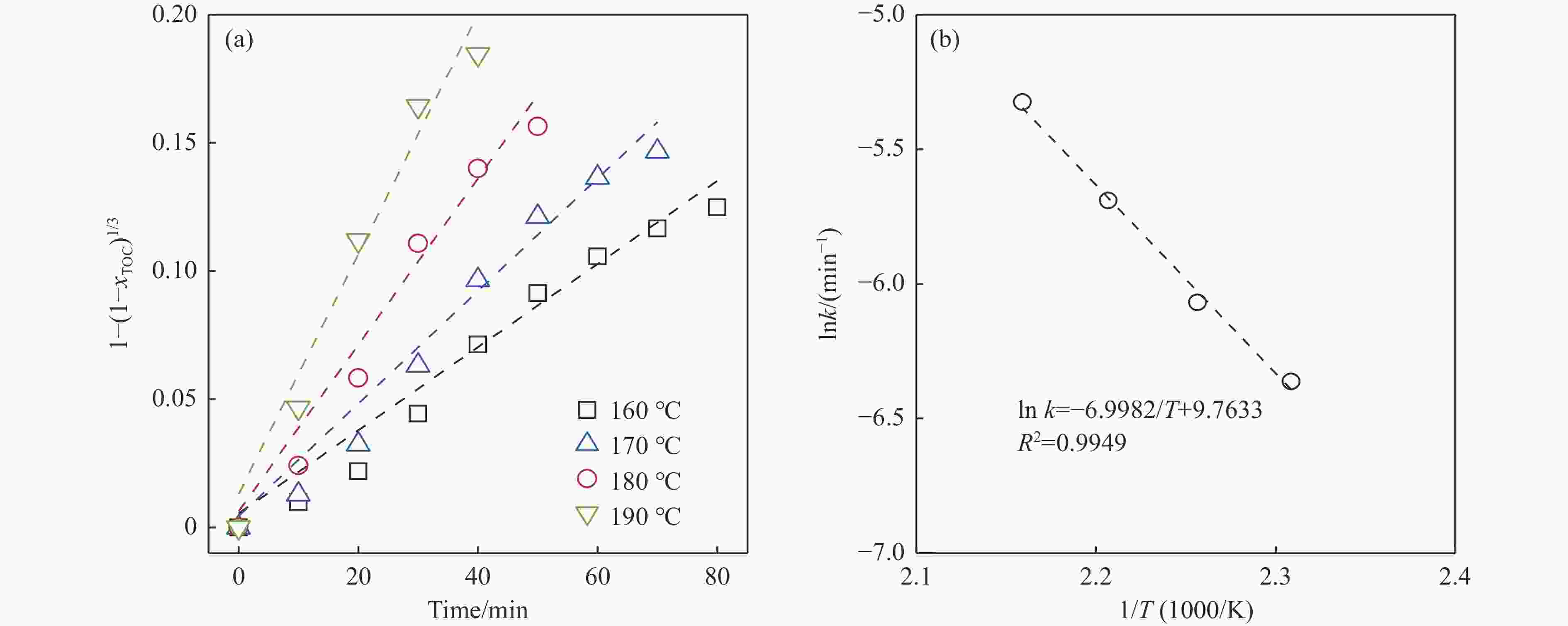
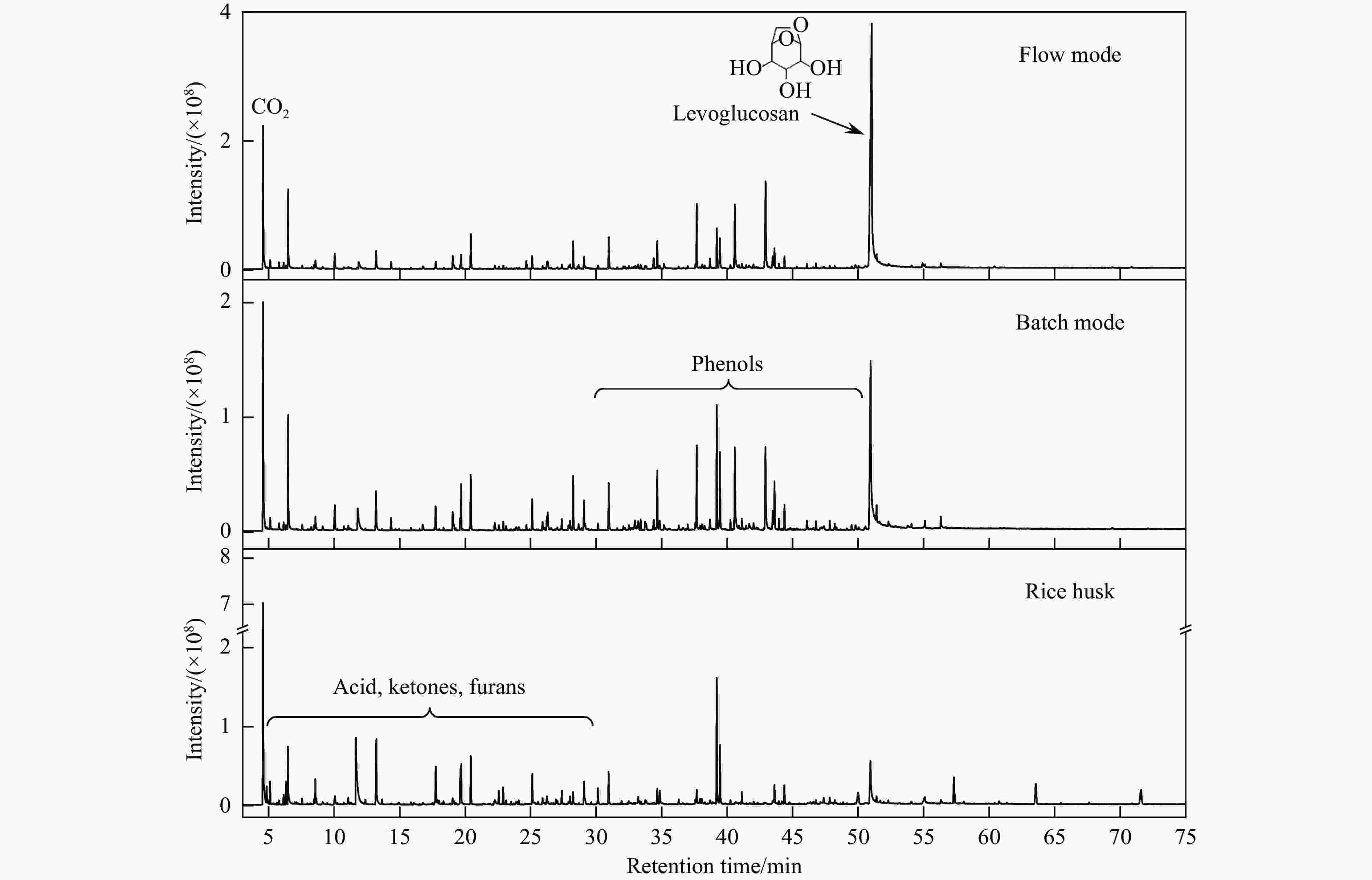
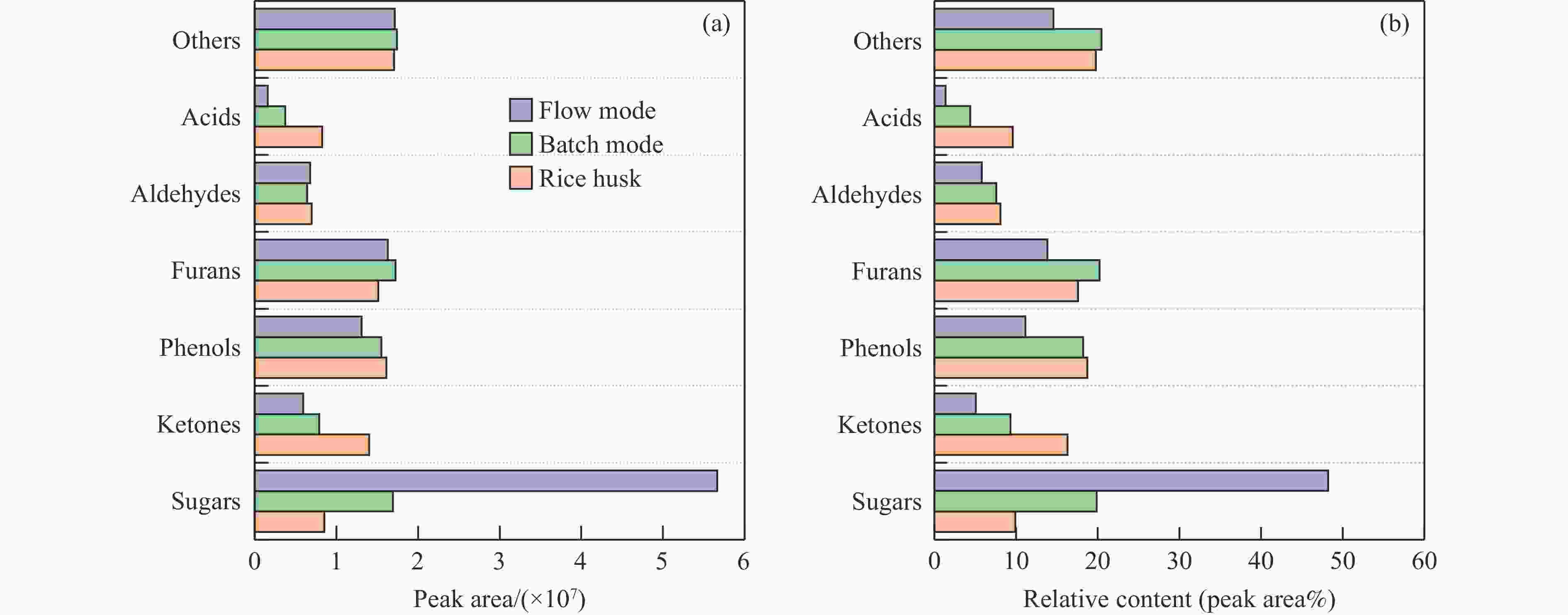
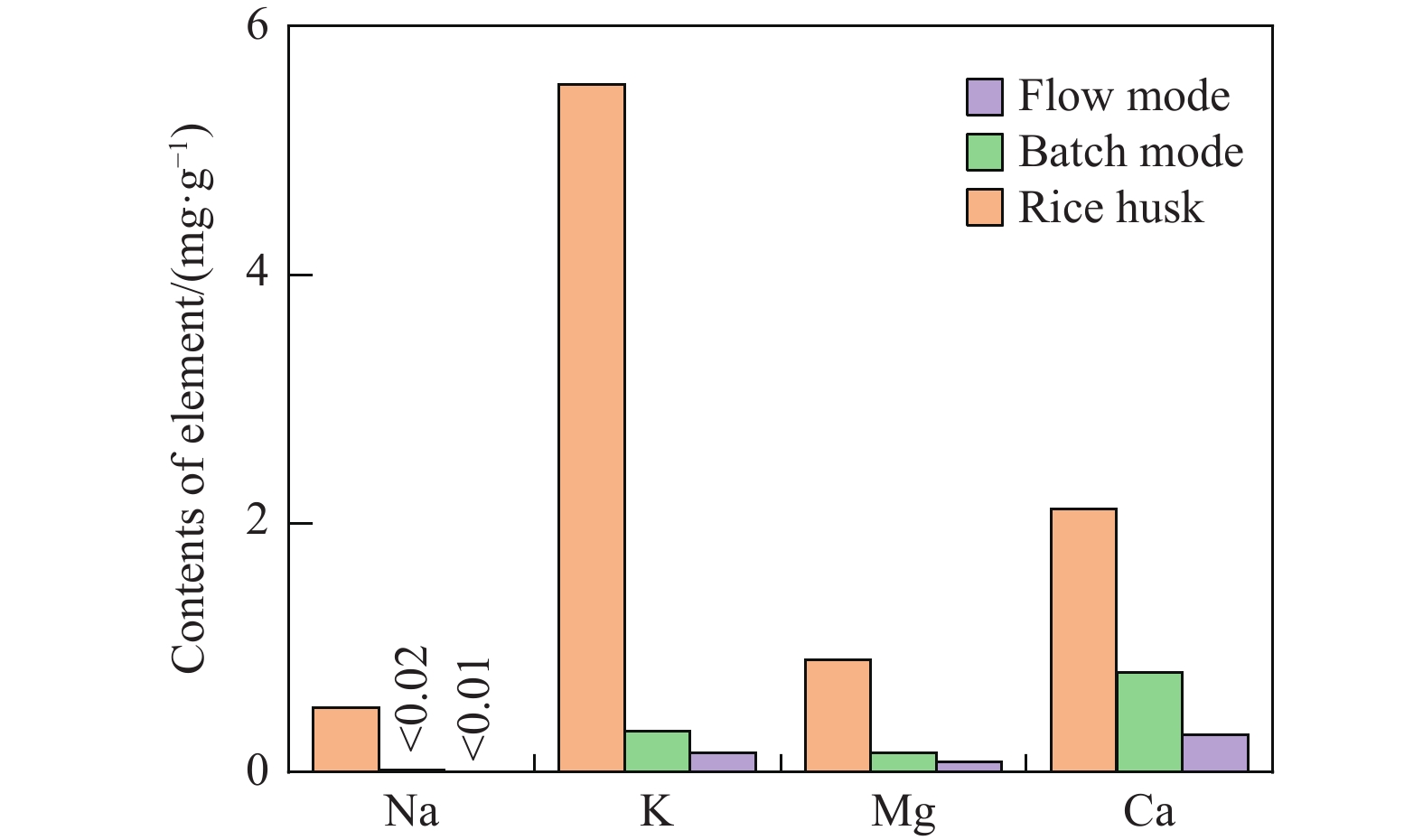
摘要: 生物质复杂的多组分体系和致密交联的化学结构是制约其高值化利用的关键,实现木质纤维组分预分离对生物质分级转化具有重要意义。实验采用连续式水热法预处理稻壳,考察了水热温度和流量对稻壳分解速率以及固相产物化学组成与热解特性的影响。结果表明,稻壳的水热分解符合表面化学反应过程控制的未反应收缩核模型,预处理在180 ℃下能脱除稻壳95%的碱及碱土金属、92%的半纤维素和59%的木质素,极大保留了纤维素组分,这使得稻壳热解产物中以左旋葡聚糖为主的脱水糖的相对含量从9.9%提高至48.2%。Abstract: The sophisticated multi-components and densed cross-link chemical structures of lignocellulosic biomass are important bottlenecks restricting its value-added utilization. The pre-fractionation of lignocellulose components is of great significance for the fractional conversion of biomass. The present study subjected rice husk (RH) to hydrothermal treatment in a flowthrough mode and investigated the effects of hydrothermal temperature and water flowrate on the decomposition rate of RH, chemical components of residual solids and their pyrolysis characteristics. It is shown that the decomposition of RH under hydrothermal conditions conformed well to the unreacted shrinking core model with phase boundary reactions rate-controlling. The pretreatment at 180 ℃ removed 95% alkali and alkaline-earth metallic species, 92% hemicellulose and 59% lignin from RH and selectively retained most of the cellulose components. As a result of the pretreatment, the relative content of anhydrosugar (mainly levoglucosan) from pyrolysis of RH at a curie-point temperature of 445 ℃ was increased from 9.9% up to 48.2%.
-
Key words:
- rice husk /
- hydrothermal pretreatment /
- anhydrosugar /
- pyrolysis
-
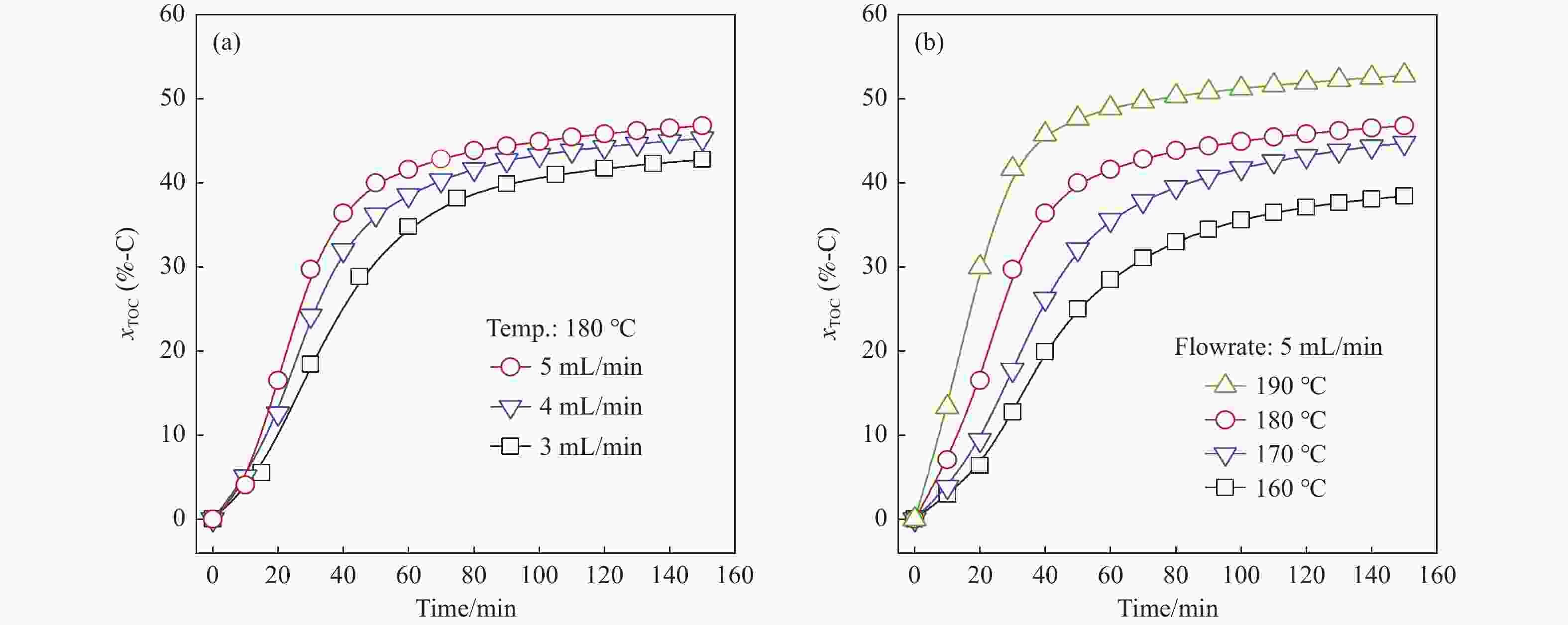
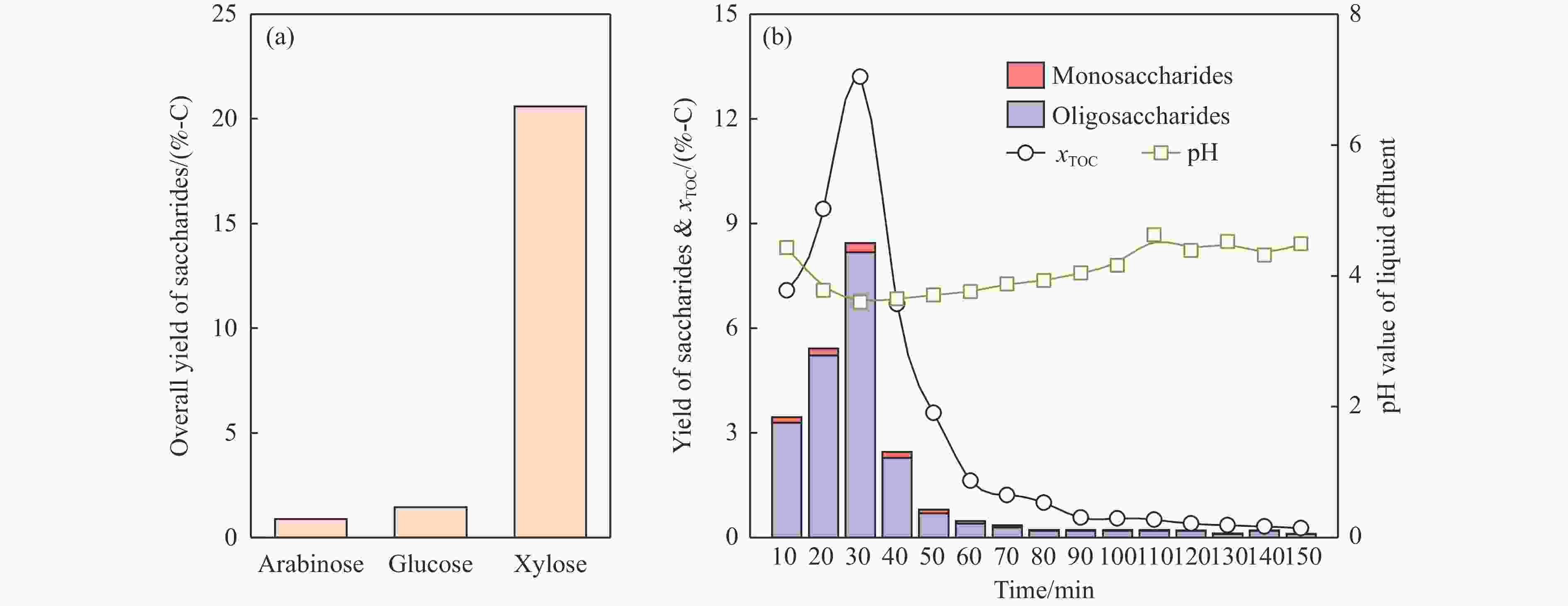
图 3 液相产物的(a)单糖收率和(b)每10 min的单糖和低聚糖收率、xTOC和pH值
Figure 3 Results of (a) overall yield of saccharides of liquid product and (b) changes in yields of mono- and oligo-saccharides, xTOC and pH values of liquid effluent collected in each 10 min (RH was pretreated at 180 ℃ and 5 mL/min water flow, the liquid product was subjected to hydrolysis for determining the overall yield of saccharides)
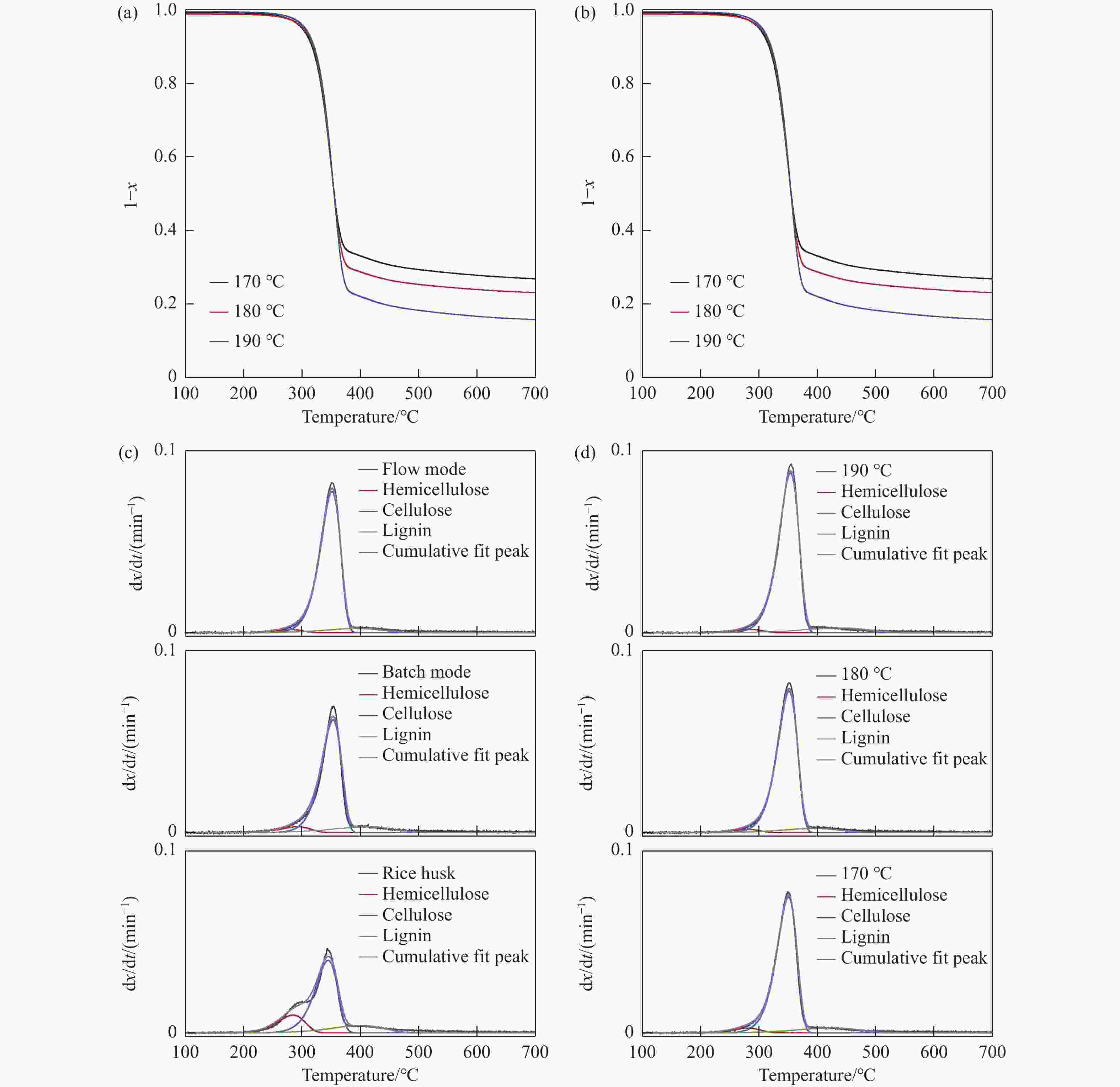
图 7 稻壳及固相产物的热重(TG)和微分热重(DTG)曲线
Figure 7 Thermogravimetric (TG) and differential thermogravimetric (DTG) profiles of RH and residual solids (Pretreatment conditions for a flow mode are 180−190 ℃ for 150 min and 5 mL/min water flow and for a batch mode are 180 ℃ for 60 min and RH/water mass ratio of 1∶40. x represents a mass-based conversion of the sample. DTG profile was deconvoluted into three peaks that were assumed to occur from the pyrolysis of hemicellulose, cellulose, and lignin)
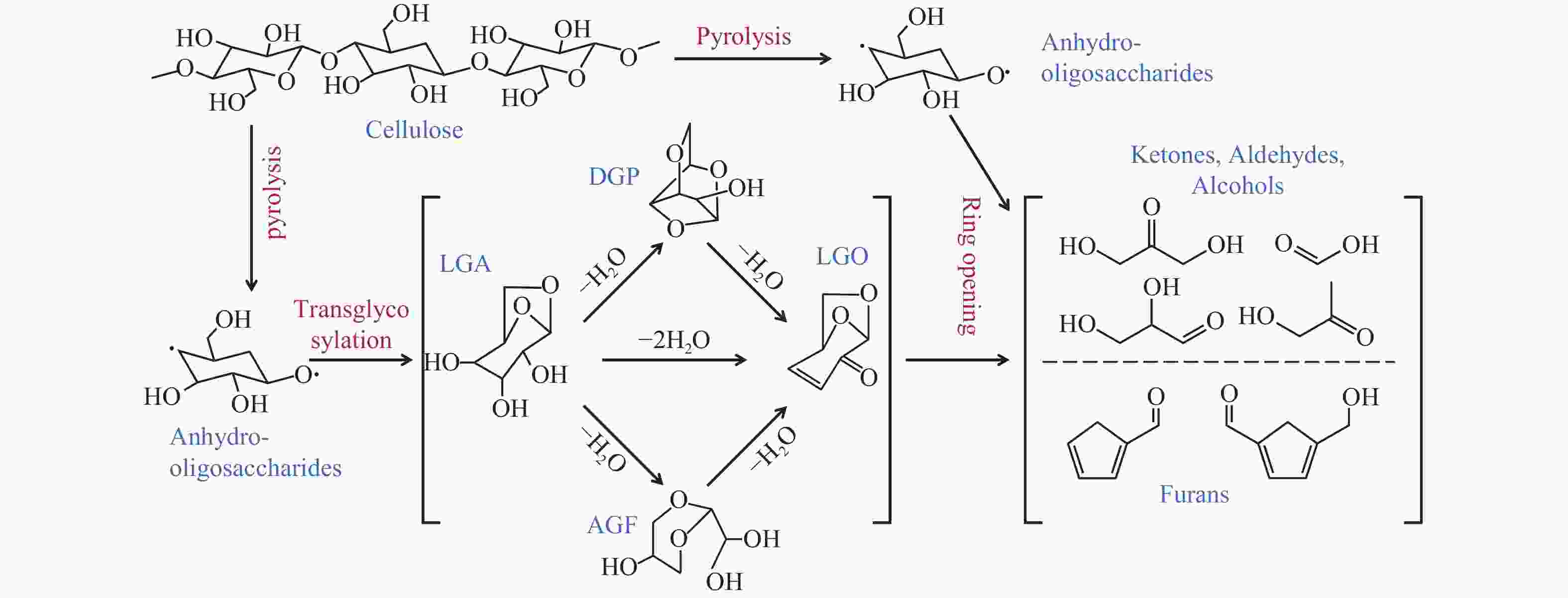
图 10 左旋葡聚糖形成与转化反应路径[23]
Figure 10 Mechanism of formation and conversion of levoglucosan (adapted from Ref. 23) (LGA, levoglucosan; DGP, 1,4:3,6-dianhydro-β-D-glucopyranose; AGF, 1,6-anhydro-β-D-glucofuranose; and LGO, levoglucosenone)
表 1 稻壳及固相产物的工业分析、元素分析和热值
Table 1 Proximate and ultimate analyses and higher heating values of RH and residual solids
Samplea Proximate analysis
wdry/%Ultimate analysis
wdaf/%bAtomic ratio HHVg/
(MJ·kg−1)A Vd FCe C H N Of H/C O/C RH 19.9 60.6 19.5 49.0 6.4 0.5 44.1 1.5483 0.6752 14.3 RH-160 19.0 66.2 14.8 50.2 6.2 0.4 43.3 1.4734 0.6475 14.7 RH-170 16.8 74.7 8.5 51.1 6.0 0.4 42.5 1.3963 0.6239 15.2 RH-180 11.9 78.9 9.2 54.3 5.4 0.3 40.1 1.1767 0.5542 16.6 RH-190 9.0 84.2 6.8 55.2 5.1 0.3 39.5 1.0967 0.5372 17.2 RH-180c 22.1 65.3 12.6 55.5 5.9 0.4 38.2 1.2689 0.5175 15.9 a: RH-x samples represent the residual solid, where x is the temperature for hydrothermal pretreatment; b: daf is the abbreviation of dry-ash-free; c: residual solid prepared by a batch mode under conditions of 180 ℃ for 1 h and 1∶40 mass ratio (RH/water); d: volatile matter; d: fixed carbon; e: calculated by difference; f: higher heating value, calculated by Dulong’s formula: Q = 0.3383C+1.442(H−0.125O), where C, H, and O are the contents of carbon, hydrogen, and oxygen, respectively. 表 2 稻壳及固相产物的化学组成分析
Table 2 Chemical components of RH and residual solids
Pretreatment Composition wsample/% Composition wRH/% ash hemi. cel. lig. extract. ash hemi. cel. lig. extract. RH 19.9 19.3 31.8 24.2 4.8 19.9 19.3 31.8 24.2 4.8 Flow mode 11.9 3.3 63.5 21.3 <0.1 5.5 1.5 29.4 9.9 <0.1 Batch mode 22.1 7.2 43.2 26.5 1.1 13.8 4.5 27.0 16.5 0.7 Hemi, cel, lig and extract are abbreviations of hemicellulose, cellulose, lignin and extractives, respectively. Pretreatment conditions for a flow mode are 180 ℃ for 150 min and 5 mL/min water flow and for a batch mode are 180 ℃ for 60 min and 1∶40 of RH/water mass ratio. 表 3 稻壳灰分SiO2纯度和比表面积分析和对比
Table 3 Results of SiO2 purity and specific surface area of RH ashes
Sample no. SiO2 puritya
/%Surface areab
/(m2·g−1)Method Ref. 1 91.17 − raw rice husk this study 2 99.70 216 flow mode, 180 ℃ 3 98.91 207 batch mode, 180 ℃ 4 99.86 218 3% HCl, room temperature 5 N.A. 164 10% HCl, 100 ℃ [26] 6 96.89 N.A. water rinsing, room temperature [27] 7 99.80 N.A. 5% HCl refluxing, 130 ℃ 8 99.52 N.A. ion liquid, room temperature 9 95.77 116 raw rice husk [28] 10 99.58 218 0.5 mol/L HCl, 60 ℃ 11 99.08 208 0.5 mol/L H2SO4, 60 ℃ 12 91.25 21 raw rice husk [29] 13 97.94 134 hydrothermo-baric treatment, 300 ℃ 14 99.77 N.A. 5% citric acid, 80 ℃ [30] a: by X-ray fluorescence spectrometry; b: calculated by the Brunauer-Emmett-Taller method; N.A., not available. -
[1] ZAKZESKI J, BRUIJNINCX P C A, JONGERIUS A L, et al. The catalytic valorization of lignin for the production of renewable chemicals[J]. Chem Rev,2010,110(6):3552−99. doi: 10.1021/cr900354u [2] 蒋叶涛, 宋晓强, 孙勇, 等. 基于木质生物质分级利用的组分优先分离策略[J]. 化学进展,2017,29(10):1273−1284.JIANG Yetao, SONG Xiaoqiang, SUN Yong, et al. Strategies of prior-fractionation for the graded utilization of lignocellulose[J]. Prog Chem.,2017,29(10):1273−1284. [3] KRUSE A, DINJUS E. Hot compressed water as reaction medium and reactant[J]. J Supercrit Fluids,2007,39(3):362−380. doi: 10.1016/j.supflu.2006.03.016 [4] BACH Q V, TRAN K Q, KHALIL R A, et al. Comparative assessment of wet torrefaction[J]. Energy Fuels,2013,27(11):6743−6753. doi: 10.1021/ef401295w [5] CHANG S, ZHAO Z, ZHENG A, et al. Effect of hydrothermal pretreatment on properties of bio-oil produced from fast pyrolysis of eucalyptus wood in a fluidized bed reactor[J]. Bioresour Technol,2013,138:321−328. doi: 10.1016/j.biortech.2013.03.170 [6] YU Q, ZHUANG X, YUAN Z, et al. Step-change flow rate liquid hot water pretreatment of sweet sorghum bagasse for enhancement of total sugars recovery[J]. Appl Energy,2011,88(7):2472−2479. doi: 10.1016/j.apenergy.2011.01.031 [7] FUNKE A, ZIEGLER F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering[J]. Biofuel Bioprod Biorefin,2010,4(2):160−177. doi: 10.1002/bbb.198 [8] SOLAR J, DE MARCO I, CABALLERO B M, et al. Influence of temperature and residence time in the pyrolysis of woody biomass waste in a continuous screw reactor[J]. Biomass Bioenergy,2016,95:416−423. doi: 10.1016/j.biombioe.2016.07.004 [9] YUAN X Z, TONG J Y, ZENG G M, et al. Comparative studies of products obtained at different temperatures during straw liquefaction by hot compressed water[J]. Energy Fuels,2009,23:3262−3267. doi: 10.1021/ef900027d [10] LENG L, ZHOU W. Chemical compositions and wastewater properties of aqueous phase (wastewater) produced from the hydrothermal treatment of wet biomass: A review[J]. Energy Sources, Part A,2018,40(22):2648−2659. doi: 10.1080/15567036.2018.1495780 [11] 余强, 庄新姝, 袁振宏, 等. 高温液态水中甜高粱渣半纤维素水解及其机理[J]. 化工学报,2012,63(2):599−605. doi: 10.3969/j.issn.0438-1157.2012.02.037YU Qiang, ZHUANG Xinshu, YUAN Zhenhong, et al. Hydrolysis of sweet sorghum bagasse hemicellulose with liquid hot water and its mechanism[J]. CIESC J,2012,63(2):599−605. doi: 10.3969/j.issn.0438-1157.2012.02.037 [12] 于建国, 姜罗, 刘李, 等. 亚熔盐低温浸取钾长石工艺过程[J]. 华东理工大学学报(自然科学版),2019,45(2):206−215. doi: 10.14135/j.cnki.1006-3080.20180322001YU Jianguo, JIANG Luo, LIU Li, et al. Leaching process of potassium feldspar by sub-molten salt at low temperature[J]. J East China Univ Sci Technol,2019,45(2):206−215. doi: 10.14135/j.cnki.1006-3080.20180322001 [13] NIU Y, LV Y, LEI Y, et al. Biomass torrefaction: Properties, applications, challenges, and economy[J]. Renewable Sustainable Energy Rev,2019,115:1−18. [14] GüLEç F, RIESCO L M G, WILLIAMS O, et al. Hydrothermal conversion of different lignocellulosic biomass feedstocks-Effect of the process conditions on hydrochar structures[J]. Fuel,2021,302:1−18. [15] BACH Q V, TRAN K Q, SKREIBERG Ø. Comparative study on the thermal degradation of dry- and wet-torrefied woods[J]. Appl Energy,2017,185:1051−1058. doi: 10.1016/j.apenergy.2016.01.079 [16] LIANG Y G, CHENG B, SI Y B, et al. Thermal decomposition kinetics and characteristics of Spartina alterniflora via thermogravimetric analysis[J]. Renew Energy,2014,68:111−117. doi: 10.1016/j.renene.2014.01.041 [17] TEKIN K, KARAGöZ S, BEKTAŞ S. A review of hydrothermal biomass processing[J]. Renewable Sustainable Energy Rev,2014,40:673−687. doi: 10.1016/j.rser.2014.07.216 [18] ZHANG B, ZHONG Z, MIN M, et al. Catalytic fast co-pyrolysis of biomass and food waste to produce aromatics: Analytical Py-GC/MS study[J]. Bioresour Technol,2015,189:30−35. doi: 10.1016/j.biortech.2015.03.092 [19] XIN X, PANG S, DE MIGUEL MERCADER F, et al. The effect of biomass pretreatment on catalytic pyrolysis products of pine wood by Py-GC/MS and principal component analysis[J]. J Anal Appl Pyrolysis,2019,138:145−153. doi: 10.1016/j.jaap.2018.12.018 [20] PATWARDHAN P R, SATRIO J A, BROWN R C, et al. Influence of inorganic salts on the primary pyrolysis products of cellulose[J]. Bioresour Technol,2010,101(12):4646−4655. doi: 10.1016/j.biortech.2010.01.112 [21] WANG K, ZHANG J, SHANKS B H, et al. The deleterious effect of inorganic salts on hydrocarbon yields from catalytic pyrolysis of lignocellulosic biomass and its mitigation[J]. Appl Energy,2015,148:115−120. doi: 10.1016/j.apenergy.2015.03.034 [22] DALLUGE D L, KIM K H, BROWN R C. The influence of alkali and alkaline earth metals on char and volatile aromatics from fast pyrolysis of lignin[J]. J Anal Appl Pyrolysis,2017,127:385−393. doi: 10.1016/j.jaap.2017.07.011 [23] LIN Y C, CHO J, TOMPSETT G A, et al. Kinetics and mechanism of cellulose pyrolysis[J]. J Phys Chem C,2009,113(46):20097−20107. doi: 10.1021/jp906702p [24] VAMVUKA D, TROULINOS S, KASTANAKI E. The effect of mineral matter on the physical and chemical activation of low rank coal and biomass materials[J]. Fuel,2006,85(12/13):1763−71. doi: 10.1016/j.fuel.2006.03.005 [25] KANIOWSKI W, TALER J, WANG X, et al. Investigation of biomass, RDF and coal ash-related problems: Impact on metallic heat exchanger surfaces of boilers[J]. Fuel,2022,326:1−11. [26] WANG W, MARTIN J C, FAN X, et al. Silica nanoparticles and frameworks from rice husk biomass[J]. ACS Appl Mater Interfaces,2012,4(2):977−981. doi: 10.1021/am201619u [27] CHEN H, WANG W, MARTIN J C, et al. Extraction of lignocellulose and synthesis of porous silica nanoparticles from rice husks: A comprehensive utilization of rice husk biomass[J]. ACS Sustainable Chem Eng,2012,1(2):254−259. [28] BAKAR R A, YAHYA R, GAN S N. Production of high purity amorphous silica from rice husk[J]. Proced Chem,2016,19:189−195. doi: 10.1016/j.proche.2016.03.092 [29] BENJAMIN IYENAGBE U, MAMAT O. Hydro thermo-baric processing and properties of nano silica from rice husk[J]. Appl Mech Mater,2012,152−154:177−182. [30] UMEDA J, KONDOH K. High-purification of amorphous silica originated from rice husks by combination of polysaccharide hydrolysis and metallic impurities removal[J]. Ind Crop Prod,2010,32(3):539−544. doi: 10.1016/j.indcrop.2010.07.002 [31] WANG W, LEMAIRE R, BENSAKHRIA A, et al. Review on the catalytic effects of alkali and alkaline earth metals (AAEMs) including sodium, potassium, calcium and magnesium on the pyrolysis of lignocellulosic biomass and on the co-pyrolysis of coal with biomass[J]. J Anal Appl Pyrolysis,2022,163:1−33. -




 下载:
下载: