Preparation of Co/Zr/Al2O3-Pt/ZSM-5 catalysts for syngas to liquid fuels
-
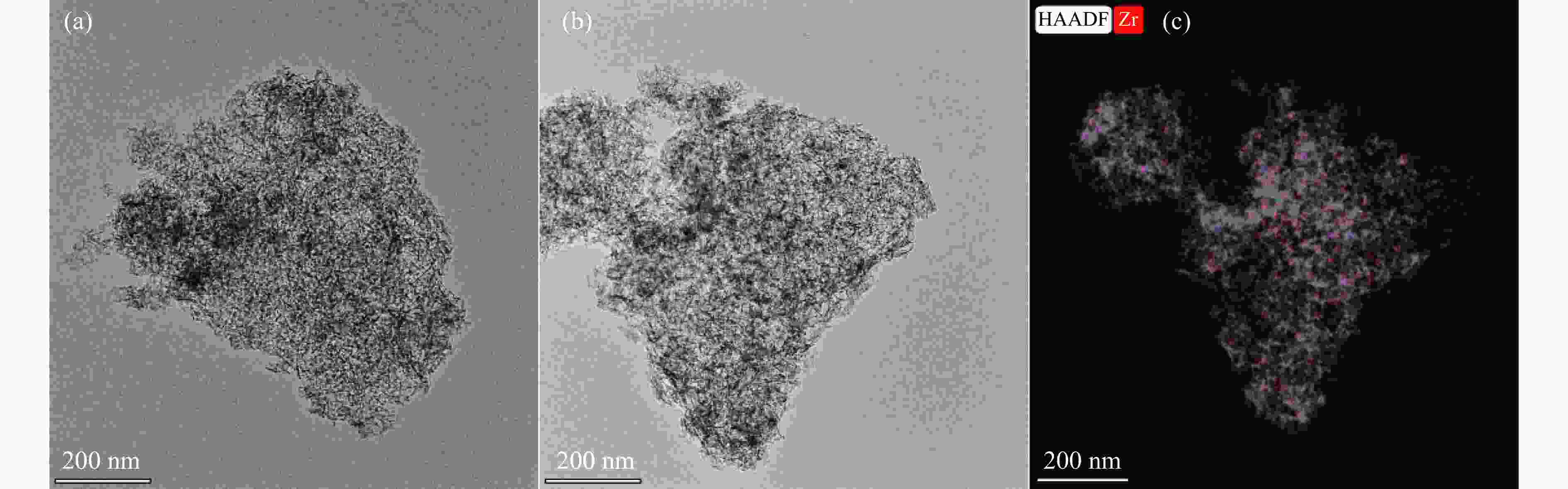
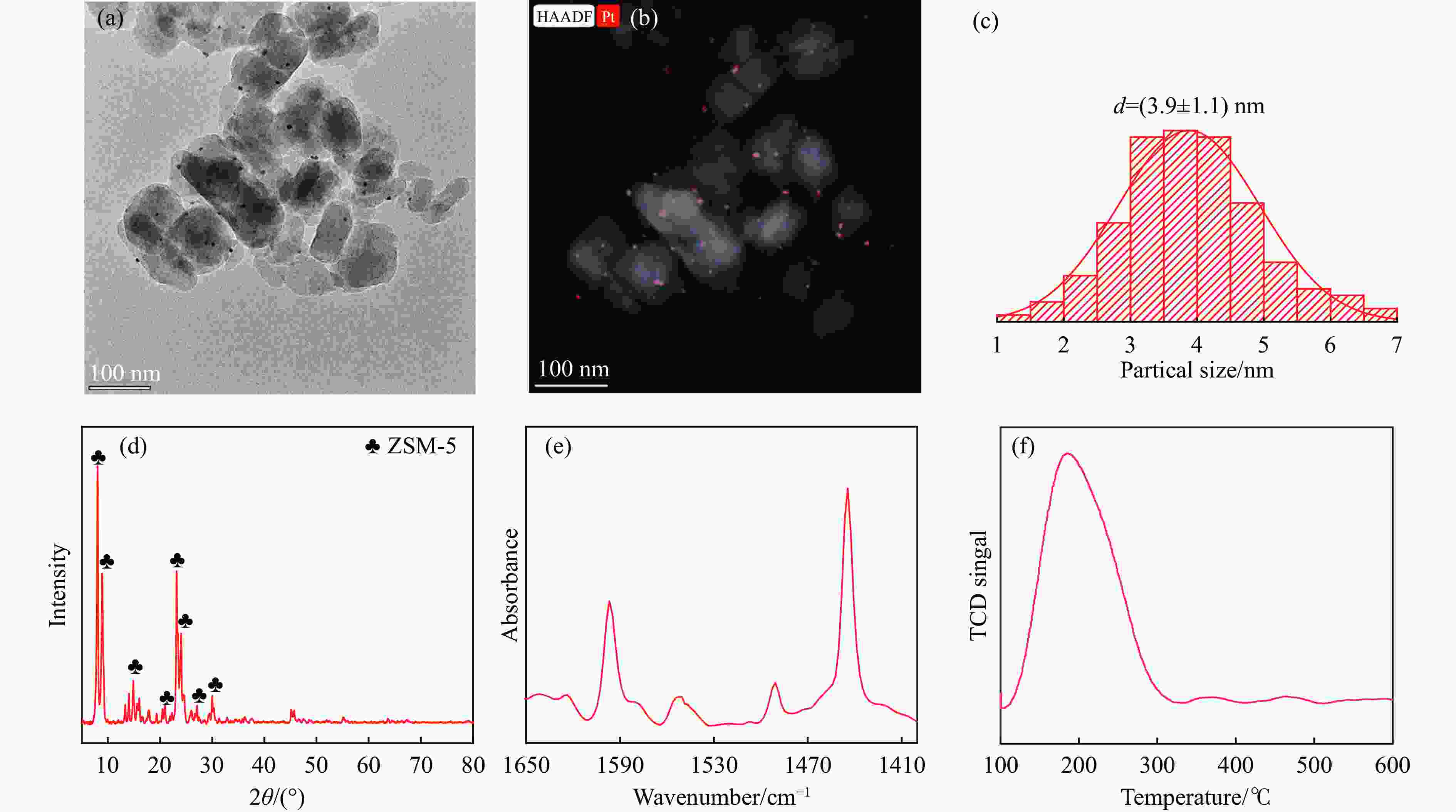
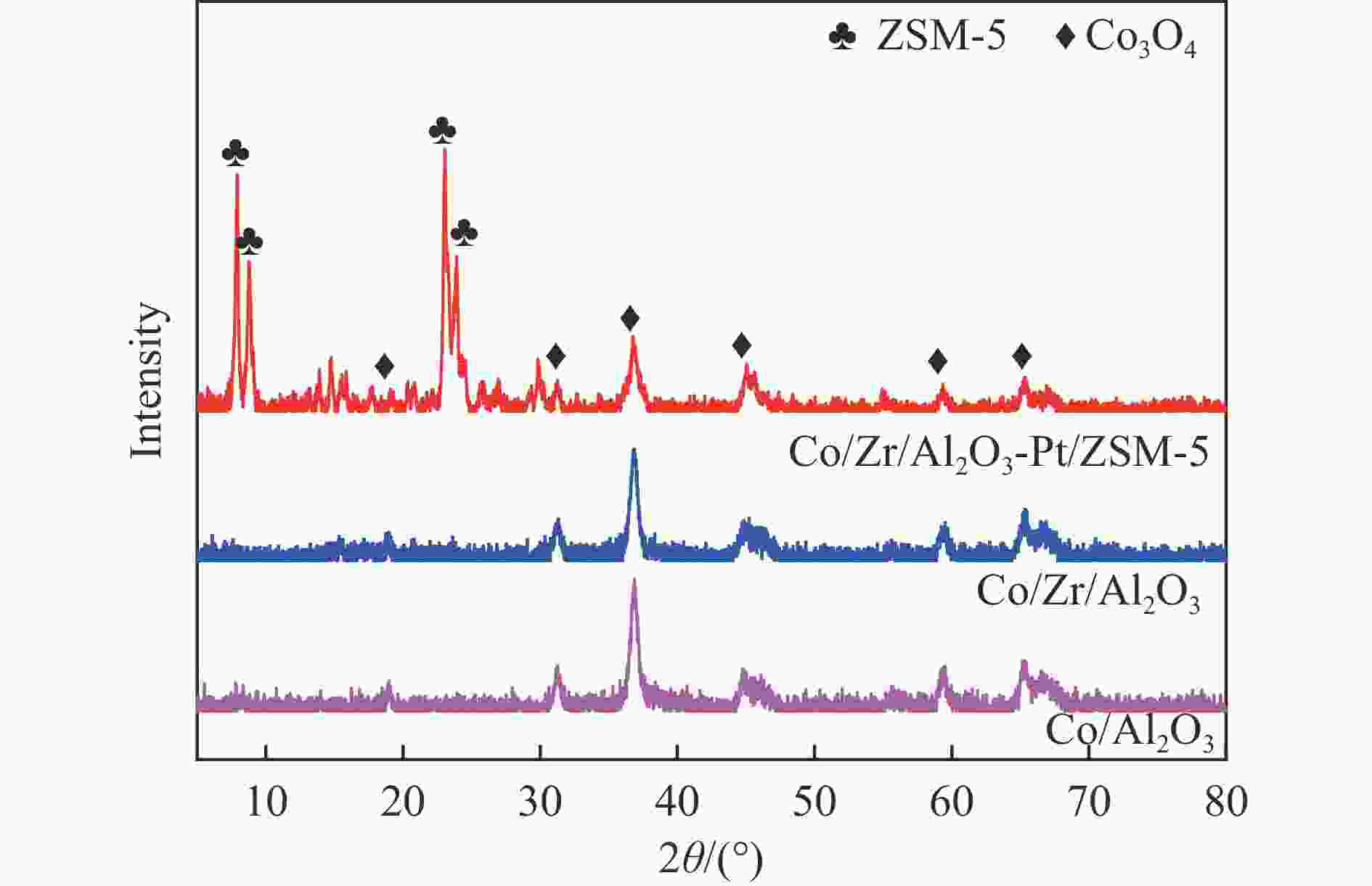
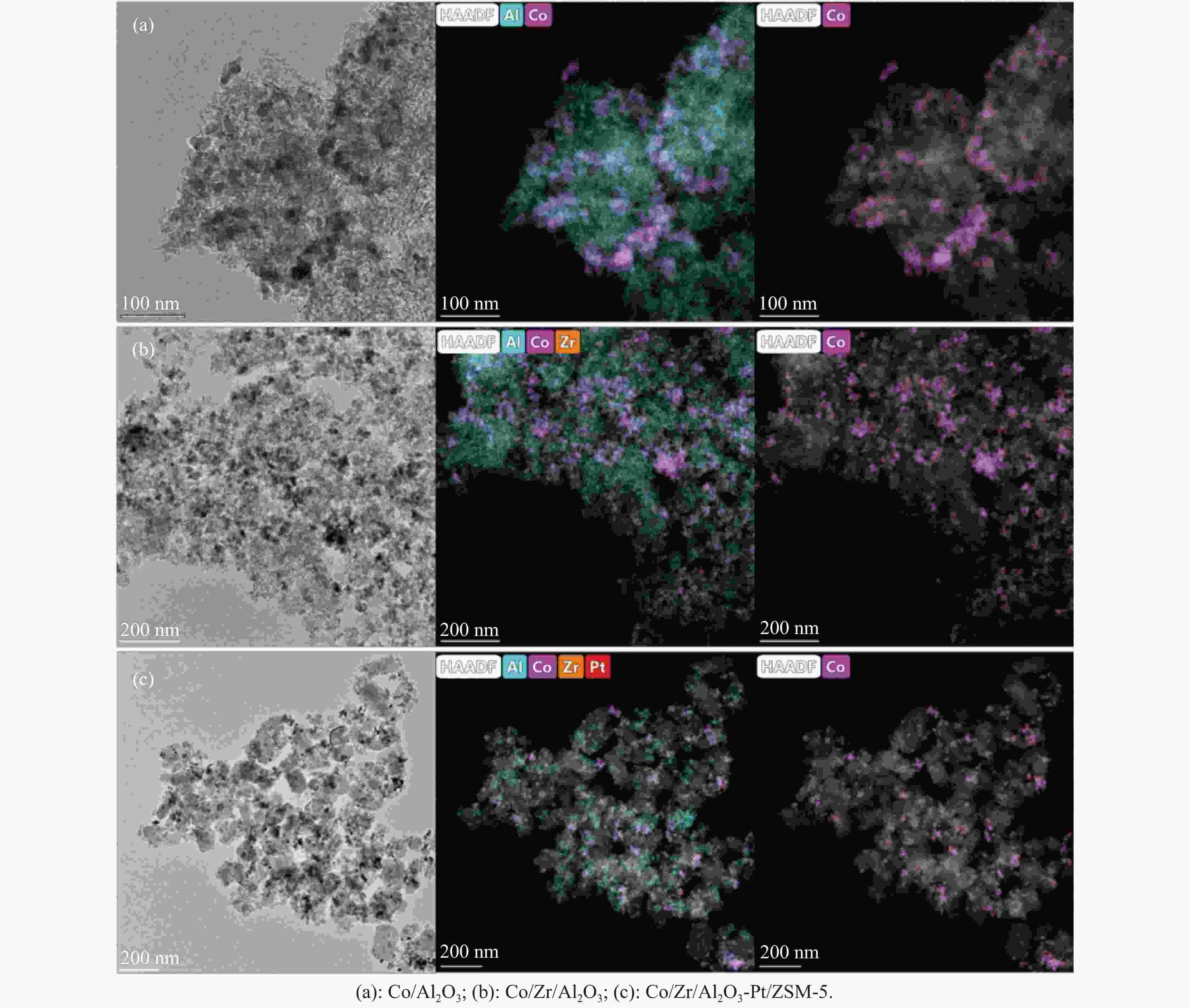
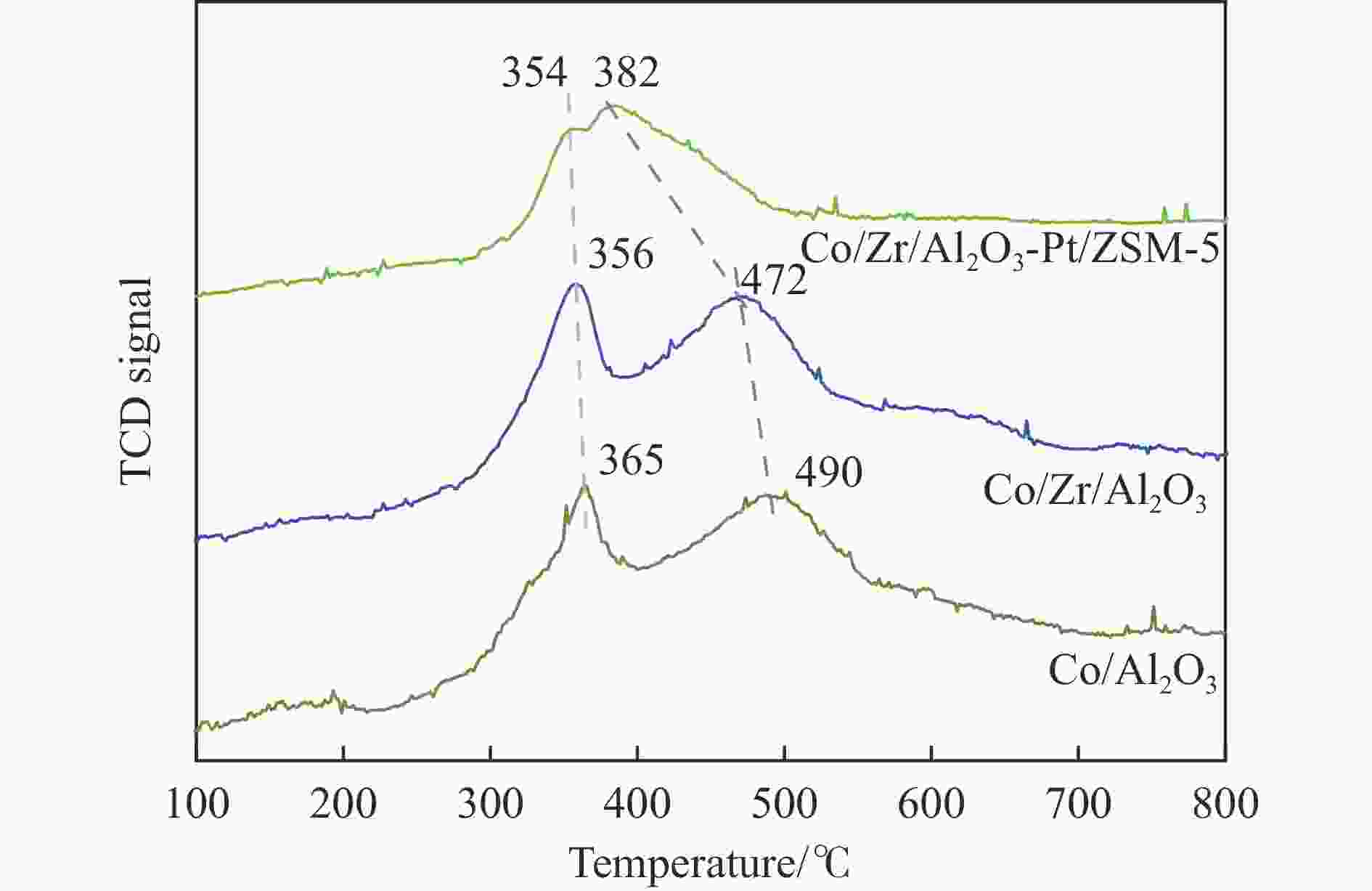
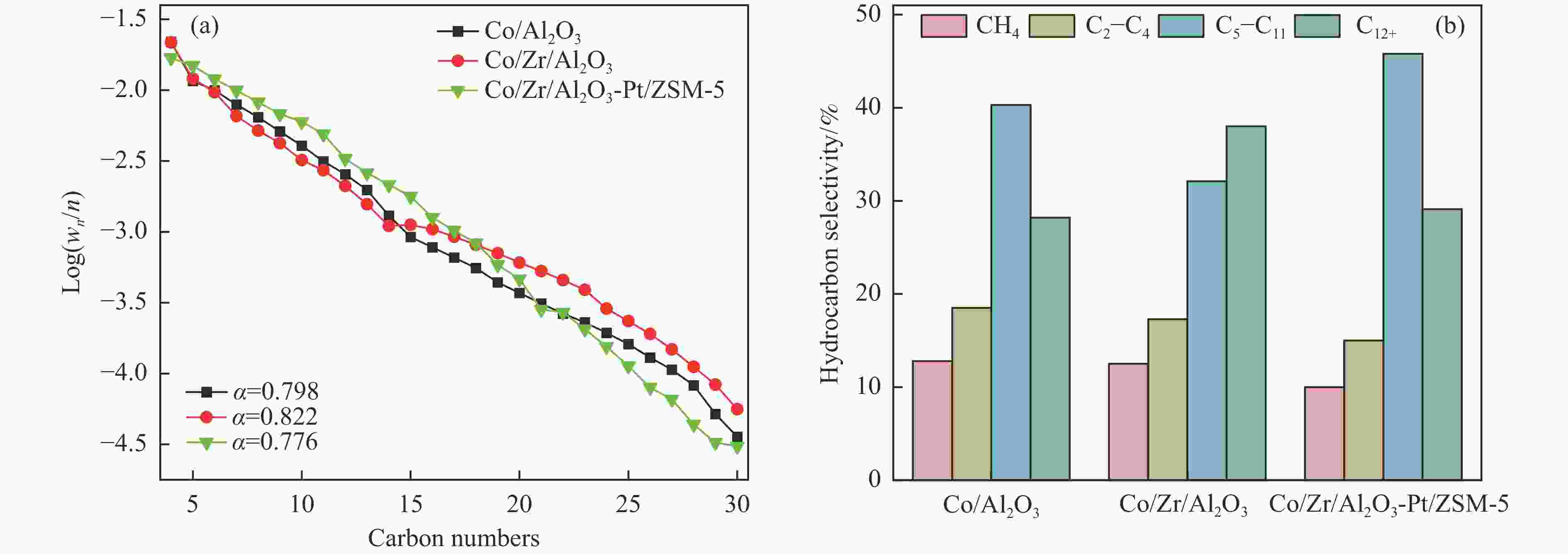
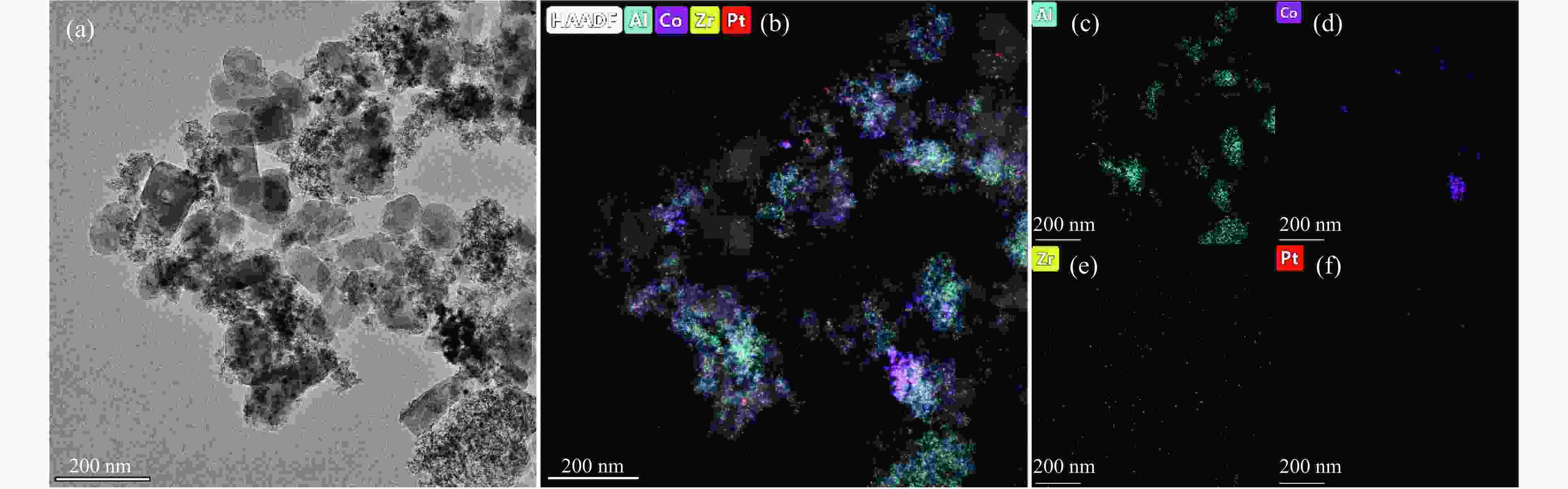
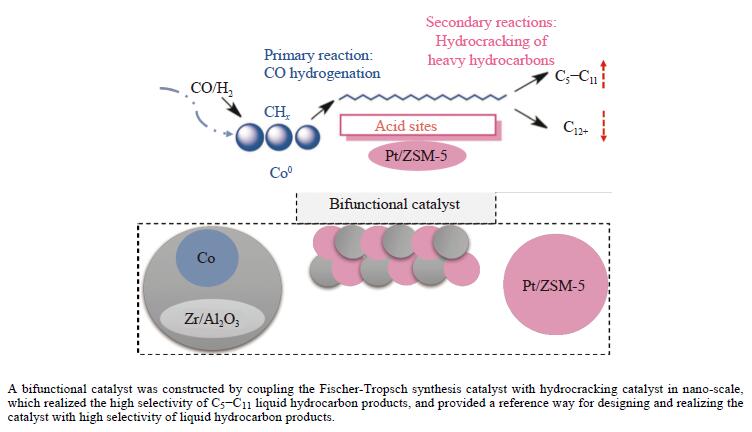
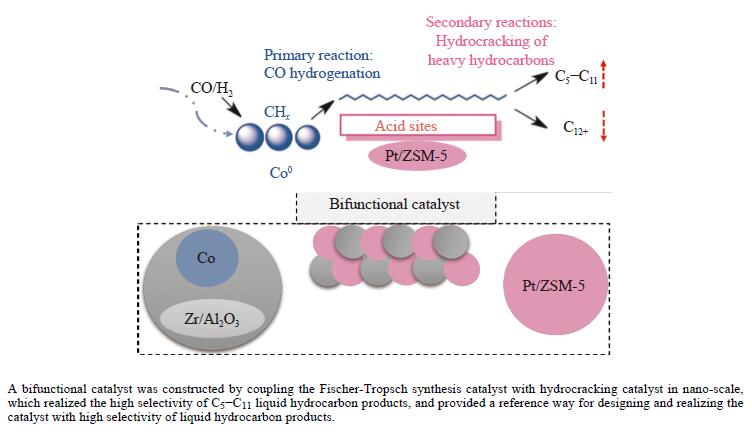
摘要: 以Co基催化剂耦合沸石分子筛催化剂应用于合成气催化转化可以有效改善催化剂的产物选择性。本研究通过浸渍法制备得到Zr/Al2O3载体和Pt/ZSM-5催化剂,再通过超声分散法制备了Co/Al2O3、Co/Zr/Al2O3和Co/Zr/Al2O3-Pt/ZSM-5催化剂。通过系列表征技术对载体和催化剂理化性质进行分析,评价了催化剂费-托合成反应性能。结果表明,Zr的引入有助于提升Co/Zr/Al2O3上Co物种的还原性,改善催化活性,增加C12+重质烃的选择性。当Co/Zr/Al2O3与Pt/ZSM-5耦合后,由于贵金属Pt的助剂效应,进一步促进Co物种的还原,Co/Zr/Al2O3-Pt/ZSM-5催化剂的CTY值提高至8.3×10−5 mmol/(g·s),同时具有较低的CH4、C2−C4产物选择性。此外,Pt/ZSM-5的酸性促进C12+产物的部分裂解,使产物分布向C5−C11液态烃偏移,C5−C11产物选择性达到45.9%。本研究为设计和制备高效的费-托合成催化剂提供了参考。Abstract: The Co catalysts exhibit high catalytic activity and low water gas shift activity, as well as excellent chain growth ability and low by-product selectivity for Fischer-Tropsch synthesis. The performance of the Co catalyst is influenced by several factors, including its structural composition and physical and chemical properties. The traditional cobalt Fischer-Tropsch catalyst follows the ASF distribution, resulting in a wide range of hydrocarbon products. This makes it difficult to achieve high selectivity for liquid hydrocarbons. Due to the presence of a large number of acidic sites on the surface of zeolite, it has excellent catalytic performance for hydrocracking. The integration of zeolite molecular sieves with Fischer-Tropsch catalysts to form a multi-component catalyst can significantly improve product selectivity, optimize liquid hydrocarbon yields and bypass conventional wax treatment steps. In this study, the catalysts Co/Al2O3, Co/Zr/Al2O3 and Co/Zr/Al2O3-Pt/ZSM-5 were prepared by ultrasonic dispersion method, and the effects of Zr promoter modification and multicomponent coupling catalyst on the activity and product selectivity of Fischer-Tropsch synthesis were investigated. In Co/Al2O3, Co/Zr/Al2O3 and Co/Zr/Al2O3-Pt/ZSM-5 catalysts, the Co species are uniformly dispersed on the support surface and have similar particle sizes. Pt and Zr were uniformly dispersed in the catalyst, Zr was mainly dispersed on the surface of Al2O3 supports, and Pt was mainly dispersed on the surface of ZSM-5 molecular sieve. The reduction of Co species was promoted by Zr-modified alumina. With the addition of Pt/ZSM-5 catalyst, the adsorbed hydrogen is more easily dissociated and converted into active hydrogen, further promoting the reduction of Co species. The catalytic performance of Fischer-Tropsch synthesis was evaluated. Compared with Co/Al2O3 catalyst, the selectivity of C12+ heavy hydrocarbon products on Co/Zr/Al2O3 catalyst increased from 28.2% to 38.1%, with a corresponding decrease in CH4, C2−C4 and C5−C11 products, indicating that Zr promoter promoted the generation of heavy hydrocarbon products. Coupled with Pt/ZSM-5 catalyst, the Co/Zr/Al2O3-Pt/ZSM-5 catalyst showed low CH4 selectivity (10.0%) and C2−C4 selectivity (15.0%), while the selectivity of C5−C11 liquid hydrocarbon products increased from 32.1% to 45.9%, the selectivity of heavy hydrocarbon products (C12+) decreased from 38.1% to 29.1%. Compared to Co/Zr/Al2O3, the TOF and CTY of Co/Zr/Al2O3-Pt/ZSM-5 catalysts are increased by 212.6% and 62.7%, respectively. The improvement in catalytic activity was mainly due to the addition of Zr promoter and Pt/ZSM-5 catalyst, which promoted the reduction of Co species. Under the synergistic effect of Zr promoter and Pt/ZSM-5 catalyst, Zr promoter promotes the formation of C12+ heavy hydrocarbons, while Pt/ZSM-5 catalyst promotes the hydrocracking of heavy hydrocarbons to C5−C11 liquid hydrocarbons, thereby improving the selectivity of Co/Zr/Al2O3-Pt/ZSM-5 catalyst for C5−C11 liquid hydrocarbons. In this study, a functional catalyst was constructed by coupling Fischer-Tropsch synthesis catalyst and hydrocracking catalyst at nanoscale, which achieved high selectivity of C5−C11 liquid hydrocarbon and low selectivity of CH4 and C2−C4 products, which provided a reference for the design and implementation of catalysts with high selectivity for liquid hydrocarbons.
-
Key words:
- Co-based catalysts /
- ZSM-5 /
- ultrasonic dispersion method /
- Fischer-Tropsch synthesis
-
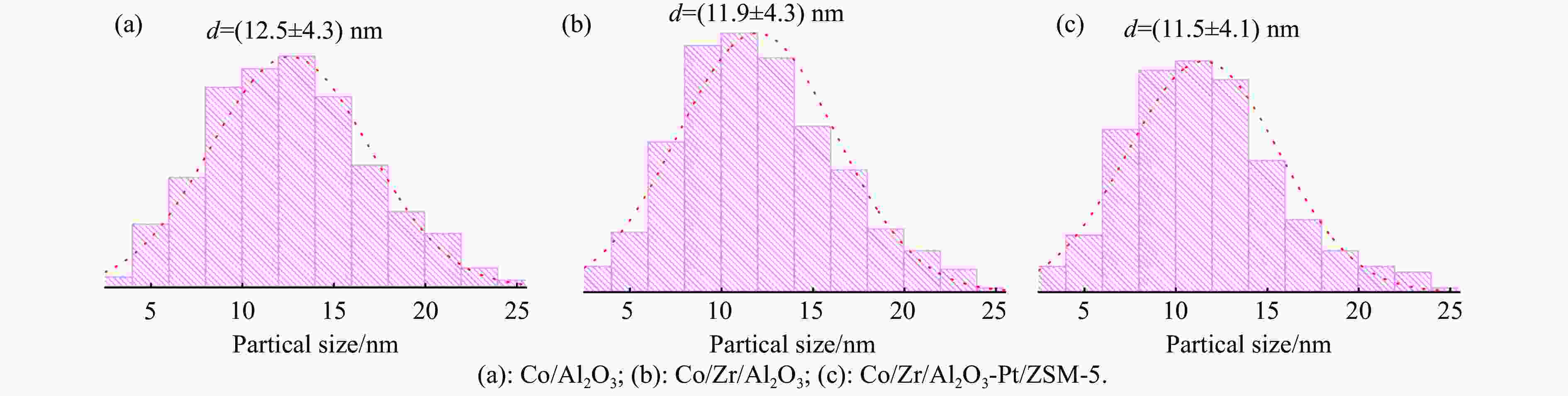
表 1 催化剂的结构参数
Table 1 Structural parameters of the catalysts
Catalyst Co3O4 d a/nm Co0 d b/nm Dispersionc/% TOFd/(10−5mmol·s−1) Co/Al2O3 12.5 9.4 10.2 46.9 Co/Zr/Al2O3 11.9 8.9 10.7 47.7 Co/Zr/Al2O3-Pt/ZSM-5 11.5 8.6 11.1 149.1 a: Obtained by particle size statistics; b: According to the formula calculation[32], d(Co0)=0.75×d(Co3O4); c: According to the formula calculation[32], Dispersion=96/d(Co0); d: According to the formula calculationon[33], TOF=rAc/nOF. 表 2 催化剂的孔结构参数
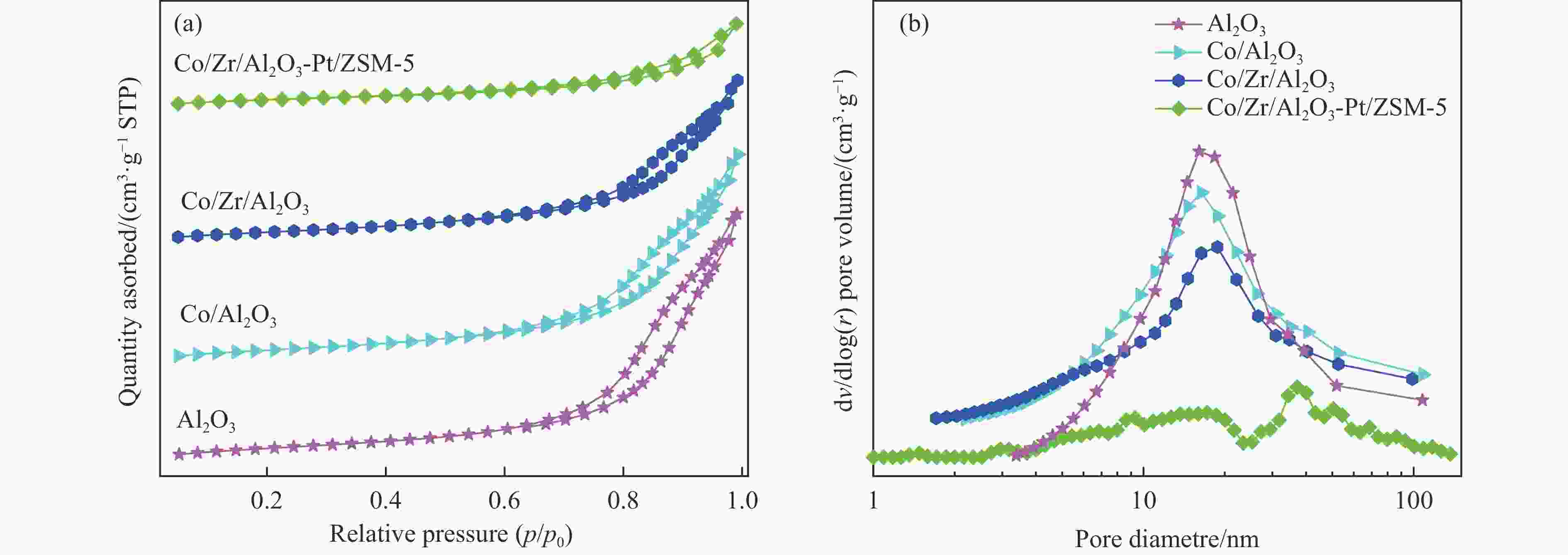
Table 2 Pore structure parameters of catalyst
Catalyst Surface area/(m2·g−1) Pore volume/(cm3·g−1) BJH pore size/nm Al2O3 242.4 1.06 17.5 Co/Al2O3 222.0 0.89 16.1 Co/Zr/Al2O3 184.4 0.70 16.3 Co/Zr/Al2O3-Pt/ZSM-5 236.0 0.42 16.1/37.1 表 3 催化剂的费-托合成反应性能
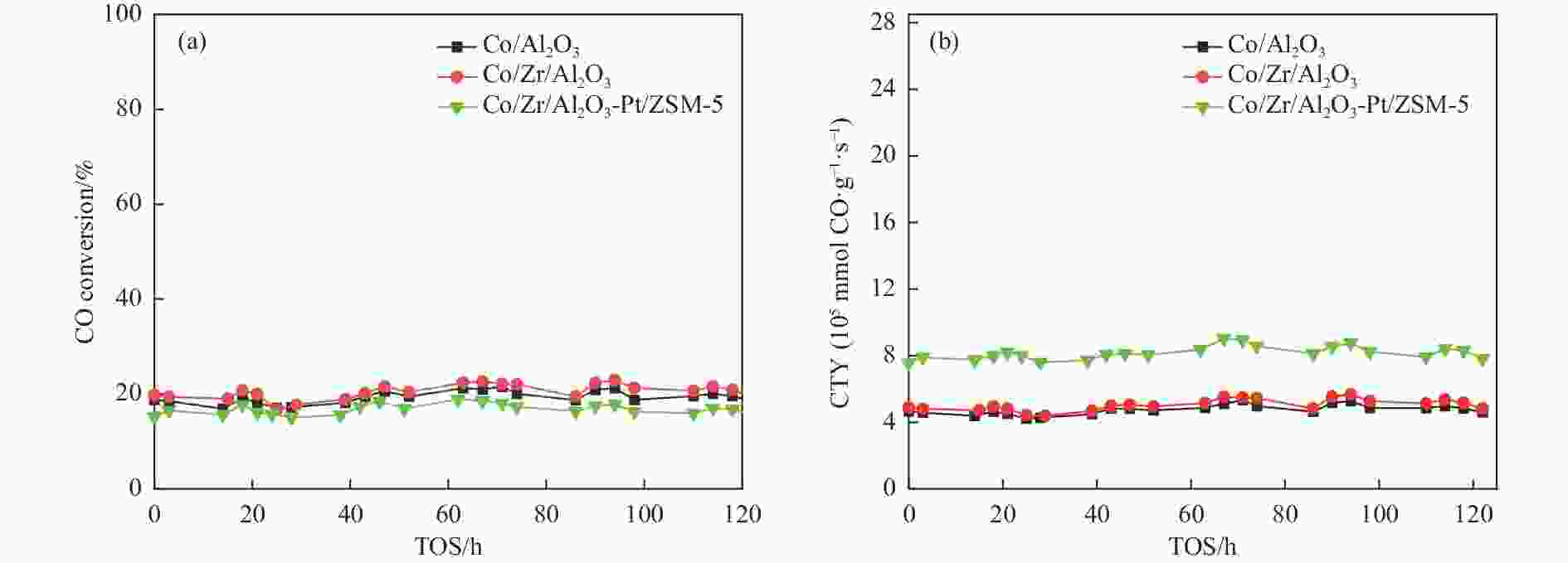
Table 3 FTS performance of the catalysts
Catalyst CO
conv./%CTY/
(10−5mmol·g−1·s−1)CO2
sel./%Hydrocarbon selectivity/% α4−30 CH4 C2−C4 C5−C11 C12+ Co/Al2O3 18.7 4.8 0.7 12.8 18.7 40.3 28.2 0.798 Co/Zr/Al2O3 19.7 5.1 0.6 12.5 17.3 32.1 38.1 0.822 Co/Zr/Al2O3-Pt/ZSM-5 16.5 8.3 0.5 10.0 15.0 45.9 29.1 0.776 -
[1] ZHANG Q, KANG J, WANG Y. Development of novel catalysts for Fischer-Tropsch synthesis: Tuning the product selectivity[J]. ChemCatChem,2010,2(9):1030−1058. doi: 10.1002/cctc.201000071 [2] SCHULZ H. Short history and present trends of Fischer-Tropsch synthesis[J]. Appl Catal A: Gen,1999,186(1/2):3−12. [3] IGLESIA E. Design, synthesis, and use of cobalt-based Fischer-Tropsch synthesis catalysts[J]. Appl Catal A: Gen,1997,161(1/2):59−78. doi: 10.1016/S0926-860X(97)00186-5 [4] 陈治平, 张智, 周文武, 等. 碳化铁的制备及其在费托合成中的应用研究进展[J]. 燃料化学学报,2022,50(11):1381−1392.CHEN Z, ZHANG Z, ZHOU W, et al. Preparation of iron carbide and its application in Fischer-Tropsch synthesis[J]. J Fuel Chem Technol,2022,50(11):1381−1392. [5] 贾留洋, 郭中山, 王峰, 等. 铁基费-托合成催化剂研究进展[J]. 工业催化,2021,29(10):12−18.JIA Liuyang, GUO Zhongshan, WANG Feng, et al. Advances in Fe-based Fischer-Tropsch synthesis catalysts[J]. Catal Ind,2021,29(10):12−18 [6] DI Z, FENG X, YANG Z, et al. Effect of iron precursor on catalytic performance of precipitated iron catalyst for Fischer-Tropsch synthesis reaction[J]. Catal Lett,2020,150(9):2640−2647. doi: 10.1007/s10562-020-03158-3 [7] ZHANG Q, GU J, CHEN J, et al. Facile fabrication of porous Fe@C nanohybrids from natural magnetite as excellent Fischer-Tropsch catalysts[J]. Chem Commum,2020,56(33):4523−4526. [8] HAN X, QING M, WANG H, et al. Effect of Fe3O4 content on the CO2 selectivity of iron-based catalyst for Fischer-Tropsch synthesis[J]. J Fuel Chem Technol,2023,51(2):155−164. doi: 10.1016/S1872-5813(22)60018-5 [9] LIU C, CHEN Y, ZHAN Y, et al. Nano-ZSM-5-supported cobalt for the production of liquid fuel in Fischer-Tropsch synthesis: Effect of preparation method and reaction temperature[J]. Fuel,2020,263:116619. doi: 10.1016/j.fuel.2019.116619 [10] YAO J, LIU J, HOFBAUER H, et al. Biomass to hydrogen-rich syngas via steam gasification of bio-oil/biochar slurry over LaCo1-x-CuxO3 perovskite-type catalysts[J]. Energy Convers Manag,2016,117:343−350. doi: 10.1016/j.enconman.2016.03.043 [11] HAO X, DONG G, YANG Y, et al. Coal to liquid (CTL): commercialization prospects in China[J]. Chem Eng Technol,2007,30(9):1157−1165. doi: 10.1002/ceat.200700148 [12] 卢文丽, 王俊刚, 孙德魁, 等. 费-托合成钴基催化剂微观结构研究进展[J]. 燃料化学学报,2022,50(4):436−445.LU W, WANG J, SUN D, et al. Research progress of microstructure for cobalt-based F-T catalysts[J]. J Fuel Chem Technol,2022,50(4):436−445. [13] TSAKOUMIS N E, RØNNING M, BORG Ø, et al. Deactivation of cobalt based Fischer-Tropsch catalysts: A review[J]. Catal Today,2010,154(3/4):162−182. doi: 10.1016/j.cattod.2010.02.077 [14] SARTIPI S, MAKKEE M, KAPTEIIN F, et al. Catalysis engineering of bifunctional solids for the one-step synthesis of liquid fuels from syngas: a review[J]. Catal Sci Technol,2014,4:893−907. doi: 10.1039/C3CY01021J [15] YAKOVENO R, SSVOST'YANOV A, NAROCHNIY G, et al. Preliminary evaluation of a commercially viable Co-based hybrid catalyst system in Fischer-Tropsch synthesis combined with hydroprocessing[J]. Catal Sci Technol,2020,10(40):7613−7629. [16] GAO P, LI S, BU X, et al. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst[J]. Nat Chem,2017,9(10):1019−1024. doi: 10.1038/nchem.2794 [17] JAVED M, CHENG S, ZHANG G, et al. A facile solvent-free synthesis strategy for Co-imbedded zeolite-based Fischer-Tropsch catalysts for direct gasoline production[J]. Chin J Catal,2020,41(4):604−612. doi: 10.1016/S1872-2067(19)63436-4 [18] WANG H, ZAHNG Z, WANG S, et al. The effect of the particle size on Fischer-Tropsch synthesis for ZSM-5 zeolite supported cobalt-based catalysts[J]. Chem Comm,2021,57(99):13522−13525. [19] WANG Y, YU J, QIAO J, et al. Effect of mesoporous ZSM-5 morphology on the catalytic performance of cobalt catalyst for Fischer-Tropsch synthesis[J]. J Energy Inst,2020,93(3):1187−1194. doi: 10.1016/j.joei.2019.11.002 [20] KANG J, CHENG K, ZHANG L, et al. Mesoporous zeolite-supported ruthenium nanoparticles as highly selective Fischer-Tropsch catalysts for the production of C5−C11 isoparaffins[J]. Angew Chem Int Ed,2011,50(22):5200−5203. doi: 10.1002/anie.201101095 [21] FAZLOLLAHI F, SARKARI M, GHAREBAGHI H et al. Preparation of Fe-Mn/K/Al2O3 Fischer-Tropsch catalyst and its catalytic kinetics for the hydrogenation of carbon monoxide[J]. Chin J Chem Eng,2013,21(5):507−519. doi: 10.1016/S1004-9541(13)60503-0 [22] ROHR F, LINDVAG O A, HOLMEN A, et al. Fischer-Tropsch synthesis over cobalt catalysts supported on zirconia-modified alumina[J]. Catal Today,2000,58(4):247−254. doi: 10.1016/S0920-5861(00)00258-3 [23] MORADI G R, BASIR M M, TAEB A, et al. Promotion of Co/SiO2 Fischer-Tropsch catalysts with zirconium[J]. Catal Commun,2003,4(1):27−32. doi: 10.1016/S1566-7367(02)00243-1 [24] XIONG H, ZHANG Y, LIEW K, et al. Catalytic performance of zirconium-modified Co/Al2O3 for Fischer-Tropsch synthesis[J]. J Mol Catal A: Chem,2005,231(1/2):145−151. doi: 10.1016/j.molcata.2004.12.033 [25] JONGSOMJIT B, PANPRANOT J, GOODWIN J. Effect of zirconia-modified alumina on the properties of Co/γ-Al2O3 catalysts[J]. J Catal,2003,215(1):66−77. doi: 10.1016/S0021-9517(02)00102-1 [26] JOKAR F, ALAVI S M, REZAEI M. Investigating the hydroisomerization of n-pentane using Pt supported on ZSM-5, desilicated ZSM-5, and modified ZSM-5/MCM-41[J]. Fuel,2022,324:124511. doi: 10.1016/j.fuel.2022.124511 [27] HUANG X, HOU B, WANG J, et al. CoZr/H-ZSM-5 hybrid catalysts for synthesis of gasoline-range isoparaffins from syngas[J]. Appl Catal A: Gen,2011,408(1/2):38−46. doi: 10.1016/j.apcata.2011.09.004 [28] LIU C, ZHANG Y, ZHAO Y, et al. The effect of the nanofibrous Al2O3 aspect ratio on Fischer-Tropsch synthesis over cobalt catalysts[J]. Nanoscale,2017,9(2):570−581. doi: 10.1039/C6NR07529K [29] TEOH G L, LIEW K Y, MAHMDDS W A. Synthesis and characterization of sol-gel alumina nanofibers[J]. J Solgel Sci Technol,2007,44:177−186. doi: 10.1007/s10971-007-1631-x [30] KIM S M, LEE Y J, JUN K W, et al. Synthesis of thermo-stable high surface area alumina powder from sol-gel derived boehmite[J]. Mater Chem Phys.,2007,104(1):56−61. doi: 10.1016/j.matchemphys.2007.02.044 [31] LIU C, HONG J, ZHANG Y, et al. Synthesis of γ-Al2O3 nanofibers stabilized Co3O4 nanoparticles as highly active and stable Fischer-Tropsch synthesis catalysts[J]. Fuel,2016,180:777−784. doi: 10.1016/j.fuel.2016.04.006 [32] MARTINEZ A, LOPEZ C, MARQUEZ F, et al. Fischer-Tropsch synthesis of hydrocarbons over mesoporous Co/SBA-15 catalysts: The influence of metal loading, cobalt precursor, and promoters[J]. J Catal,2003,220(2):486−499. doi: 10.1016/S0021-9517(03)00289-6 [33] ABDELDAYEM H, FAIZ M, HASSAN S, et al. Rare earth oxides doped NiO/γ-Al2O3 catalyst for oxidative dehydrogenation of cyclohexane[J]. J Rare Earths,2015,33(6):611−618. doi: 10.1016/S1002-0721(14)60461-0 [34] 张萌, 刘佳, 张煜华, 等. 硅球负载高分散钴基催化剂的制备及其费-托合成催化性能研究[J]. 燃料化学学报(中英文),2023,51(5):608−615. doi: 10.1016/S1872-5813(22)60078-1ZHANG Meng, LIU Jia, ZHANG Yuhua, et al. Preparation of highly dispersed silicon spheres supported cobalt-based catalysts and their catalytic performance for Fischer-Tropsch synthesis[J]. J Fuel Chem Technol,2023,51(5):608−615. doi: 10.1016/S1872-5813(22)60078-1 [35] ARNOLDY P, MOULIJIN J. A. Temperature-programmed reduction of CoO/AI2O3 catalysts[J]. J Catal,1985,97(1):38−54. [36] XU D, LI W, DUAN H, et al. Reaction performance and characterization of Co/Al2O3 Fischer-Tropsch catalysts promoted with Pt, Pd and Ru[J]. Catal Lett,2005,102(3/4):229−235. doi: 10.1007/s10562-005-5861-7 [37] LI Z, WU J, YU J, et al. Effect of incorporation manner of Zr on the Co/SBA-15 catalyst for the Fischer-Tropsch synthesis[J]. J Mol Catal A: Chem,2016,424:384−392. doi: 10.1016/j.molcata.2016.09.025 [38] MA W, JACOBS G, GAO P, et al. Fischer-Tropsch synthesis: Pore size and Zr promotional effects on the activity and selectivity of 25%Co/Al2O3 catalysts[J]. Appl Catal A: Gen,2014,475:314−324. doi: 10.1016/j.apcata.2014.01.016 [39] KANG J, ZHOU W, WANG Y, et al. Iridium boosts the selectivity and stability of cobalt catalysts for syngas to liquid fuels[J]. Chem,2022,8(4):1050−1066. doi: 10.1016/j.chempr.2021.12.016 [40] PENG X, CHENG K, KANG J, et al. Impact of hydrogenolysis on the selectivity of the Fischer-Tropsch synthesis: diesel fuel production over mesoporous zeolite-Y-supported cobalt nanoparticles[J]. Angew Chem Int Ed,2015,54(15):4553−4556. doi: 10.1002/anie.201411708 -




 下载:
下载: