Reconstruction of copper nanoparticles catalysts and its catalytic performance for synthesis of dimethyl carbonate
-
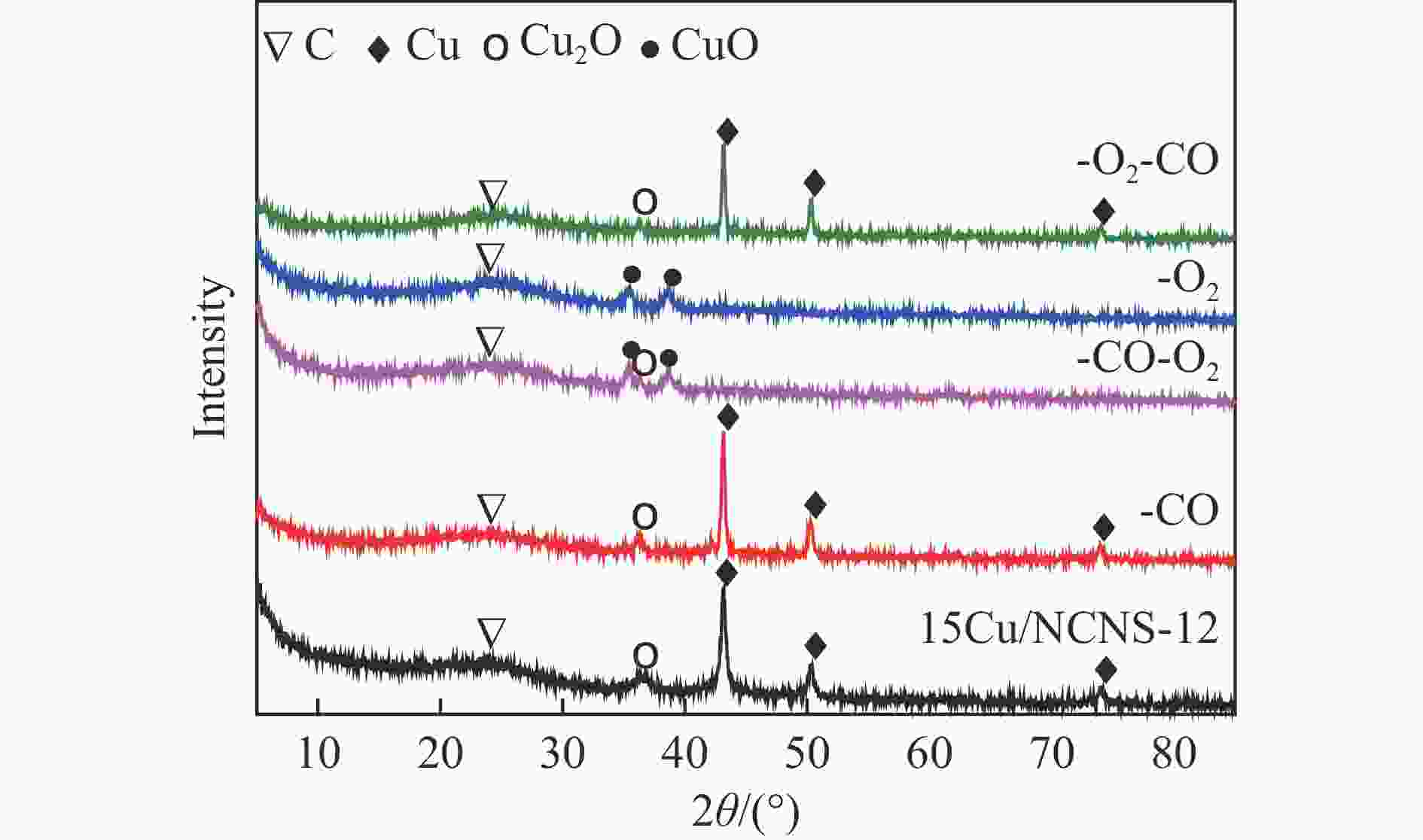
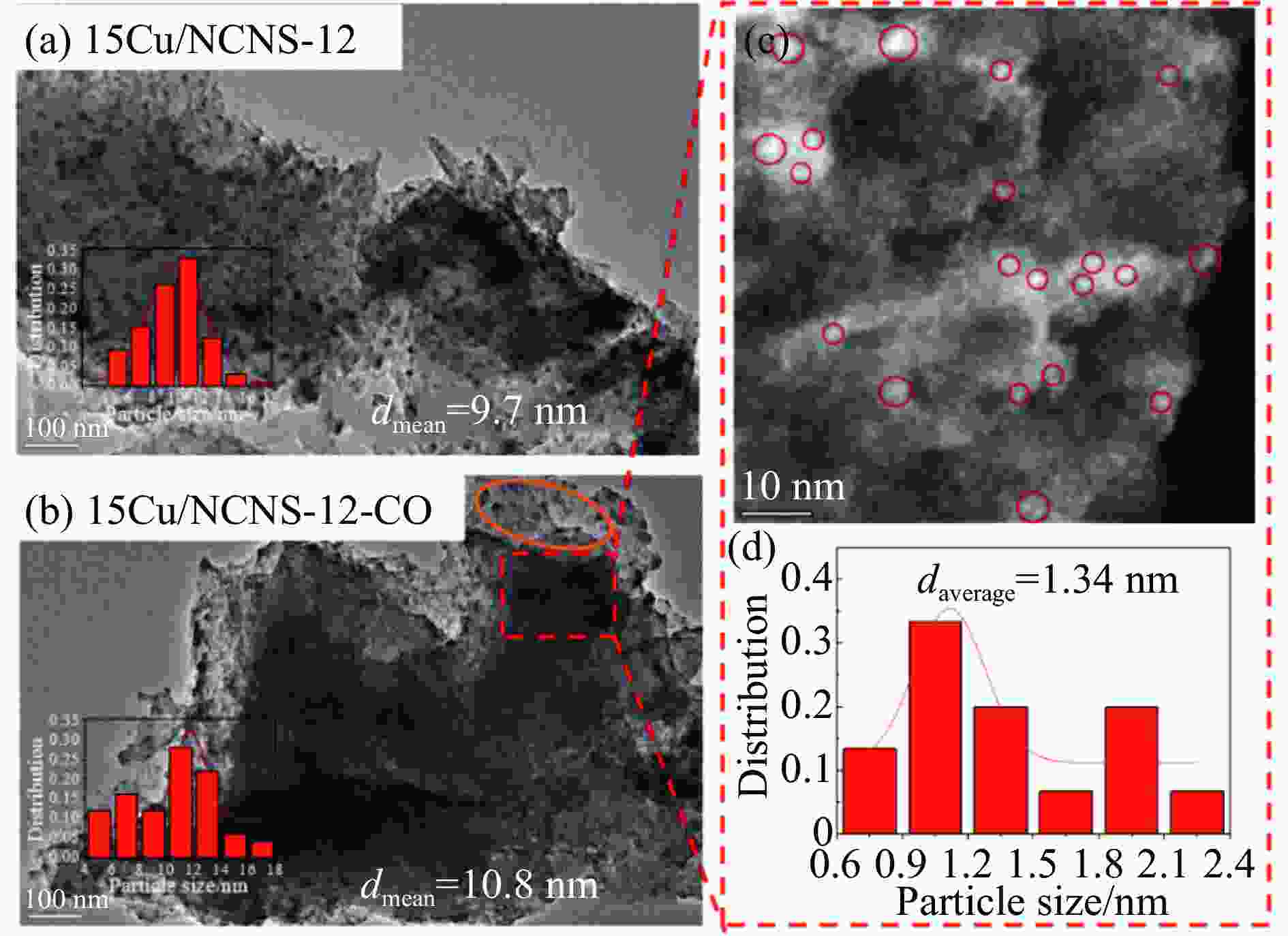
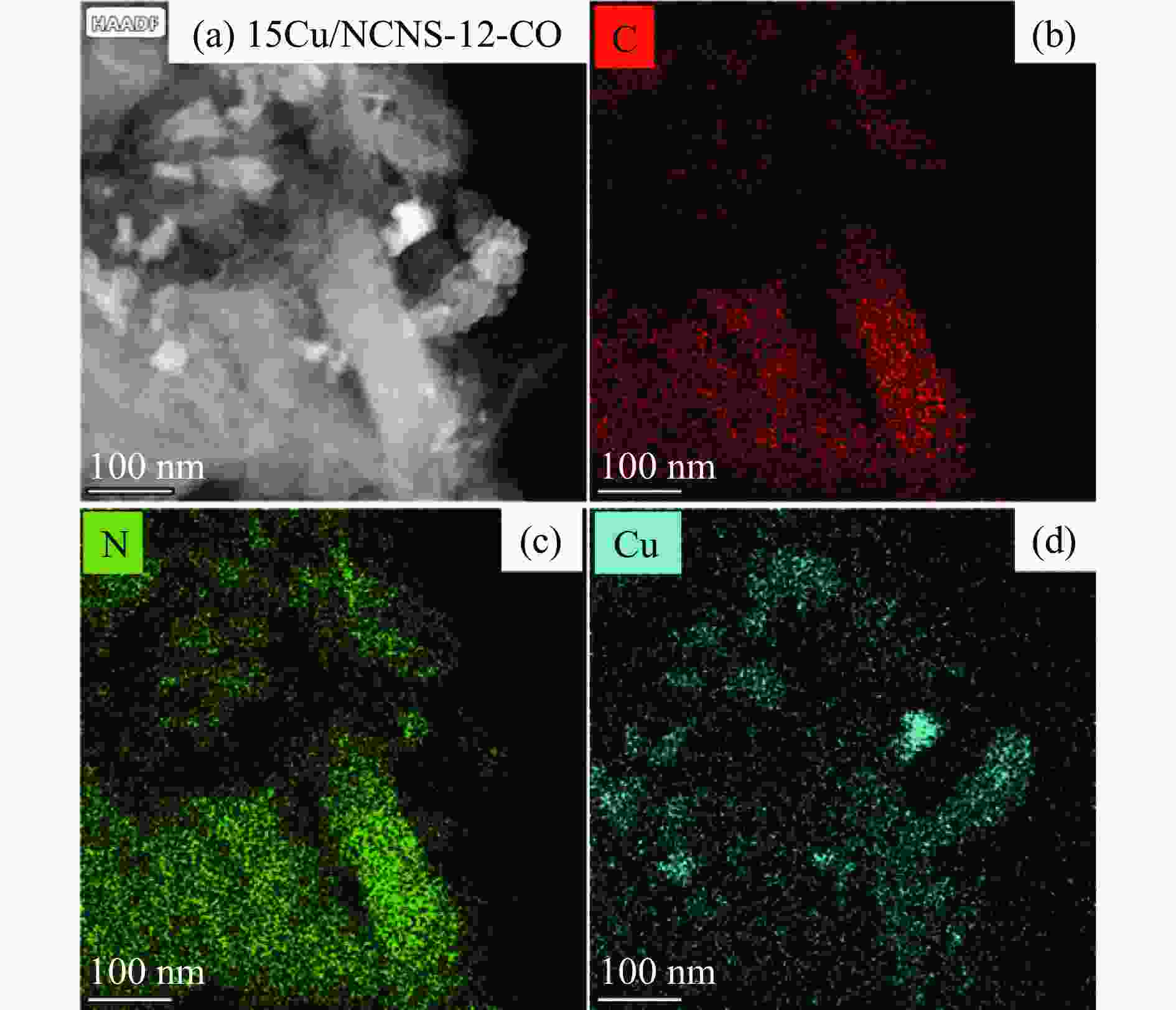
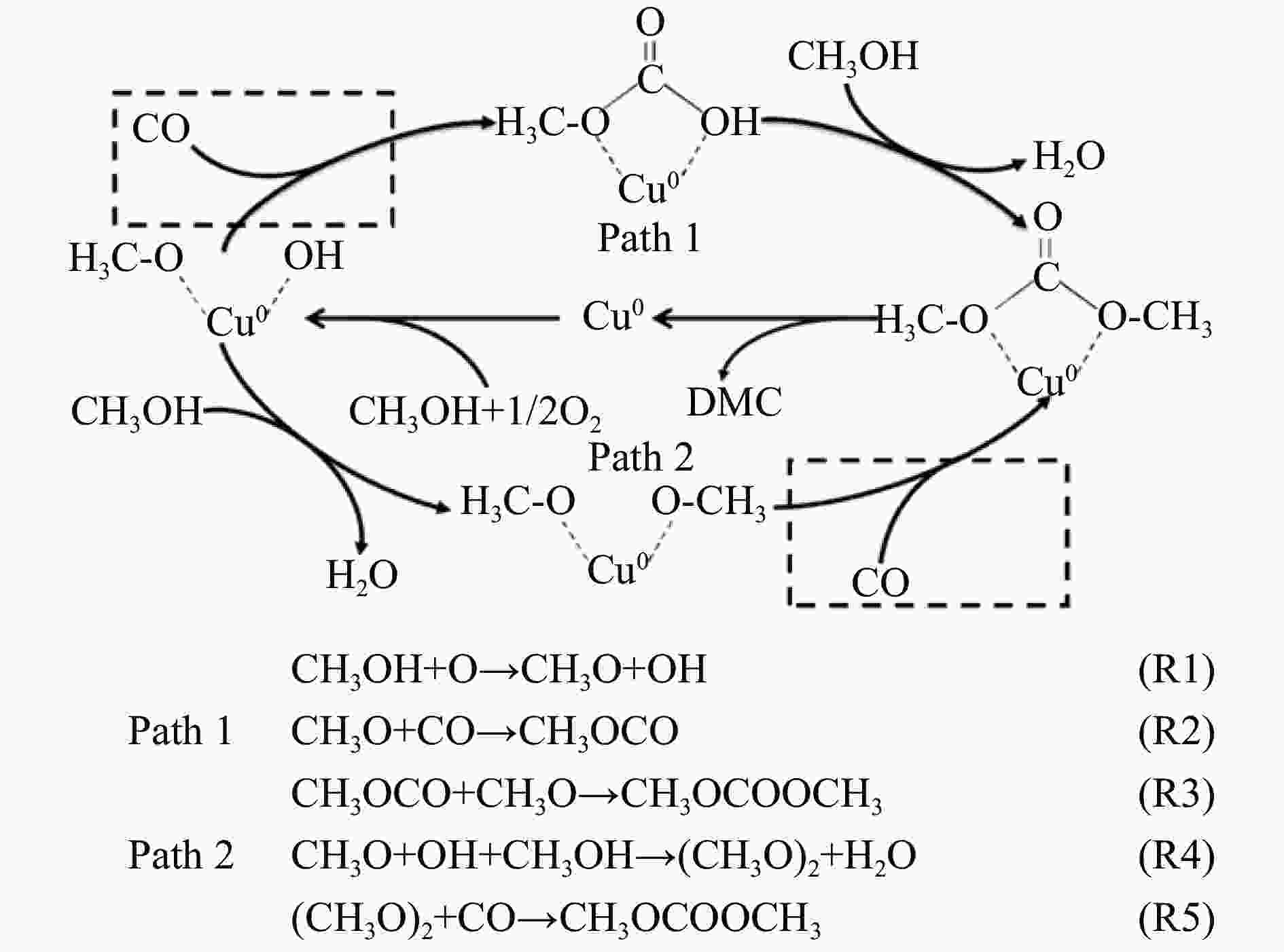
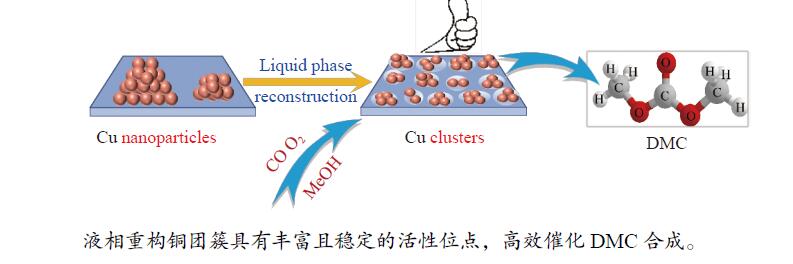
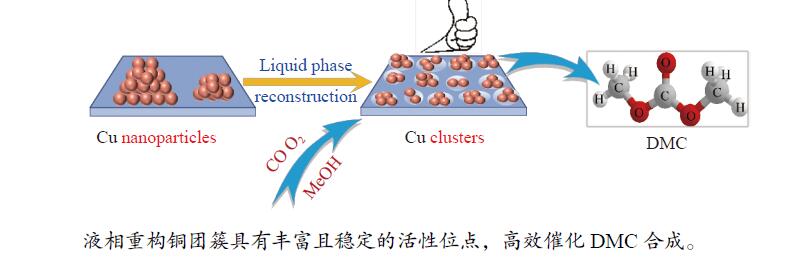
摘要: 甲醇氧化羰基化合成碳酸二甲酯(DMC)是中国重点开发的现代煤化工路线,其关键在于高性能催化剂的设计研发。本研究采用液相重构法,通过调变溶剂种类、气氛和压力制得高效铜团簇催化剂,在DMC合成反应中时空收率(STYDMC)高达3520 mg/(g·h),经过10次循环,STYDMC下降21.8%。N2吸附-脱附、XRD、TEM、STEM等表征手段表明,强还原性的CO不但可使铜纳米颗粒(~9.7 nm)部分重构为亚纳米团簇(~1.34 nm),同时有效维持了Cu0物种的存在,从而提升了催化活性和稳定性。进一步研究表明,铜重构的发生取决于气氛-金属-载体三者的相互作用,且随氧化、还原性气氛的变化呈可逆状态。Abstract: Dimethyl carbonate (DMC) is an important and environmentally friendly chemical intermediate to meet the growing demand for a clean and sustainable energy supply. Among several routes for DMC synthesis, the oxidative carbonylation of methanol has attracted much attention with the advantages of a high utilization rate of carbon source, moderate operating conditions and environmental benefits. More importantly, the oxidative carbonylation of methanol is an important development route of modern coal chemical industry in China, and the key lies in the design of highly efficient catalysts. Copper-based catalysts have been used extensively in this reaction. Problems, such as reactor corrosion and catalyst deactivation, occur with chlorine-containing catalysts. The development of chlorine-free catalysts is the focus of current research. Recently, Cu-based catalysts supported on carbon materials have been used extensively in DMC synthesis because of its high activity, high selectivity and facile preparation process. However, the carbon-supported Cu catalysts suffer from the leaching and aggregation of Cu nanoparticles (NPs) in the harsh reaction conditions of high temperature, high pressure as well as severe stirring, leading to the deactivation. It has become a key scienctific problem that needs to be addressed urgently. In our previous studies, several strategies have been attempted to solve the deactivation problem caused by these reasons. For instance, encapsulating Cu NPs with hollow porous carbon spheres or mesoporous carbon materials. Besides, the introduction of N species in the carbon framework or sulfonic acid groups and oxygen-containing groups on the surface of carbon materials leads to an anchoring effect on Cu NPs. Great progress has been made via these methods, yet still unsatisfactory. Supported metal clusters have adjacent metal sites, countable numbers of atoms in each clusters, and limited size range (normally smaller than 2 nm). Benefiting from these distinct geometric and electronic structures, supported metal clusters can trigger synergistic effects among every metal atom, and thus exhibiting enhanced catalytic activity and selectivity in catalysis. Besides, the strong metal-support interaction on supported metal clusters improve the stability of metal clusters, enhancing the catalytic stability. To prevent the aggregation of metal clusters, the metal loading of supported metal clusters catalysts are generally kept at a low level (≤1%). However, catalysts with insufficient numbers of active sites always lead to compromised mass-activity, which greatly restrict them from industrial applications. Hence, the synthesis of supported metal clusters with high metal loading and high stability is a great challenge. In this study, the Cu clusters catalysts with high Cu loading were synthesized via liquid phase reconstruction method under the condition of water and CO. The optimal 15Cu/NCNS-12-CO exhibited superb activity with STYDMC of 3520 mg/(g·h) and stability with the loss rate of 28% after 10 cycles. A series of characterization showed that the strongly reducing CO not only resulted in the partial reconstruction of copper nanoparticles (from ~9.7 nm to ~1.34 nm), but also effectively maintained the existence of Cu0 species, improving the catalytic activity and stability. Further investigation showed that the reconstruction of Cu nanoparticles was dependent on the interaction of atmosphere-metal-support, and was reversible under the oxidation and reducing atmosphere.
-
表 1 不同预处理条件下制备的催化剂在DMC合成中的催化性能
Table 1 Catalytic performance of various catalysts prepared in different pretreatment conditions in the synthesis of DMC
No. Sample Solvent Pressure/
MPaAtmosphere CMeOH/
%SDMC/
%STYDMC/
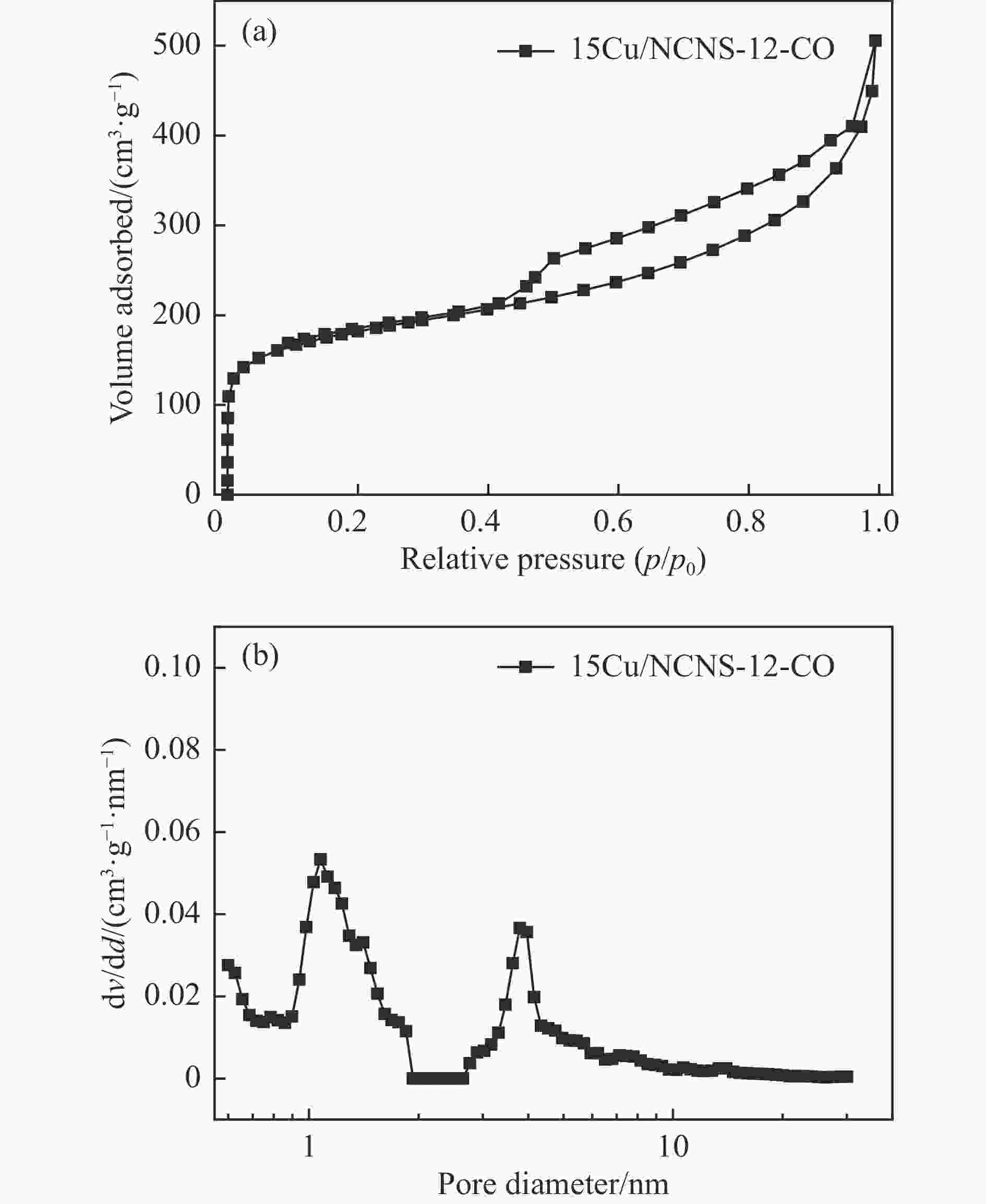
(mg·g−1·h−1)1 15Cu/NCNS-12-MeOH methanol 1 CO 6.47 96.1 3464 2 15Cu/NCNS-12-CO water 1 CO 6.67 94.84 3520 3 15Cu/NCNS-12-3CO water 3 CO 5.70 94.70 2995 4 15Cu/NCNS-12-H2 water 1 H2 6.42 91.16 3261 5 15Cu/NCNS-12-N2 water 1 N2 6.30 94.21 3307 6 15Cu/NCNS-12-CO2 water 1 CO2 6.54 89.83 3272 7 15Cu/NCNS-12-O2 water 1 O2 1.85 91.93 1562 表 2 15Cu/NCNS-12-CO催化剂的孔结构参数和实际铜负载量
Table 2 Structural properties and practical Cu loading of 15Cu/NCNS-12-CO catalysts
Sample Cu loading/% SBET/(m2·g−1) vtotal/(cm3·g−1) vmicro/(cm3·g−1) vmeso/(cm3·g−1) 15Cu/NCNS-12-CO 17.4 594 0.71 0.16 0.55 表 3 NCNS-12的CHN元素分析
Table 3 Carbon, hydrogen, and nitrogen element analysis of NCNS-12
Sample C/% O/% H/% N/% NCNS-12 75.6 18.5 2.1 3.8 表 4 15Cu/NCNS-12、15Cu/NCNS-12-CO和CuCl催化剂的活性及稳定性评价结果
Table 4 Catalytic results for DMC synthesis on the 15Cu/NCNS-12, 15Cu/NCNS-12-CO and CuCl catalysts
No. Sample xMeOH/% sDMC/% sDMM/% sMF/% STYDMC/(mg·g−1·h−1) 1 15Cu/NCNS-12 7.1 91.2 0.7 8.1 3604 2 15Cu/NCNS-12-10run 4.3 99.3 0.7 0 2382 3 15Cu/NNS-12-CO 6.7 94.8 1.3 3.9 3520 4 15Cu/NCNS-12-CO-10run 5.3 92.7 1.3 6.0 2753 5 CuCl 8.2 85.0 6.9 8.1 16677 6 CuCl-3run 0.63 65.1 20.7 14.2 976 -
[1] 孙旭东, 张蕾欣, 张博. 碳中和背景下我国煤炭行业的发展与转型研究[J]. 中国矿业,2021,30(2):1−6.SUN Xudong, ZHANG Leixin, ZHANG Bo. Research on the development and transformation of China's coal industry under the background of carbon neutrality[J]. Min Mag,2021,30(2):1−6. [2] TAN H , WANG Z , XU Z , et al. Review on the synthesis of dimethyl carbonate[J]. Catal Today,2018,316:2−12. [3] REN M, REN J, HAO P, et al. Influence of microwave irradiation on the structural properties of carbon-supported hollow copper nanoparticles and their effect on the synthesis of dimethyl carbonate[J]. ChemCatChem,2016,8(4):861−871. [4] ZHANG G, LI Z, ZHENG H, et al. Influence of the surface oxygenated groups of activated carbon on preparation of a nano Cu/AC catalyst and heterogeneous catalysis in the oxidative carbonylation of methanol[J]. Appl Catal B: Environ,2015,179:95−105. doi: 10.1016/j.apcatb.2015.05.001 [5] 王瑞玉, 李忠, 郑华艳等. 无氯Cu/AC催化剂的制备及其催化气相甲醇氧化羰基化反应性能[J]. 催化学报,2010,31(7):851−856.WANG Ruiyu, LI Zhong, ZHENG Huayan, et al. Preparation of chlorine-free Cu/AC catalyst and its catalytic properties for vapor phase oxidative carbonylation of methanol[J]. Chin J Catal,2010,31(7):851−856. [6] WANG Y, JIANG R, ZHAO X, et al. Synthesis of dimethyl carbonate by gas-phase oxidative carbonylation of methanol over activated carbon-supported copper catalysts[J]. J Nat Gas Chem,2000,9(3):205−211. [7] MERZA G, LÁSZLÓ B, OSZKÓ A, et al. The synthesis of dimethyl carbonate by the oxicarbonylation of methanol over Cu supported on carbon norit[J]. Catal Lett,2014,145(3):881−892. [8] ZHANG G, ZHAO D, YAN J, et al. The promotion and stabilization effects of surface nitrogen containing groups of CNT on Cu-Based nanoparticles in the oxidative carbonylation reaction[J]. Appl Catal A: Gen,2019,579(79):18−29. [9] SHI R, WANG J, ZHAO J, et al. Cu nanoparticles encapsulated with hollow carbon spheres for methanol oxidative carbonylation: Tuning of the catalytic properties by particle size control[J]. Appl Surf Sci,2018,459:707−715. doi: 10.1016/j.apsusc.2018.08.032 [10] ZHANG G, ZHAO D, YAN J, et al. The promotion and stabilization effects of surface nitrogen containing groups of CNT on Cu-based nanoparticles in the oxidative carbonylation reaction[J]. Appl Catal A: Gen,2019,579:18−29. doi: 10.1016/j.apcata.2019.04.012 [11] WANG J, SHI R, HAO P, et al. Influence of oxygen-containing groups of activated carbon aerogels on copper/activated carbon aerogels catalyst and synthesis of dimethyl carbonate[J]. J Mater Sci,2018,53(3):1833−1850. doi: 10.1007/s10853-017-1639-8 [12] ZHAO J, SHI R, QUAN Y, et al. Highly efficient synthesis of dimethyl carbonate over copper catalysts supported on resin-derived carbon microspheres[J]. Chem Eng Sci.,2019,207:1060−1071. doi: 10.1016/j.ces.2019.07.039 [13] SHI R, ZHAO J, LIU S, et al. Nitrogen-doped graphene supported copper catalysts for methanol oxidative carbonylation: enhancement of catalytic activity and stability by nitrogen species[J]. Carbon,2018,130:185−195. doi: 10.1016/j.carbon.2018.01.011 [14] REN J, HAO P, SUN W, et al. Ordered mesoporous silica-carbon-supported copper catalyst as an efficient and stable catalyst for catalytic oxidative carbonylation[J]. Chem Eng J,2017,328:673−682. doi: 10.1016/j.cej.2017.07.101 [15] SHI R, REN M, LI H, et al. Graphene supported cu nanoparticles as catalysts for the synthesis of dimethyl carbonate: Effect of carbon black intercalation[J]. Mol Catal,2018,445:257−268. doi: 10.1016/j.mcat.2017.12.002 [16] PENG M, DONG C, GAO R, et al. Fully Exposed Cluster Catalyst (FECC): Toward rich surface sites and full atom utilization efficiency[J]. ACS Cent Sci,2020,7(2):262−273. [17] DONG C, LI Y, CHENG D, et al. Supported metal clusters: Fabrication and application in heterogeneous catalysis[J]. ACS Catal,2020,10:11011−11045. doi: 10.1021/acscatal.0c02818 [18] HU Q, HAN Z, WANG X, et al. Facile synthesis of sub-Nanometric copper clusters by double confinement enables selective reduction of carbon dioxide to methane[J]. Angew Chem,2020,59(43):19054−19059. doi: 10.1002/anie.202009277 [19] ZHANG Z, SU J, MATIAS ANA SANZ, et al. Enhanced and stabilized hydrogen production from methanol by ultrasmall Ni nanoclusters immobilized on defect-rich h-BN nanosheets[J]. PANS,2020,117(47):29442−29452. doi: 10.1073/pnas.2015897117 [20] HERZING A A, KIELY C J, CARLEY A F, et al. Identification of active gold nanoclusters on iron oxide supports for CO oxidation[J]. Science,2008,321(5894):1331−1335. doi: 10.1126/science.1159639 [21] KADEN W, WU T, KUNKEL W, et al. Electronic structure controls reactivity of size-selected Pd clusters adsorbed on TiO2 surfaces[J]. Science,2009,326(5954):826−830. doi: 10.1126/science.1180297 [22] WATANABE Y, WU X, HIRATA H, et al. Size-dependent catalytic activity and geometries of size-selected Pt clusters on TiO2(110) surfaces[J]. Catal Sci Technol,2011,1:1490−1495. doi: 10.1039/c1cy00204j [23] GUAN E, CISTON J, BARE S R, et al. Supported metal pair-site catalysts[J]. ACS Catal,2020,10(16):9065−9085. doi: 10.1021/acscatal.0c02000 [24] YIN P, LUO X, MA Y, et al. Sulfur stabilizing metal nanoclusters on carbon at high temperatures[J]. Nat Commun,2021,12:3435. doi: 10.1038/S41467-021-23426-Z [25] PEI Y, QUAN Y, WANG X, et al. Surface reconstruction induced highly efficient N-doped carbon nanosheet supported copper cluster catalysts for dimethyl carbonate synthesis[J]. Appl Catal B: Environ,2022,300:120718. doi: 10.1016/j.apcatb.2021.120718 [26] YU X, ZHAO J, LV R, et al. Facile synthesis of nitrogen-doped carbon nanosheets with hierarchical porosity for high performance supercapacitors and lithium-sulfur batteries[J]. J Mater Chem A,2015,3(36):18400−18405. doi: 10.1039/C5TA05374A [27] HUANG W, ZHANG H, HUANG Y, et al. Hierarchical porous carbon obtained from animal bone and evaluation in electric double-layer capacitors[J]. Carbon N Y,2011,49(3):838−843. doi: 10.1016/j.carbon.2010.10.025 [28] XU G, HAN J, DING B, et al. Biomass-derived porous carbon materials with sulfur and nitrogen dual-doping for energy storage[J]. Green Chem,2015,17(3):1668−1674. doi: 10.1039/C4GC02185A [29] ZHAO Y Q, LU M, TAO P Y, et al. Hierarchically porous and heteroatom doped carbon derived from tobacco rods for supercapacitors[J]. J Power Sources,2016,307:391−400. doi: 10.1016/j.jpowsour.2016.01.020 [30] CHEN J, WANG H, WANG Z, et al. Redispersion of Mo-based catalysts and the rational design of super small-sized metallic Mo species[J]. ACS Catal,2019,9:5302−5307. doi: 10.1021/acscatal.8b04634 [31] LI R, XU X, ZHU B, et al. In situ identification of the metallic state of Ag nanoclusters in oxidative dispersion[J]. Nat Commun,2021,12:1406. doi: 10.1038/s41467-021-21552-2 [32] BLIEM R, HOEVEN V, HULVA J, et al. Dual Role of CO in the Stability of Subnano Pt Clusters at the Fe3O4(001) Surface[J]. Proc Natl Acad Sci U S A,2016,113(32):8921−8926. doi: 10.1073/pnas.1605649113 [33] NAGAI Y, DOHMAE K, IKEDA Y, et al. In situ redispersion of platinum autoexhaust catalysts: An on-line approach to increasing catalyst lifetimes?[J]. Angew Chem,2008,47(48):9303−9306. doi: 10.1002/anie.200803126 [34] WELLER S, MONTAGNA A. O2 chemisorption at high temperatures on platinum-alumina and platinum-zeolite[J]. J Catal,1971,20(3):394−407. doi: 10.1016/0021-9517(71)90102-3 [35] KARTUSCH C, KRUMEICH F, SAFONOVA O, et al. Redispersion of gold multiple-twinned particles during liquid-phase hydrogenation[J]. ACS Catal,2012,2(7):1394−1403. doi: 10.1021/cs300075k -




 下载:
下载: