Effect of zinc content on the structure of Zn species and catalytic properties over Zn/ZSM-5
-
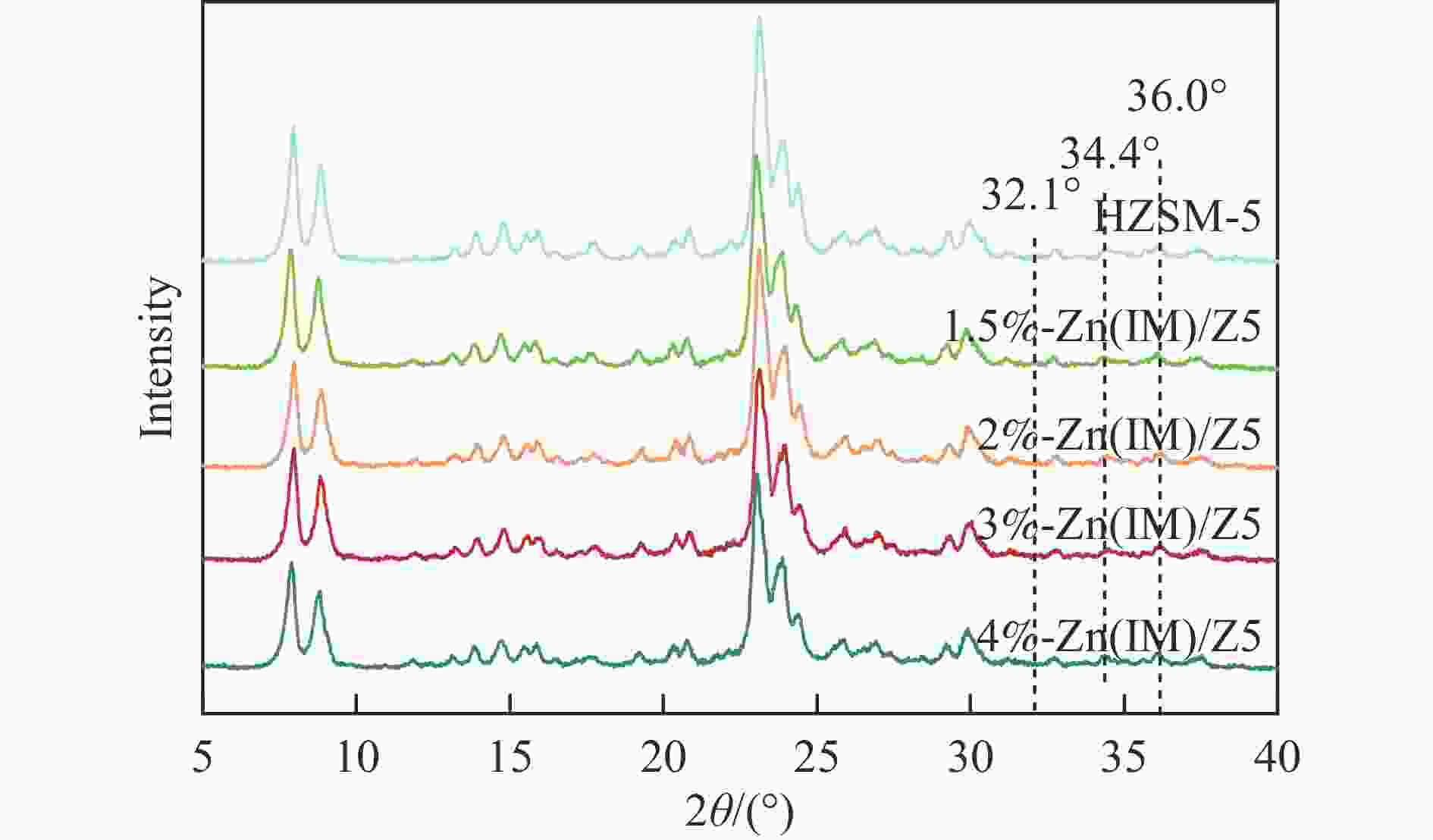
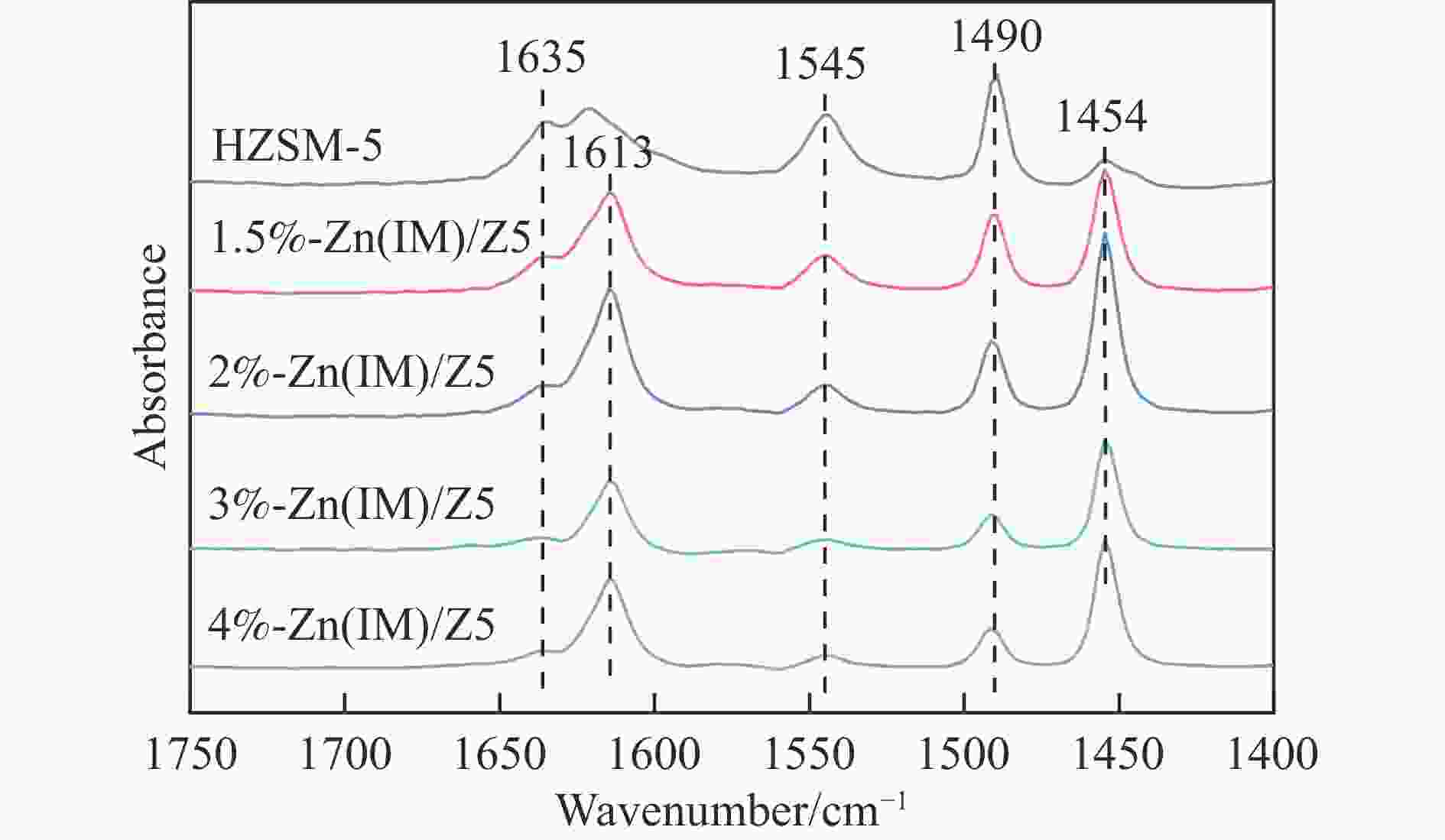
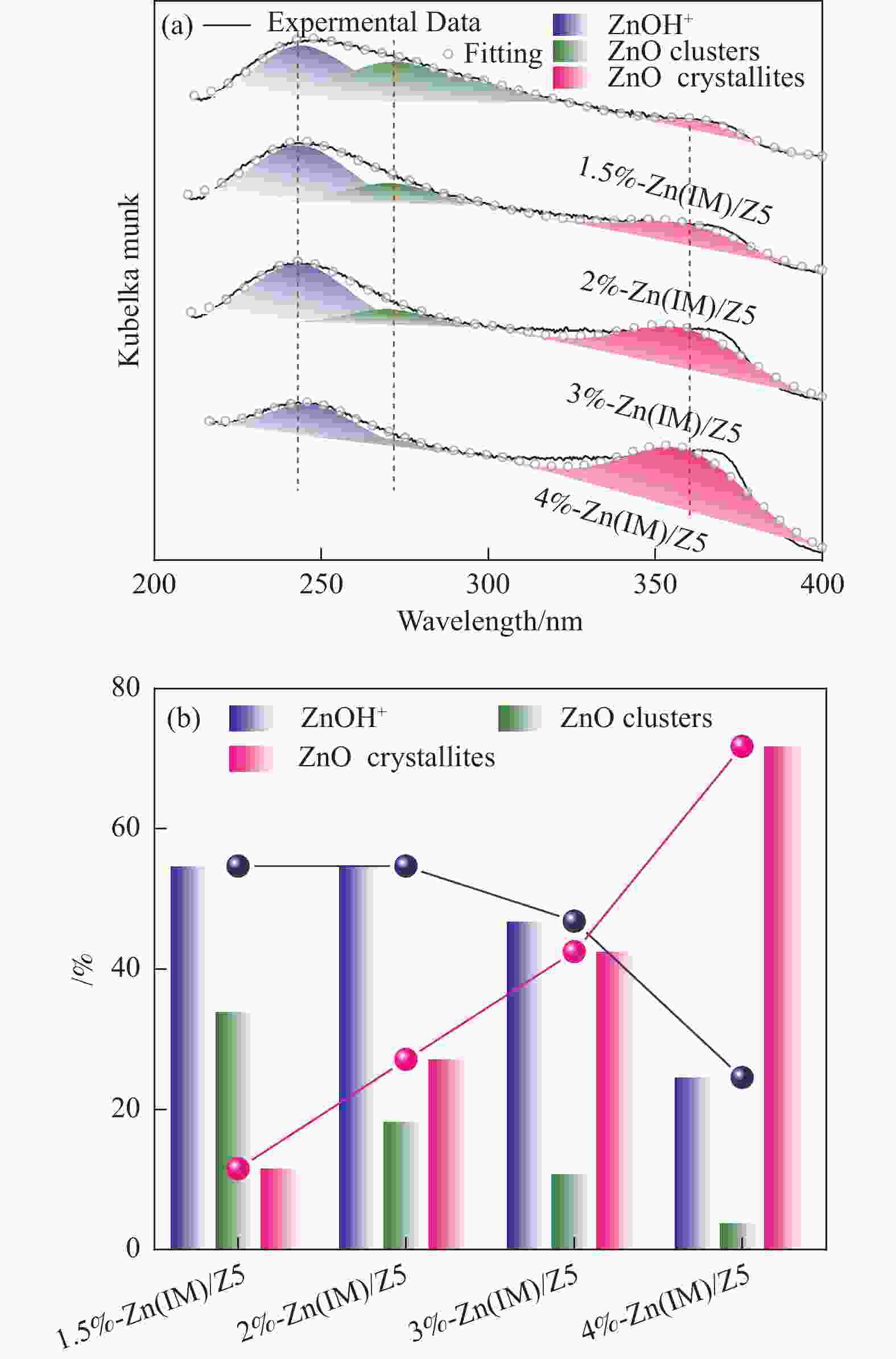
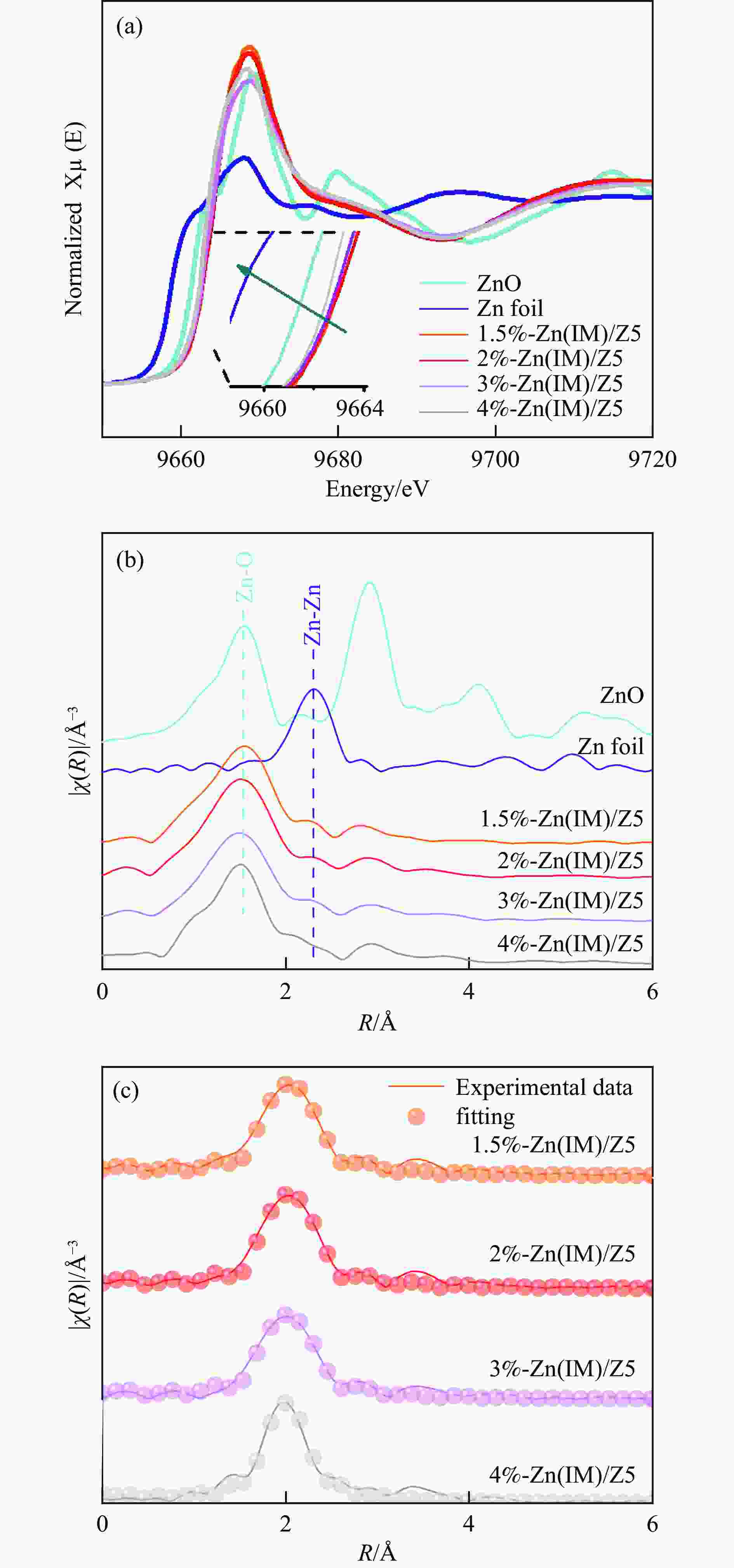
摘要: HZSM-5(Si/Al=30)为载体,用等体积浸渍法合成了系列不同Zn负载量的双功能Zn/ZSM-5催化剂,考察了Zn负载量在乙烯芳构化过程中的催化性能。用X射线粉末衍射(XRD)、N2吸附-脱附和吡啶吸附红外光谱(Py-FTIR)方法考察了催化剂的结构和酸性,用电感耦合等离子发射光谱(ICP)、紫外可见光谱(UV-vis DRS)、X射线吸收精细结构(XAFS)技术解析了Zn物种的结构及其流失行为。结果表明,Zn含量对其在HZSM-5上的存在状态及催化乙烯芳构化反应性能均有明显的影响,具有较多活性六配位ZnOH+物种的1.5%-Zn(IM)/Z5催化剂表现出较高的芳烃选择性和催化剂稳定性,且表现出较低的Zn流失速率。Abstract: Studying the status and distribution of Zn species on Zn/ZSM-5 zeolite catalysts were of great significance for determining the active centers and establishing structure-activity relationships in the ethylene aromatization process. The effect of zinc contents of Zn/ZSM-5 zeolites prepared by incipient-wetness impregnation method on catalytic performances in ethylene aromatization were investigated. The structures and acidic properties of the catalyst were studied through X-ray powder diffraction (XRD), N2 adsorption/desorption, and infrared spectra for pyridine adsorption (Py-FTIR). Besides, inductively coupled plasma-atomic emission spectrum (ICP), diffuse reflectance ultraviolet-visible spectrum (UV-vis DRS), extended X-ray absorption fine structure (EXAFS) and linear combination fitting (LCF) analysis on X-ray Absorption near edge spectra (XANES) had finely analyzed the structure and transition of Zn species and the losing rate of Zn species on HZSM-5 molecular sieve catalyst during ethylene aromatization process. The results showed that the introduction of Zn was advantage to improve the selectivity of aromatics hydrocarbon, and Zn contents of the catalyst had obvious influence on the structures, acidic properties, and the status of Zn species, as well as the catalytic performance of Zn/ZSM-5 catalysts. At low zinc loading, 1.5%-Zn(IM)/Z5 catalyst with more active 6-fold coordinated ZnOH+ species (55%) showed the highest selectivity to aromatics and catalyst stability. With the increase of zinc amount, the excessive Zn contents not only covered the acid sites and blocked the pore channel, but also changed the local coordination structure and state of Zn species. It was confirmed that the oxidizability of Zn species and the coordination number around Zn sites decreased, accompanied by a weakening of the interaction between Zn and zeolite, leading to the formation of large amounts of 4-fold coordinated ZnO clusters and ZnO crystallites. In the 4%-Zn(IM)/Z5 catalyst, Zn species composed of multi characteristics from ZnOH+, ZnO clusters inside the pores, and ZnO crystals on the external surface with relative contributions of 23.5%, 56.1%, and 20.4%, respectively. It meant that ZnO clusters and ZnO crystallites became the main component at the high Zn content. Furthermore, ZnO species located on the outer surface of Zn/ZSM-5 catalysts were easily reduced by H2 and then transported as zinc vapor to the outer surface, which eventually lead to the loss of Zn species from the catalyst and the decline of the catalytic performance of Zn/ZSM-5 catalyst. The relative proportion of ZnOH+ decreased with that of ZnO clusters and ZnO crystallites correspondingly increased considerably with the increase of Zn loading on ZSM-5, accompanied with the elevated rate for Zn losing, and shortened catalyst life. Therefore, a positively correlated between the content of ZnOH+ obtained through the UV-vis DRS and LCF analysis on XANES and the rate of aromatics formation was established, further confirming the catalytic nature of ZnOH+ as the active center, which played an important role in the aromatization reaction that enhancing the formation of aromatic hydrocarbons. Meanwhile, ZnO on the outer surface of Zn/ZSM-5 catalysts was the main species that losing from catalyst, and influenced the catalytic properties on a certain degree.
-
Key words:
- Zn/ZSM-5 /
- incipient-wetness impregnation /
- Zn content /
- Zn species /
- ethylene aromatization
-
表 1 HZSM-5和x%-Zn(IM)/Z5催化剂的结构性质和酸性
Table 1 Textural properties and acidic properties of the HZSM-5 and x%-Zn(IM)/Z5 catalysts
Catalyst Surface area/
(m2·g−1)Pore volume/
(cm3·g−1)Acidity by
Py-FTIRBET total micro meso L/B HZSM-5 385 0.379 0.113 0.266 0.30 1.5%-Zn(IM)/Z5 364 0.366 0.110 0.256 2.23 2%-Zn(IM)/Z5 345 0.350 0.105 0.245 3.96 3%-Zn(IM)/Z5 348 0.360 0.106 0.254 4.64 4%-Zn(IM)/Z5 337 0.352 0.105 0.247 5.61 表 2 不同Zn负载量新鲜和失活x%-Zn(IM)/Z5催化剂上Zn含量
Table 2 Zn contents of the fresh an deactivation x%-Zn(IM)/Z5 catalysts
Catalyst Zn content w/% Reaction time/h Zn loss rate/(%·h−1) fresh catalysts deactivation catalysts 1.5%-Zn(IM)/Z5 1.54 1.40 78.00 0.12 2%-Zn(IM)/Z5 1.81 1.64 54.00 0.17 3%-Zn(IM)/Z5 2.78 2.51 56.00 0.17 4%-Zn(IM)/Z5 3.70 3.14 48.00 0.32 表 3 ZnO和x%-Zn(IM)/Z5催化剂的XAFS拟合参数
Table 3 EXAFS fit parameters of ZnO and x%-Zn(IM)/Z5 catalysts
Sample E0/eV Zn K-edge EXAFS fit parameters a contribution CN R/Å ${{S}}_0^2 $ σ/Å2 R-factor Zn 9658.7 − − − − − − ZnO 9661.7 Zn-O 4.0(±0.6) 1.97(±0.01) 0.904 0.004(±0.002) 0.007 1.5%-Zn(IM)/Z5 9664.1 Zn-O 5.4(±0.3) 2.05(±0.01) 0.904 0.011(±0.002) 0.004 2%-Zn(IM)/Z5 9664.4 Zn-O 5.1(±0.7) 2.05(±0.01) 0.904 0.009(±0.003) 0.005 3%-Zn(IM)/Z5 9663.4 Zn-O 4.7(±0.7) 2.02(±0.02) 0.904 0.011(±0.003) 0.006 4%-Zn(IM)/Z5 9663.1 Zn-O 4.6(±0.4) 2.00(±0.01) 0.904 0.010(±0.002) 0.006 a: CN = coordination number, R =Interatomic distances, σ= Debye-Waller factor, and R-factor=∑i(datai -fiti)2/(datai)2. -
[1] BARADARAN S, SOHRABI M, BIJANI P, et al. Isobutane aromatization in the presence of propane as a co-reactant over H-ZSM-5 catalysts using different crystallization times[J]. J Ind Eng Chem,2015,27(1):354−361. [2] NIU X, GAO J, WANG K, et al. Influence of crystal size on the catalytic performance of H-ZSM-5 and Zn/H-ZSM-5 in the conversion of methanol to aromatics[J]. Fuel Process Technol,2017,157:99−107. doi: 10.1016/j.fuproc.2016.12.006 [3] PIDKO E A, HENSEN E J M, VAN SANTEN R A. Anionic oligomerization of ethylene over Ga/ZSM-5 Zeolite: A theoretical study[J]. J Phys Chem C,2008,112(49):19604−19611. doi: 10.1021/jp8069767 [4] PINILLA-HERRERO I, BORFECCHIA E, CORDERO-LANZAC T, et al. Finding the active species: The conversion of methanol to aromatics over Zn-ZSM-5/alumina shaped catalysts[J]. J Catal,2021,394:416−428. doi: 10.1016/j.jcat.2020.10.024 [5] YUAN Y, LOBO R F. Zinc speciation and propane dehydrogenation in Zn/H-ZSM-5 catalysts[J]. ACS Catal,2023,13(7):4971−4984. doi: 10.1021/acscatal.2c05898 [6] QIU B, ZHANG Y, ZHANG Y. A stable zinc zeolite catalyst for dehydrogenation of ethane to aromatics and ethylene[J]. Catal Lett,2022,152(5):1372−1385. doi: 10.1007/s10562-021-03726-1 [7] NIU X, GAO J, MIAO Q, et al. Influence of preparation method on the performance of Zn-containing HZSM-5 catalysts in methanol-to-aromatics[J]. Microporous Mesoporous Mater,2014,197:252−261. doi: 10.1016/j.micromeso.2014.06.027 [8] GAO D, ZHI Y B, CAO L Y, et al. Influence of Zn state on the catalyst properties of Zn/HZSM-5 zeolite in 1-hexene aromatization and cyclohexane dehydrogenation[J]. Chin J Chem Eng,2022,43:124−134. doi: 10.1016/j.cjche.2022.01.005 [9] TRIWAHYONO S, JALIL A A, MUKTI R R, et al. Hydrogen spillover behavior of Zn/HZSM-5 showing catalytically active protonic acid sites in the isomerization of n-pentane[J]. Appl Catal A: Gen,2011,407:91−99. doi: 10.1016/j.apcata.2011.08.027 [10] KOLYAGIN Y G, ORDOMSKY V V, KHIMYAK Y Z, et al. Initial stages of propane activation over Zn/MFI catalyst studied by in situ NMR and IR spectroscopic techniques[J]. J Catal,2006,238:122−133. doi: 10.1016/j.jcat.2005.11.037 [11] 位春蕾, 高洁, 王凯, 等. 氢预处理对Zn/HZSM-5分子筛催化乙烯芳构化反应性能的影响[J]. 物理化学学报,2017,33(7):1483−1491. doi: 10.3866/PKU.WHXB201704133WEI Chun-lei, GAO Jie, WANG Kai, et al. Effect of hydrogen pre-treatment on the catalytic properties of Zn/HZSM-5 zeolite for ethylene aromatization reaction[J]. Acta phys-Chim Sin,2017,33(7):1483−1491 doi: 10.3866/PKU.WHXB201704133 [12] LYTLE F W. Applications of Synchrotron Radiation[M]. New York: GordonBreach, 1989: 135−223. [13] HOFFMANN M M, DARAB J G, HEALD S M, et al. New experimental developments for in situ XAFS studies of chemical reactions under hydrothermal conditions[J]. Chem geol,2000,167:89−103. doi: 10.1016/S0009-2541(99)00202-8 [14] TAKEKOH R, OKUBO M, ARAKI T, et al. Quantitative chemical mapping of nanostructured “Onionlike” poly(methyl methacrylate)/polystyrene composite particles by soft X-ray microscopy[J]. Macromolecules,2005,38:542−551. doi: 10.1021/ma048609y [15] GENG R, LIU Y, GUO Y, et al. Structure evolution of Zn species on fresh, deactivated and regenerated Zn/ZSM-5 catalysts in ethylene aromatization[J]. ACS Catal,2022,12:14735−14747. doi: 10.1021/acscatal.2c04074 [16] GENG R, LIU Y, GAO J, et al. The migration of Zn species on Zn/ZSM-5 catalyst during the process of ethylene aromatization[J]. Catal Sci Technol,2022,12:4201−4210. doi: 10.1039/D2CY00661H [17] 刘亚聪, 董 梅, 樊卫斌, 等. 用于乙烯芳构化反应的 Zn /HZSM-5 催化剂失活机制研究[J]. 燃料化学学报,2018,46(7):826−834.LIU Yacong, DONG Mei, FAN Weibin, et al. The deactivation mechanism of Zn /HZSM-5 zeolites in ethylene aromatization reaction[J]. J Fuel Chem Technol,2018,46(7):826−834 [18] CHEN X, DONG M, NIU X, et al. Influence of Zn species in HZSM-5 on ethylene aromatization[J]. Chin J Catal,2015,36(6):880−888. doi: 10.1016/S1872-2067(14)60289-8 [19] MADEIRA F, BEN T, PINARD L, et al. Ethanol transformation into hydrocarbons on ZSM-5 zeolites: Influence of Si/Al ratio on catalytic performances and deactivation rate. Study of the radical species role[J]. Appl Catal A: Gen,2012,443:171−180. [20] BI Y, WANG Y, CHEN X, YU Z, et al. Methanol aromatization over HZSM-5 catalysts modified with different zinc salts[J]. Chin J Catal,2014,35:1740−1751. doi: 10.1016/S1872-2067(14)60145-5 [21] SMIEŠKOVÁ A, ROJASOVÁ E, HUDEC P, et al. Aromatization of light alkanes over ZSM-5 catalysts: Influence of the particle properties of the zeolite[J]. Appl Catal A: Gen,2004,268:235−240. doi: 10.1016/j.apcata.2004.03.043 [22] MADEIRA F F, TAYEB K B, PINARD L, et al. Ethanol transformation into hydrocarbons on ZSM-5 zeolites: Influence of Si/Al ratio on catalytic performances and deactivation rate. Study of the radical species role[J]. Appl Catal A: Gen, 2012, 443–444 : 171−180. [23] PAN T, WU Z, ZHOU K. In situ incorporation of Zn into hierarchical ZSM-5 zeolites for olefin hydroisomerization[J]. Ind Eng Chem Res,2020,59:12371−12380. doi: 10.1021/acs.iecr.0c01506 [24] SHEN X, KANG J, NIU W, et al. Impact of hierarchical pore structure on the catalytic performances of MFI zeolites modified by ZnO for the conversion of methanol to aromatics[J]. Catal Sci Technol,2017,7:3598−3612. doi: 10.1039/C7CY01041A [25] KIM Y, LEE K, LEE J. The effect of pre-coking and regeneration on the activity and stability of Zn/ZSM-5 in aromatization of 2-methyl-2-butene[J]. Catal Today,2011,178:72−78. doi: 10.1016/j.cattod.2011.07.002 [26] ZHENG H, MA D, BAO X, et al. Direct observation of the active center for methane dehydroaromatization using an ultrahigh field Mo-95 NMR spectroscopy[J]. J Am Chem Soc,2008,130:3722−3723. doi: 10.1021/ja7110916 [27] LUO Y J, MIAO C X, YUE Y H, et al. ZnO supported on silicalite-1 as an efficient catalyst for isobutane dehydrogenation to isobutene assisted by CO2[J]. Microporous Mesoporous Mater,2019,294:109864. -





 下载:
下载: