Preparation and oxidation desulfurization performance of zirconium oxychloride based ternary deep eutectic solvent
-
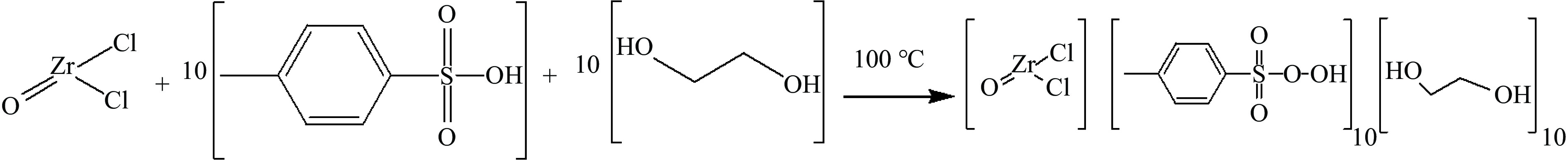
摘要: 通过简单加热乙二醇,对甲苯磺酸和八水氯氧化锆混合物制备了三元低共熔溶剂。采用傅里叶变换红外光谱(FT-IR)和核磁共振氢谱(1H NMR)验证了低共熔溶剂成功合成。分别采用紫外-可见吸收光谱和旋转式黏度计对其酸性和黏度进行测试。以双氧水作为氧化剂,以合成的低共熔溶剂为萃取剂和催化剂构成萃取-氧化脱硫系统,考察了低共熔溶剂的组成、反应温度、氧硫比、剂油比以及不同硫化物等对脱硫率的影响。实验结果表明,在氯氧化锆、乙二醇和对苯甲磺酸物质的量比为1∶10∶10,反应温度50 ℃、剂油比为1∶5、氧硫比为8的最佳反应条件下,二苯并噻吩(DBT)、4,6-二甲基二苯并噻吩(4,6-DMDBT)、苯并噻吩(BT)模拟油的脱硫率分别为100%、92.2%、60%,且低共熔溶剂重复使用五次后脱硫率仍可达到96.2%,最后对氧化脱硫的机理进行了探讨。Abstract: A zirconium oxychloride based ternary deep eutectic solvent (DES) was prepared by simply heating mixture of ethylene glycol, p-toluenesulfonic acid and octahydrate zirconium oxychloride. The successful synthesis of deep eutectic solvents was verified using Fourier transform infrared spectroscopy (FT-IR) and nuclear magnetic resonance hydrogen spectroscopy (1H NMR). The acidity and viscosity were tested using UV-visible absorption spectroscopy and rotary viscometer, respectively. The extraction-oxidation desulfurization system was composed of hydrogen peroxide as the oxidant, deep eutectic solvent as the extractant and catalyst. The effects of the composition of the deep eutectic solvent, reaction temperature, oxygen sulfur ratio, solvent oil ratio, and different sulfides on the desulfurization rate were investigated. The experimental results showed that the desulfurization rates of dibenzothiophene (DBT), 4,6-dimethyldibenzothiophene (4,6-DMDBT), and benzothiophene (BT) simulated oil were 100%, 92.2%, and 60%, respectively, under the optimal reaction conditions of a molar ratio of 1:10:10 between zirconium oxychloride, ethylene glycol, and p-benzenesulfonic acid components, 50 ℃, a solvent oil ratio of 1∶5, and an oxygen sulfur ratio of 8. After repeated use of the deep eutectic solvent for 5 times, the desulfurization rate could still reach 96.2%. The mechanism of oxidative desulfurization was explored.
-
Key words:
- deep eutectic solvent /
- oxidative desulfurization /
- DBT /
- zirconium oxychloride
-
表 1 低共熔溶剂酸度的参数
Table 1 Parameters of acidity of deep eutectic solvents
Entry Substance Amax I (HI)+ H0 1 4-nitrodiphenylamine 4.89 100 0 − 2 TsOH 4.49 91.6 8.4 1.04 3 ZOC/TsOH/EG 4.57 93.4 6.4 1.16 4 TsOH/ZOC 4.51 92.2 7.8 1.07 5 TsOH/EG 4.43 90.5 9.5 0.98 -
[1] 纪桂杰, 张耀兵, 付宁宁, 等. Mn/Al-SBA-15 的制备及吸附脱硫性能[J]. 燃料化学学报,2015,43(4):449−455.JI Guijie, ZHANG Yaobing, FU Ningning, et al. Preparation and desulfurization performance of Mn/Al-SBA -15[J]. J Fuel Chem Technol,2015,43(4):449−455. [2] RAJENDRAN A, CUI T, FAN H, et al. A comprehensive review on oxidative desulfurization catalysts targeting clean energy and environment[J]. J Mater Chem A,2020,8(5):2246−2285. doi: 10.1039/C9TA12555H [3] 周隆昌, 刘汉林, 李秀萍, 等. 直接煅烧法制备二氧化钛及其氧化脱硫性能[J]. 辽宁石油化工大学学报,2021,41(5):17−22.ZHOU Longchang, LIU Hanlin, LI Xiuping, et al. Preparation of titanium dioxide by direct calcination and its oxidative desulfurization properties.[J]. Liaoning Univ Pet Chem Technol,2021,41(5):17−22. [4] 郝阳阳, 李秀萍, 赵荣祥. MoO3/MIL-101(Cr)负载型催化剂的制备及其氧化脱硫性能[J]. 化学工程,2019,47(9):24−28. doi: 10.3969/j.issn.1005-9954.2019.09.005HAO Yangyang, LI Xiuping, ZHAO Rongxiang. Preparation and oxidative desulfurization performance of Moo3/mil -101(CR) supported catalyst[J]. Chem Eng,2019,47(9):24−28. doi: 10.3969/j.issn.1005-9954.2019.09.005 [5] ZHANG K, LIU Y, TIAN S, et al. Preparation of bifunctional NiPb/ZnO-diatomite-ZSM-5 catalyst and its reactive adsorption desulfurization coupling aromatization performance in FCC gasoline upgrading process[J]. Fuel,2013,104:201−207. doi: 10.1016/j.fuel.2012.08.052 [6] KOBAYASHI T, LI Y Y. Performance and characterization of a newly developed self-agitated anaerobic reactor with biological desulfurization[J]. Bioresour Technol,2011,102(10):5580−5588. doi: 10.1016/j.biortech.2011.01.077 [7] AGUIAR A, RIBEIRO S, SILVA A M N, et al. An efficient eco sustainable oxidative desulfurization process using µ-oxo-bridged Fe (Ⅲ) complex of meso-tetrakis (pentafluorophenyl) porphyrin[J]. Appl Catal A: Gen,2014,478:267−274. doi: 10.1016/j.apcata.2014.04.002 [8] HOU L, ZHAO R, LI X, et al. Preparation of MoO2/g-C3N4 composites with a high surface area and its application in deep desulfurization from model oil[J]. Appl Surf Sci,2018,434:1200−1209. doi: 10.1016/j.apsusc.2017.10.076 [9] CERUTTI M L M, HACKBARTH F V, MAASS D, et al. Copper-exchanged Y zeolites for gasoline deep-desulfurization[J]. Adsorption,2019,25(8):1595−1609. doi: 10.1007/s10450-019-00153-y [10] DUAN C, DONG L, LI F, et al. Room-temperature rapid synthesis of two-dimensional metal-organic framework nanosheets with tunable hierarchical porosity for enhanced adsorption desulfurization performance[J]. Ind Eng Chem Res,2020,59(42):18857−18864. doi: 10.1021/acs.iecr.0c02437 [11] WANG Q, ZHANG T, ZHANG S, et al. Extractive desulfurization of fuels using trialkylamine-based protic ionic liquids[J]. Sep Purif Technol,2020,231:115923. doi: 10.1016/j.seppur.2019.115923 [12] ABBASI A, FEYZI F. Oxidation/extraction desulfurization with carboxylic acid-based deep eutectic solvents[J]. Pet Sci Technol,2021,42:1−16. [13] MAKOS P, BOCZKAJ G. Deep eutectic solvents based highly efficient extractive desulfurization of fuels-Eco-friendly approach[J]. J Mol Liq,2019,296:111916. doi: 10.1016/j.molliq.2019.111916 [14] BAI J, SONG Y, WANG C, et al. Engineering the electronic structure of Mo sites in Mn–Mo–O mixed-metal oxides for efficient aerobic oxidative desulfurization[J]. Energy Fuels,2021,35(15):12310−12318. doi: 10.1021/acs.energyfuels.1c01476 [15] JIANG W, XIAO J, GAO X, et al. In situ fabrication of hollow silica confined defective molybdenum oxide for enhanced catalytic oxidative desulfurization of diesel fuels[J]. Fuel,2021,305:121470. doi: 10.1016/j.fuel.2021.121470 [16] AGARWAL P, SHARMA D K. Comparative studies on the bio-desulfurization of crude oil with other desulfurization techniques and deep desulfurization through integrated processes[J]. Energy Fuels,2010,24(1):518−524. doi: 10.1021/ef900876j [17] Zhang Q, KDO V, HU X, et al. Deep eutectic solvents: syntheses, properties and applications[J]. Chem Soc Rev,2012,41(21):7108−7146. doi: 10.1039/c2cs35178a [18] CHANDRAN D, KHALID M, WALVEKAR R, et al. Deep eutectic solvents for extraction-desulphurization: A review[J]. J Mol Liq,2019,275:312−322. doi: 10.1016/j.molliq.2018.11.051 [19] TAHIR S, QAZI U Y, NASEEM Z, et al. Deep eutectic solvents as alternative green solvents for the efficient desulfurization of liquid fuel: A comprehensive review[J]. Fuel,2021,305:121502. doi: 10.1016/j.fuel.2021.121502 [20] JIANG W, ZHU K, JIA H, et al. Synthesis of task-specific ternary deep eutectic solvents for deep desulfurization via reactive extraction[J]. Chem Eng Process,2022,171:108754. doi: 10.1016/j.cep.2021.108754 [21] YU G, JIN D, ZHANG F, et al. Oxidation-extraction desulfurization of fuel with a novel green acidic deep eutectic solvent system[J]. Fuel,2022,329:125495. doi: 10.1016/j.fuel.2022.125495 [22] JIN D, YU G, LI X, et al. One-pot extractive and oxidative desulfurization of fuel with ternary dual-acid deep eutectic solvent[J]. Fuel,2022,329:125513. doi: 10.1016/j.fuel.2022.125513 [23] XU L, JIA H, ZHU D, et al. Hydrogen bonding boosted oxidative desulfurization by ZnCl2/boric acid/polyethylene glycol-based ternary deep eutectic solvents[J]. J Mol Liq,2022,368:120725. doi: 10.1016/j.molliq.2022.120725 [24] GANO Z S, MJALLI F S, AL-WAHAIBI T, et al. Solubility of thiophene and dibenzothiophene in anhydrous FeCl3-and ZnCl2-based deep eutectic solvents[J]. Ind Eng Chem Res,2014,53(16):6815−6823. doi: 10.1021/ie500466g [25] MAO C, ZHAO R, LI X. Propionic acid-based deep eutectic solvents: Synthesis and ultra-deep oxidative desulfurization activity[J]. RSC Adv,2017,7(67):42590−42596. doi: 10.1039/C7RA05687G [26] GANO Z S, MJALLI F S, AL-WAHAIBI T, et al. Extractive desulfurization of liquid fuel with FeCl3-based deep eutectic solvents: experimental design and optimization by central-composite design[J]. Chem Eng Process,2015,93:10−20. doi: 10.1016/j.cep.2015.04.001 [27] RAHMATPOUR A. ZrOCl2·8H2O as a highly efficient, eco-friendly and recyclable Lewis acid catalyst for one-pot synthesis of N-substituted pyrroles under solvent-free conditions at room temperature[J]. Appl Organomet Chem,2011,25(8):585−590. doi: 10.1002/aoc.1806 [28] KHALILI B, MAHMOODI N, AFRAND H. Efficient synthesis of 1-Aryl-1H-tetrazols in presence of ZrOCl2. 8H2O and quantum chemical study of the products using DFT[J]. Russ J Appl Chem,2019,14(52):199−216. [29] HALIMEHJANI A Z, KESHAVARZI N. One-pot three-component route for the synthesis of functionalized 4H-chromenes catalyzed by ZrOCl2·8H2O in water[J]. J Heterocycl Chem,2018,55(2):522−529. doi: 10.1002/jhet.3081 [30] SING H R, JAKHAR K, SHARMA P. ZrOCl2. 8H2O: An efficient catalyst for the synthesis of N, N’-disubstituted ureas from biuret under solvent free conditions[J]. Chem Sci,2017,6(1):135−140. [31] REDDY M V, REDDY G C S, JEONG Y T. Polystyrene-supported p-toluenesulfonic acid (PS/PTSA): as a highly active and reusable heterogeneous bronsted acid catalyst for the synthesis of novel 1H-indol-3-yl-4H-chromene-3-carbonitriles under neat conditions[J]. Tetrahedron Lett,2016,57(11):1289−1292. doi: 10.1016/j.tetlet.2016.02.032 [32] LIU W, LI T, YU G, et al. One-pot oxidative desulfurization of fuels using dual-acidic deep eutectic solvents[J]. Fuel,2020,265:116967. doi: 10.1016/j.fuel.2019.116967 [33] HAO L, WANG M, SHAN W, et al. L-proline-based deep eutectic solvents (DESs) for deep catalytic oxidative desulfurization (ODS) of diesel[J]. J Hazard Mater,2017,339:216−222. doi: 10.1016/j.jhazmat.2017.06.050 [34] 张红, 余肇誉, 苏远海. 微反应器耦合离子液体强化萃取过程的研究进展[J]. 化工进展,2020,39(12):4908−4918.ZHANG Hong, YU Zhaoyu, SU Yuanhai. Research progress in enhanced extraction process by coupling ionic liquids in microreactors[J]. Prog Chem,2020,39(12):4908−4918. [35] GHAEDI H, AYOUB M, SUFIAN S, et al. Thermal stability and FT-IR analysis of phosphonium-based deep eutectic solvents with different hydrogen bond donors[J]. J Mol Liq,2017,242:395−403. doi: 10.1016/j.molliq.2017.07.016 [36] DUTTA A, GARG A, BORAH J, et al. Deep eutectic solvent mediated controlled and selective oxidation of organic sulfides and hydroxylation of arylboronic acids[J]. Curr Opin Green Sustainable Chem,2021,4:100107. doi: 10.1016/j.crgsc.2021.100107 [37] JIANG W, JIA H, LI H, et al. Boric acid-based ternary deep eutectic solvent for extraction and oxidative desulfurization of diesel fuel[J]. Green Chem,2019,21(11):3074−3080. doi: 10.1039/C9GC01004A [38] ALOMAR M K, HAYYAN M, ALSAADI M A, et al. Glycerol-based deep eutectic solvents: physical properties[J]. J Mol Liq,2016,215:98−103. doi: 10.1016/j.molliq.2015.11.032 [39] 赵岩, 李秀萍, 赵荣祥. 苯酚型低共熔溶剂中硫酸钛作为催化剂高效氧化脱硫[J]. 化工学报,2021,72(8):4391−4400.ZHAO Yan, LI Xiuping, ZHAO Rongxiang. High efficient oxidative desulfurization using titanium sulfate as catalyst in phenol-based low eutectic solvent[J]. J Chem Eng,2021,72(8):4391−4400. [40] 王韵淇, 李珊珊, 汤梦蝶, 等. OA-ZnCl2/SG催化剂的合成及其氧化脱硫性能[J]. 辽宁石油化工大学学报,2022,42(4):11−16.WANG Yunqi, LI Shanshan, TANG Mengdie, et al. Synthesis and oxidative desulfurization of OA-ZnCl2/SG Catalyst[J]. Liaoning Univ. Pet Chem Technol.,2022,42(4):11−16. [41] ZHU W, WU P, YANG L, et al. Pyridinium-based temperature-responsive magnetic ionic liquid for oxidative desulfurization of fuels[J]. Chem Eng J,2013,229:250−256. doi: 10.1016/j.cej.2013.05.115 [42] ZHANG C, PAN X, WANG F, et al. Extraction-oxidation desulfurization by pyridinium-based task-specific ionic liquids[J]. Fuel,2012,102:580−584. doi: 10.1016/j.fuel.2012.07.040 [43] LV H, GAO J, JIANG Z, et al. Ultra-deep desulfurization of diesel by selective oxidation with [C18H37N (CH3)3]4[H2NaPW10O36] catalyst assembled in emulsion droplets[J]. J Catal,2006,239(2):369−375. doi: 10.1016/j.jcat.2006.01.025 [44] SHIRAISHI Y, TACHIBANA K, HIRAI T, et al. Desulfurization and denitrogenation process for light oils based on chemical oxidation followed by liquid-liquid extraction[J]. Ind Eng Chem Res,2002,41(17):4362−4375. doi: 10.1021/ie010618x [45] LIU H, CHEN S, LI X, et al. Preparation of DEP/2C3H4O4 DESs and its oxidative desulfurization performance[J]. Sep Sci Technol,2021,56(3):558−566. doi: 10.1080/01496395.2020.1717532 -





 下载:
下载: