The research progress of formation and control on the N-containing compound of biomass pyrolysis gas
-
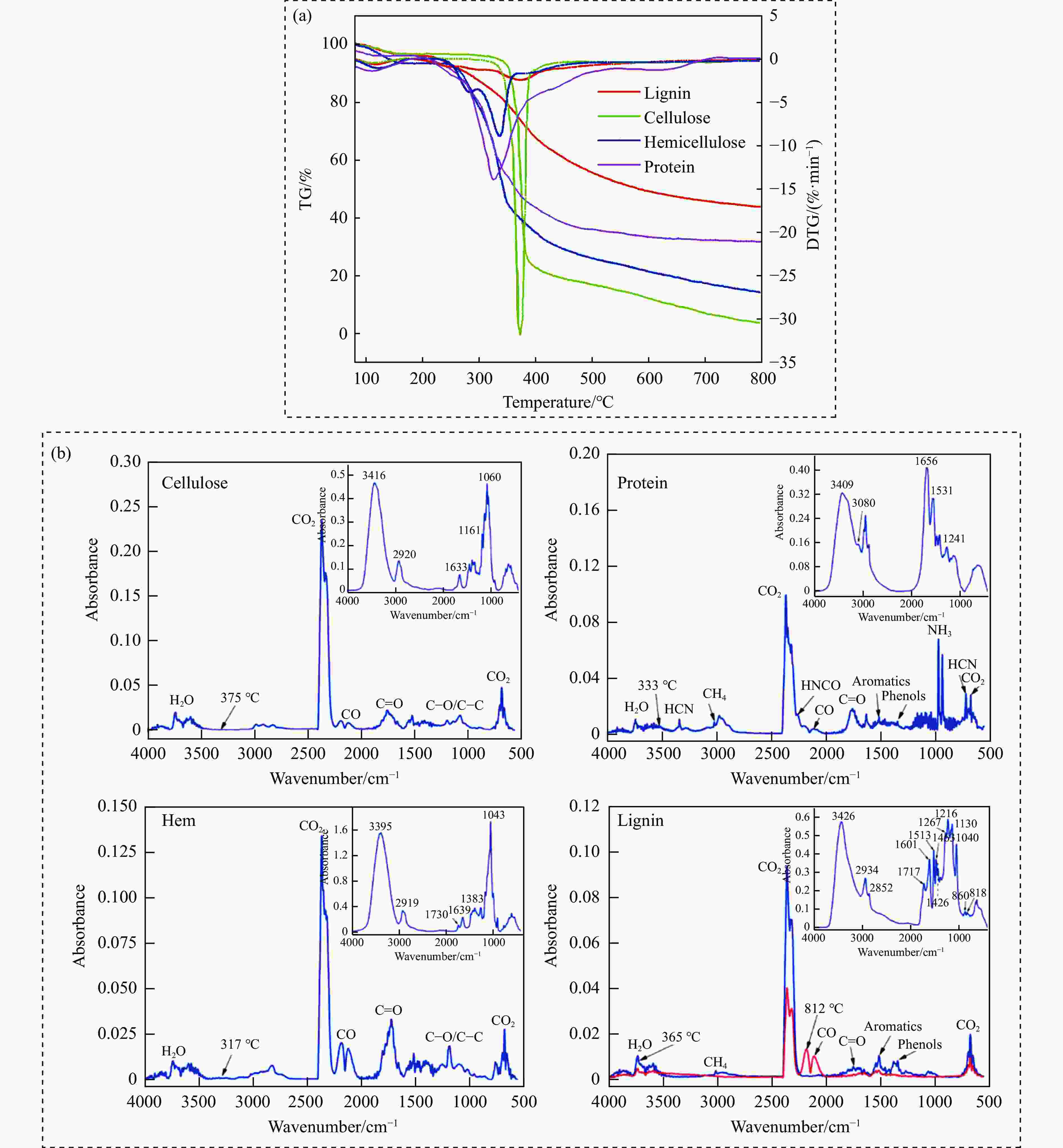
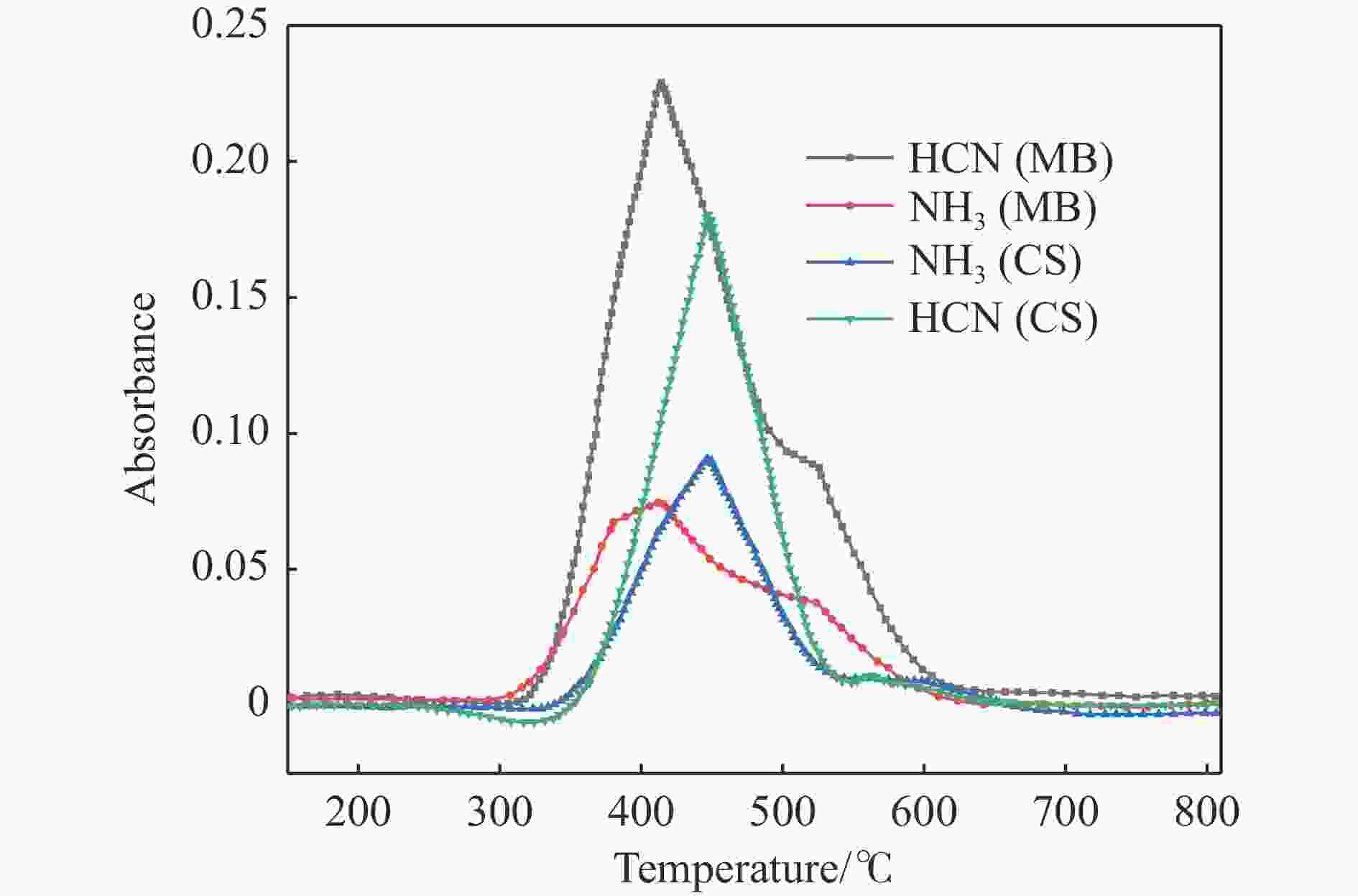
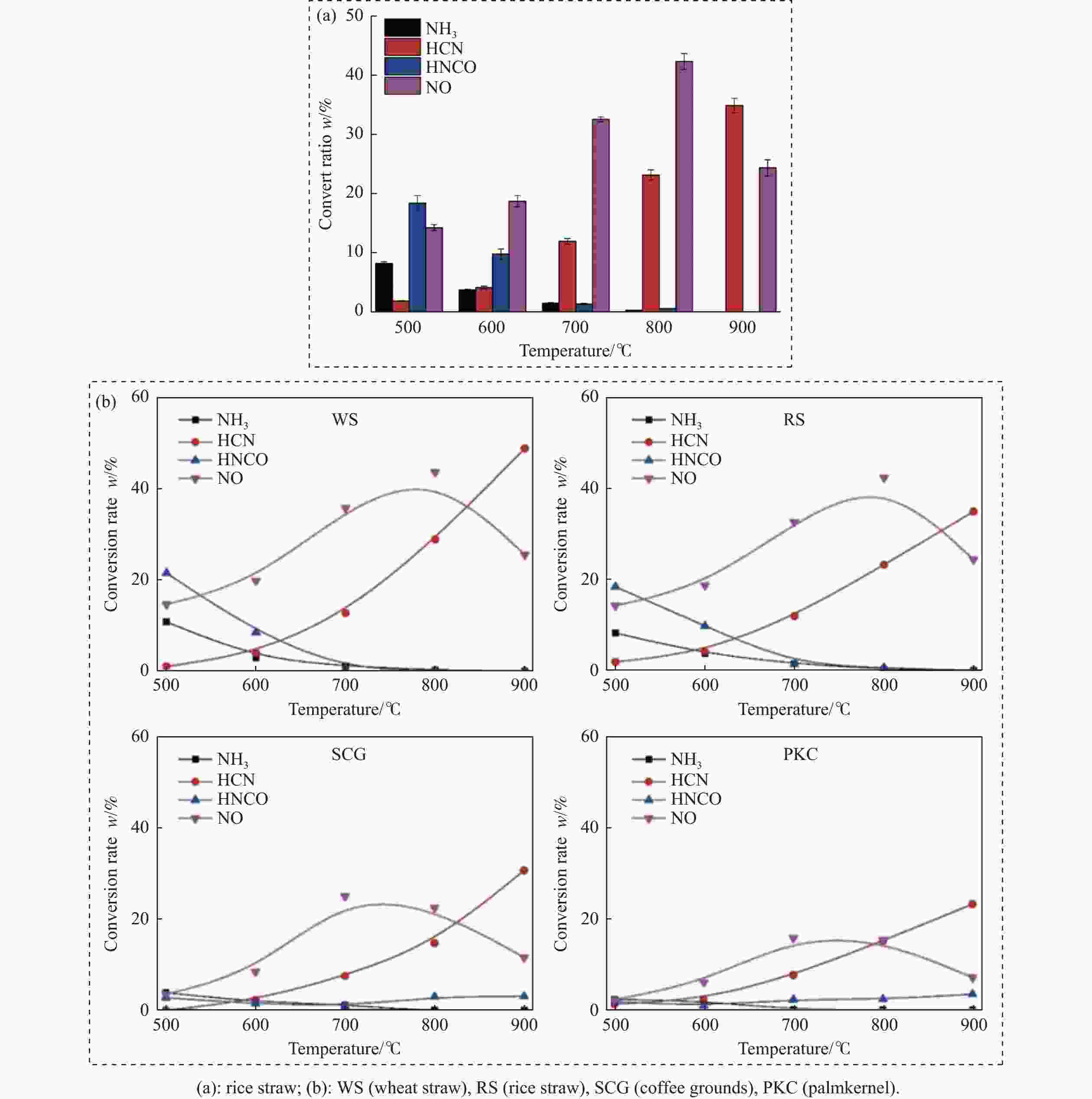
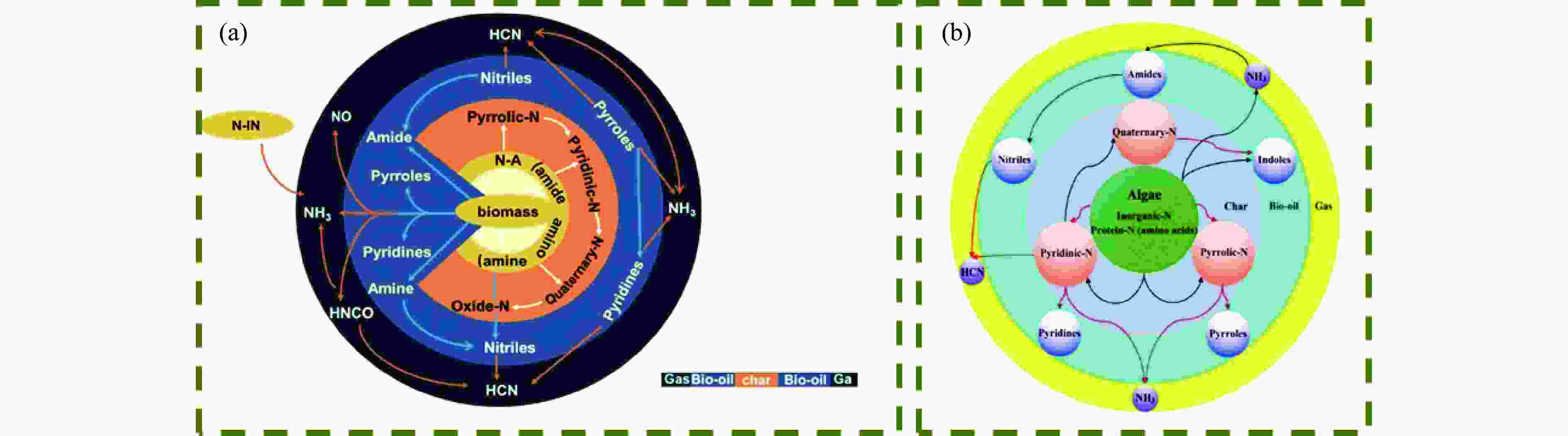
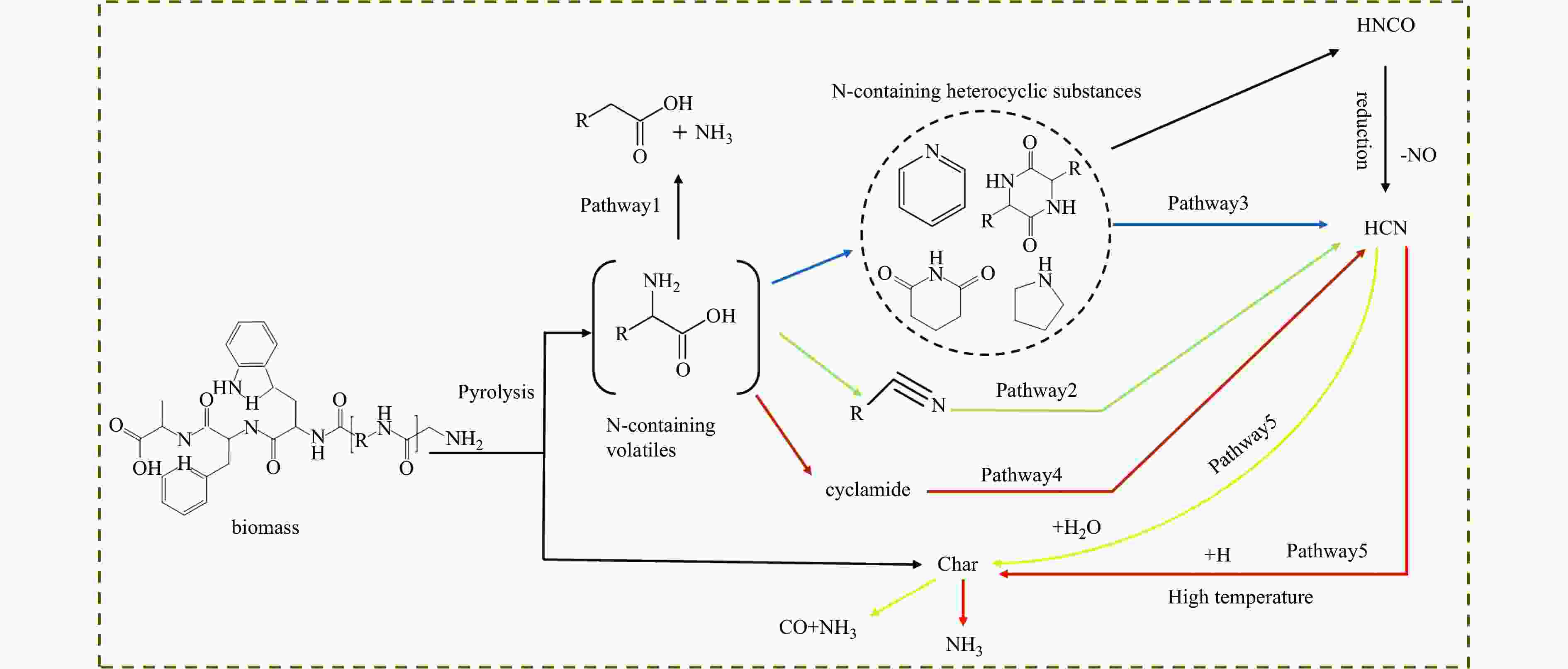
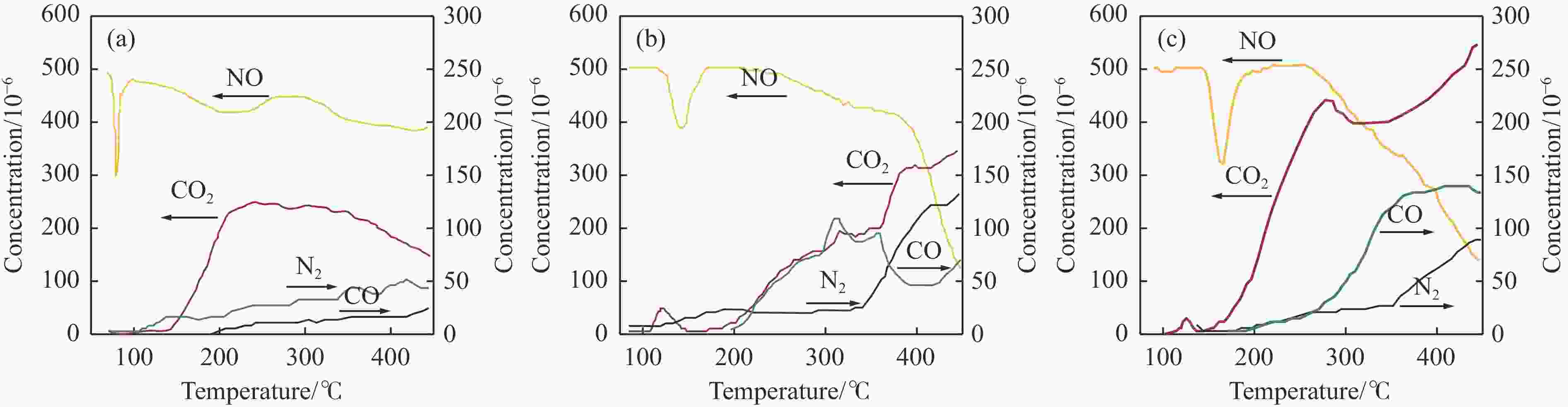
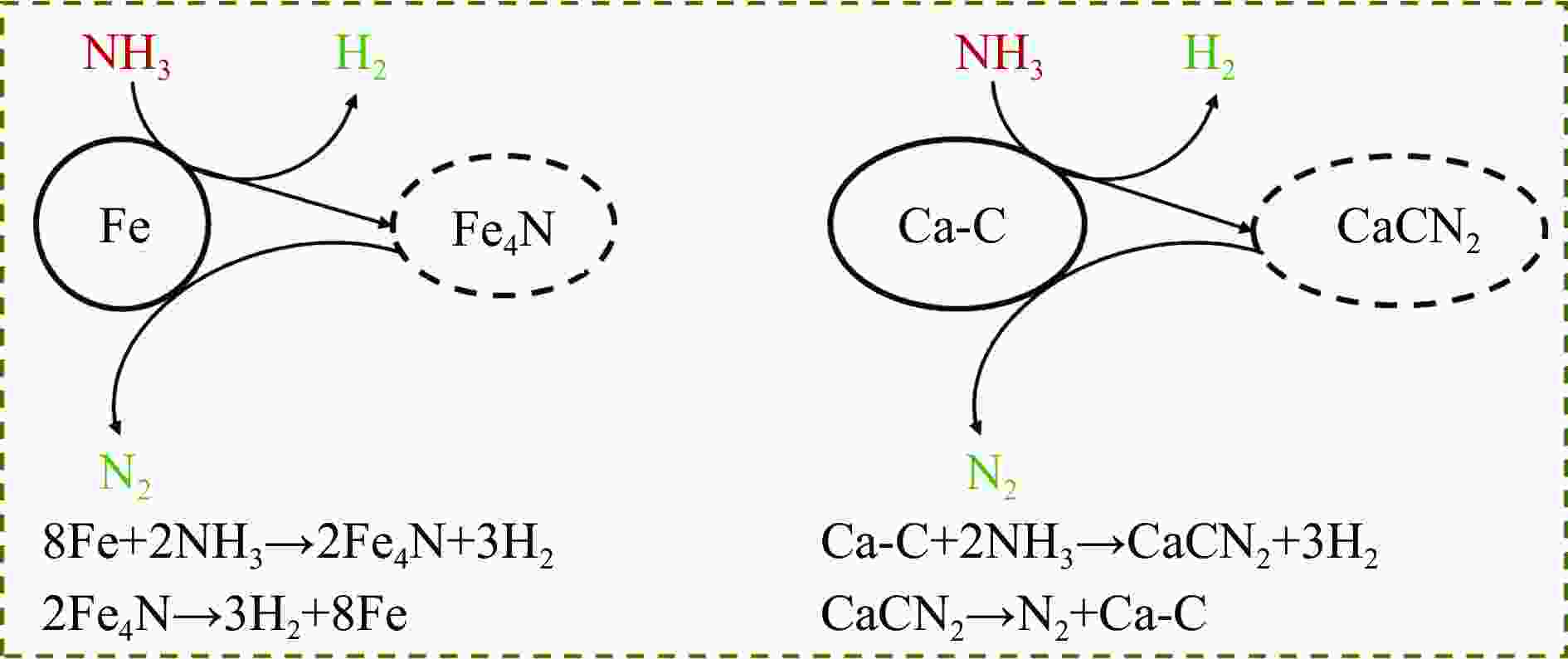
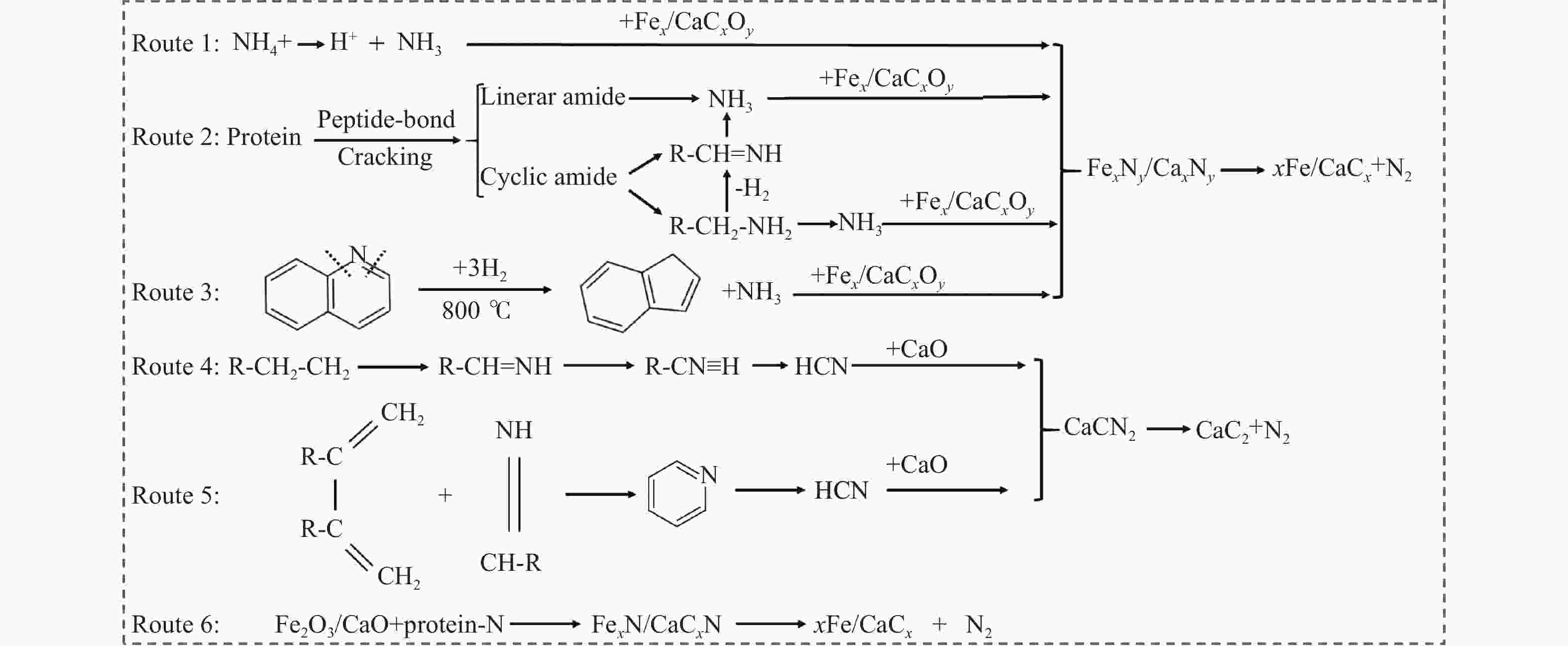
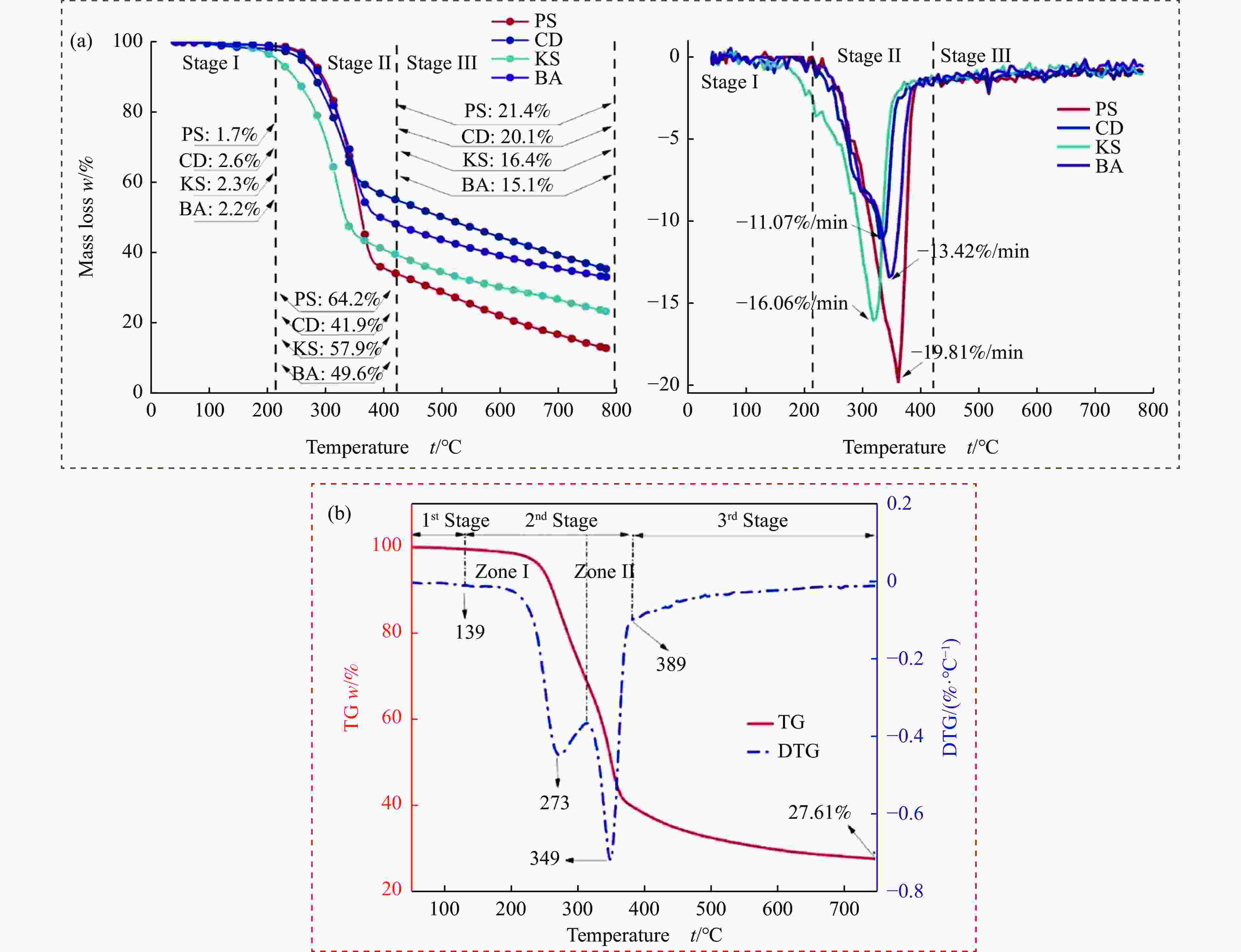
摘要: 热解是利用生物质能的一种高效且经济的方式,但生物质热解气中的含氮化合物使热解气品质低且燃烧导致空气二次污染。本工作总结了生物质热解气中的含氮化合物研究现状,主要综述了典型生物质热失重行为,探讨了生物质热解气中含氮化合物的生成机理,分析了含氮化合物的分布状况和控制的研究进展。同时,指出了含氮化合物控制在实际应用中面临的困难挑战,进一步展望了含氮化合物控制工艺优化及经济性分析的重点研究方向,为生物质热解气净化提供理论依据和技术支持。Abstract: Biomass energy plays an important role in combating global warming and the depletion of fossil energy sources. Although different recovery technologies of biomass energy were utilized industrially, the development level of different recovery technologies varies. The application of biomass energy includes technologies such as combustion, pyrolysis, gasification, and fermentation. The pyrolysis technology is an efficient and economical method to utilize biomass energy, which combines the advantage of energy recovery and product diversification. However, the N-containing compounds in the biomass pyrolysis gas make the pyrolysis gas of low quality, which combustion leads to secondary pollution of air. This review summaries the research status of N-containing compounds in the biomass pyrolysis gas, mainly reviewing the differences in the thermos-gravimetric behavior of typical biomass and the four compositions in biomass (cellulose, hemicellulose, lignin, and proteins). There were significant differences in the thermos-gravimetric behavior of biomass with different material compositions, but the whole TG curve can be divided into three stages: in the first stage, the pyrolysis of easily decomposable components in biomass releases small molecule gases and steam; in the second stage, the pyrolysis of cellulose, hemicellulose, and lignin in biomass released a large amount of O-containing bio-oil; in the third stage, the volatile components attached to the surface of the bio-char were cracked again and condensation reaction occurs. The nitrogen content in biomass was high, and during the pyrolysis process, nitrogen migrated into the solid-liquid-gas three-phase, and the migration transformation process was extremely complex. This review also discussed the generation mechanism of N-containing compounds in biomass pyrolysis gas and analyzed the distribution and control research of N-containing compounds. The NH3 in the low-temperature pyrolysis gas was mainly derived from the direct pyrolysis of protein in biomass. With the increase of pyrolysis temperature, the biomass pyrolysis volatiles were cracked secondly to generate N-containing heterocyclic substances, nitriles, and cyclic amides, and further cracked to produce HCN. Under the high-temperature atmosphere, partial HCN reacts with ·H and generates NH3 with the biomass char catalysis, leading to a decrease in the concentration of HCN. The N-containing heterocyclic substances from the second cracking of volatiles were the main resource of HCNO, and HCNO has a relatively lower concentration and is easily reduced to HCN and NO. Thus, with the pyrolysis temperature increase, the main components of N-containing compounds in the pyrolysis gas were gradually converted from NH3 and HCNO to NO and HCN. When the temperature was 800 ℃, the concentration of NO accounted for 40% of the N-containing compounds in pyrolysis gas. While, at 900 ℃, NH3 and HNCO were barely detectable. At the same time, it pointed out the difficulties and challenges faced in the practical application of N-containing compound removal. It is necessary to establish a generalized mechanism for nitrogen conversion during the thermal conversion of biomass. The nitrogen transport and control mechanisms during biomass pyrolysis need to be further improved. And, the key research directions in the process optimization and economic analysis of N-containing control are further anticipated. This review aims to provide a theoretical basis and technology support for biomass pyrolysis gas purification.
-
Key words:
- biomass /
- pyrolysis /
- N-containing compound /
- generation mechanism /
- removal
-
表 1 部分典型生物质化学组成
Table 1 chemical composition of partial typical biomass
Material Proximate analysis/% Ultimate analysis/% QHHV/(MJ·kg−1) Reference M V A FC N C H S Oa Almond shell (ar) 11.0 69.6 1.30 18.10 0.50 49.38 5.23 − 44.76 17.92 [12] Pine sawdust (ar) 1.67 84.27 1.02 13.04 0.72 34.65 4.13 − 45.8 − [13] Cow dung (ar) 4.35 73.71 21.40 0.54 1.53 24.39 3.08 − 40.2 − Kidney bean stura (ar) 4.25 82.4 1.84 11.51 1.67 38.19 6.10 − 54.01 − Bamboo (ar) 3.37 71.81 16.58 8.24 0.90 27.22 3.42 − 43.8 − Penicillin residue(db) − 78.51 8.09 13.40 8.04 48.07 6.96 0.57 36.36 19.28 [14] Hygromycin residue(db) − 73.09 14.85 11.25 10.93 50.60 7.17 0.81 30.49 19.33 Soybean straw(db) 77.77 5.32 16.91 1.40 46.74 6.59 0.06 45.21 − [15] Fibreboard(db) 83.56 0.30 16.13 7.49 44.79 6.16 0.01 41.55 − Cellulose (ar) 4.76 96.66 0.05 3.34 0.00 43.44 6.42 0.00 50.14 − [16] Hemicellulose (ar) 4.32 90.81 0.12 9.19 0.01 41.76 6.72 0.00 51.51 − Lignin (ar) 5.33 65.74 16.40 34.26 0.03 61.48 5.86 3.08 29.55 − Protein (ar) 8.78 83.25 4.59 16.75 14.90 51.07 7.72 1.12 25.19 − -: no tested,a: indicated difference calculation, ar: as received,db: dry basis. -
[1] SENNECA O, CERCIELLO F. Kinetics of combustion of lignocellulosic biomass: Recent research and critical issues[J]. Fuel,2023,347:128310. doi: 10.1016/j.fuel.2023.128310 [2] ZHANG Y, WANG J, WEI J, et al. Biomass catalytic pyrolysis over CaO microspheres: Relationship between the production of bio-oil components and CO2 capture[J]. Fuel Process Technol,2023,247:107775. doi: 10.1016/j.fuproc.2023.107775 [3] FAIZAN M, SONG H. Critical review on catalytic biomass gasification: State-of-Art progress, technical challenges, and perspectives in future development[J]. J Clean Prod,2023,408:137224. doi: 10.1016/j.jclepro.2023.137224 [4] YU S, YANG X, LI Q, et al. Breaking the temperature limit of hydrothermal carbonization of lignocellulosic biomass by decoupling temperature and pressure[J]. Green Energy Environ,2023,8(4):1216. doi: 10.1016/j.gee.2023.01.001 [5] LI J, LIU X, ZHU C, et al. Bacterial dynamics and functions driven by biomass wastes to promote rural toilet blackwater absorption and recycling in an ectopic fermentation system[J]. Chemosphere,2023,316:137804. doi: 10.1016/j.chemosphere.2023.137804 [6] VUPPALADADIYAM A K, VUPPALADADIYAM S S V, AWASTHI A, et al. Biomass pyrolysis: A review on recent advancements and green hydrogen production[J]. Bioresour Technol,2022,364:128087. doi: 10.1016/j.biortech.2022.128087 [7] HANSSON K-M, SAMUELSSON J, TULLIN C, et al. Formation of HNCO, HCN, and NH3 from the pyrolysis of bark and nitrogen-containing model compounds[J]. Combust Flame,2004,137(3):265−277. doi: 10.1016/j.combustflame.2004.01.005 [8] 刘亮, 郑扬, 黄思彪, 等. 生物质热解过程中氮迁移转化机理研究进展[J]. 农业工程学报,2022,38(19):227−236.LIU liang, ZHENG yang, HUANG sibiao, et al. Review of nitrogen migration and transformation during biomass pyrolysis[J]. Trans Chin Soci Agric Eng,2022,38(19):227−236. [9] NAIK G G and DHARMADHIKARI H M. Methods for reducing NO x and PM emissions in compression ignition engine: A review[J]. Mater Today: Proc,2023,72:1406−1412. doi: 10.1016/j.matpr.2022.09.339 [10] HERREROS J M, GEORGE P, UMAR M, et al. Enhancing selective catalytic reduction of NO x with alternative reactants/promoters[J]. Chem Eng J,2014,252:47−54. doi: 10.1016/j.cej.2014.04.095 [11] SHAFIZADEH A, RASTEGARI H, SHAHBEIK H, et al. A critical review of the use of nanomaterials in the biomass pyrolysis process[J]. J Clean Prod,2023,400:136705. doi: 10.1016/j.jclepro.2023.136705 [12] RASOOL T, NAJAR I, SRIVASTAVA V C, et al. Pyrolysis of almond (Prunus amygdalus) shells: Kinetic analysis, modelling, energy assessment and technical feasibility studies[J]. Bioresour Technol,2021,337:125466. doi: 10.1016/j.biortech.2021.125466 [13] LI J, YAO X, GE J, et al. Investigation on the pyrolysis process, products characteristics and BP neural network modelling of pine sawdust, cattle dung, kidney bean stalk and bamboo[J]. Process Saf Environ Prot,2022,162:752−764. doi: 10.1016/j.psep.2022.04.055 [14] 詹昊, 林均衡, 黄艳琴, 等. 抗生素菌渣热解N官能团变化特征及其与NO x 前驱物关系研究[J]. 燃料化学学报,2017,45(10):1219−1229.ZHAN hao, LIN junheng, HUANG yanqin, et al. Evolution of nitrogen functionalities and their relation to NO x precursors during pyrolysis of antibiotic mycelia wastes[J]. J Fuel Chem Technol,2017,45(10):1219−1229. [15] 张晓鸿, 詹昊, 阴秀丽, 等. 富氮生物质原料热解过程中NO x 前驱物释放特性研究[J]. 燃料化学学报,2016,44(12):1464−1472.ZHANG xiaohong, ZHAN hao, YIN xiuli, et al. Release characreristic of NO x precursors during the pyrolysis of nitrogen-rich biomass[J]. J Fuel Chem Technol,2016,44(12):1464−1472. [16] ZONG P, JIANG Y, TIAN Y, et al. Pyrolysis behavior and product distributions of biomass six group components: Starch, cellulose, hemicellulose, lignin, protein and oil[J]. Energy Convers Manag,2020,216:112777. doi: 10.1016/j.enconman.2020.112777 [17] SINGH R K, RUJ B, SADHUKHAN A K, et al. A TG-FTIR investigation on the co-pyrolysis of the waste HDPE, PP, PS and PET under high heating conditions[J]. J Energy Inst,2020,93(3):1020−1035. doi: 10.1016/j.joei.2019.09.003 [18] MA Z, WANG J, YANG Y, et al. Comparison of the thermal degradation behaviors and kinetics of palm oil waste under nitrogen and air atmosphere in TGA-FTIR with a complementary use of model-free and model-fitting approaches[J]. J Anal Appl Pyrolysis,2018,134:12−24. doi: 10.1016/j.jaap.2018.04.002 [19] WANG F, GAO N, MAGDZIARZ A, et al. Co-pyrolysis of biomass and waste tires under high-pressure two-stage fixed bed reactor[J]. Bioresour Technol,2022,344:126306. doi: 10.1016/j.biortech.2021.126306 [20] 王锐, 高明洋, 曹景沛. 碱/碱土金属催化松木屑快速热解机制[J]. 应用化学,2022,39(2):289−297.WANG rui, GAO mingyang, CAO jingpei. Effects of alkali/alkaline earth metals on fast pyrolysis of pine sawdust[J]. Chin J Appl Chem,2022,39(2):289−297. [21] GAO N, LI A, QUAN C, et al. TG-FTIR and Py-GC/MS analysis on pyrolysis and combustion of pine sawdust[J]. J Anal Appl Pyrolysis,2013,100:26−32. doi: 10.1016/j.jaap.2012.11.009 [22] MA Z, YANG Y, MA Q, et al. Evolution of the chemical composition, functional group, pore structure and crystallographic structure of bio-char from palm kernel shell pyrolysis under different temperatures[J]. J Anal Appl Pyrolysis,2017,127:350−359. doi: 10.1016/j.jaap.2017.07.015 [23] MA Z, CHEN D, GU J, et al. Determination of pyrolysis characteristics and kinetics of palm kernel shell using TGA-FTIR and model-free integral methods[J]. Energy Convers Manag,2015,89:251−259. doi: 10.1016/j.enconman.2014.09.074 [24] LIANG F, WANG R, HONGZHONG X, et al. Investigating pyrolysis characteristics of moso bamboo through TG-FTIR and Py-GC/MS[J]. Bioresour Technol,2018,256:53−60. doi: 10.1016/j.biortech.2018.01.140 [25] LAOUGÉ Z B, MERDUN H. Investigation of thermal behavior of pine sawdust and coal during co-pyrolysis and co-combustion[J]. Energy,2021,231:120895. doi: 10.1016/j.energy.2021.120895 [26] VASSILEV S V, BAXTER D, ANDERSEN L K, et al. An overview of the organic and inorganic phase composition of biomass[J]. Fuel,2012,94:1−33. doi: 10.1016/j.fuel.2011.09.030 [27] 童金华, 林占熺, 林应兴. 菌草栽培平菇2种出菇方式的蛋白质营养评价[J]. 福建轻纺,2023(07):7−11+17.TONG jinhua, LIN zhanxi, LIN yingxing. Nutritional evaluation of proteins in two types of mushroom production by mycorrhizal cultivation of flat mushrooms[J]. Light Textile Ind Fujian,2023,(07):7−11+17. [28] GE L, ZHAO C, ZUO M, et al. Effects of Fe addition on pyrolysis characteristics of lignin, cellulose and hemicellulose[J]. J Energy Inst,2023,107:101177. doi: 10.1016/j.joei.2023.101177 [29] HUANG Y, SEKYERE D T, ZHANG J, et al. Fast pyrolysis behaviors of biomass with high contents of ash and nitrogen using TG-FTIR and Py-GC/MS[J]. J Anal Appl Pyrolysis,2023,170:105922. doi: 10.1016/j.jaap.2023.105922 [30] WEI X, MA X, PENG X, et al. Comparative investigation between co-pyrolysis characteristics of protein and carbohydrate by TG-FTIR and Py-GC/MS[J]. J Anal Appl Pyrolysis,2018,135:209−218. doi: 10.1016/j.jaap.2018.08.031 [31] REN Q, ZHAO C. NO x and N2O precursors (NH3 and HCN) from biomass pyrolysis: Interaction between amino acid and mineral matter[J]. Appl Energy,2013,112:170−174. doi: 10.1016/j.apenergy.2013.05.061 [32] LIU X, LUO Z, YU C, et al. Release mechanism of Fuel-N into NO x and N2O precursors during pyrolysis of rice straw[J]. Energies,2018,11(3):520. doi: 10.3390/en11030520 [33] LIU X, LUO Z, YU C, et al. Conversion mechanism of fuel-N during pyrolysis of biomass wastes[J]. Fuel,2019,246:42−50. doi: 10.1016/j.fuel.2019.02.042 [34] YUAN S, ZHOU Z J, LI J, et al. HCN and NH3 released from biomass and soybean cake under rapid pyrolysis[J]. Energy Fuels,2010,24(11):6166−6171. doi: 10.1021/ef100959g [35] WANG Y, DONG B, FAN Y, et al. Nitrogen transformation during pyrolysis of oilfield sludge with high polymer content[J]. Chemosphere,2019,219:383−389. doi: 10.1016/j.chemosphere.2018.11.171 [36] BURDOVÁ H, KWOCZYNSKI Z, NEBESKÁ D, et al. The influence of diesel contaminated soil on miscanthus x giganteus biomass thermal utilization and pyrolysis products composition[J]. J Clean Prod,2023,406(5):136984. [37] PENG X, MA X, LIN Y, et al. Co-pyrolysis between microalgae and textile dyeing sludge by TG-FTIR: Kinetics and products[J]. Energy Convers Manag,2015,100:391−402. doi: 10.1016/j.enconman.2015.05.025 [38] UZUN B B, PÜTÜN A E, PÜTÜN E. Fast pyrolysis of soybean cake: Product yields and compositions[J]. Bioresour Technol,2006,97(4):569−576. doi: 10.1016/j.biortech.2005.03.026 [39] 陆强, 赵微, 夏源谷, 等. 生物质热解过程中氮元素迁移转化机制研究进展[J]. 燃料化学学报(中英文),2023,51(8):1047−1059+1025.LU qiang, ZHAO wei, XIA yuangu, et al. Research on the migration and transformation mechanism of nitrogen during biomass pyrolysis[J]. J Fuel Chem Technol,2023,51(8):1047−1059+1025. [40] HU J, SONG Y, LIU J, et al. Synergistic effects, gaseous products, and evolutions of NO x precursors during (co-)pyrolysis of textile dyeing sludge and bamboo residues[J]. J Hazard Mater,2021,401:123331. doi: 10.1016/j.jhazmat.2020.123331 [41] LI D, SHI F, FU M, et al. Insight into the synergistic reaction mechanism of biomass pseudocomponents and polyamide by TG-MS and Py-GC/MS: Pyrolysis properties, reaction kinetics and N-containing species evolution[J]. Ind Crops Prod,2023,195:116428. doi: 10.1016/j.indcrop.2023.116428 [42] ZHU X, YANG S, WANG L, et al. Tracking the conversion of nitrogen during pyrolysis of antibiotic mycelial fermentation residues using XPS and TG-FTIR-MS technology[J]. Environ Pollut,2016,211:20−27. doi: 10.1016/j.envpol.2015.12.032 [43] TIAN Y, ZHANG J, ZUO W, et al. Nitrogen conversion in relation to NH3 and HCN during microwave pyrolysis of sewage sludge[J]. Environ Sci Technol,2013,47(7):3498−3505. doi: 10.1021/es304248j [44] LI J, WANG Z, YANG X, et al. Evaluate the pyrolysis pathway of glycine and glycylglycine by TG-FTIR[J]. J Anal Appl Pyrolysis,2007,80(1):247−253. doi: 10.1016/j.jaap.2007.03.001 [45] CHEN W, CHEN Y, YANG H, et al. Investigation on biomass nitrogen-enriched pyrolysis: Influence of temperature[J]. Bioresour Technol,2018,249:247−253. doi: 10.1016/j.biortech.2017.10.022 [46] LI C, ZHU L, MA Z, et al. Optimization of the nitrogen and oxygen element distribution in microalgae by ammonia torrefaction pretreatment and subsequent fast pyrolysis process for the production of N-containing chemicals[J]. Bioresour Technol,2021,321:124461. doi: 10.1016/j.biortech.2020.124461 [47] PENG K-H, WU Q, TIPENG W, et al. Generation mechanism of NO x and N2O precursors (NH3 and HCN) from aspartic acid pyrolysis: A DFT study[J]. Int J Agric Biol Eng,2016,9:166−176. [48] WEI F, CAO J-P, ZHAO X-Y, et al. Nitrogen evolution during fast pyrolysis of sewage sludge under inert and reductive atmospheres[J]. Energy Fuels,2017,31(7):7191−7196. doi: 10.1021/acs.energyfuels.7b00920 [49] TIAN K, LIU W-J, QIAN T-T, et al. Investigation on the evolution of N-Containing organic compounds during pyrolysis of sewage sludge[J]. Environ Sci Technol,2014,48(18):10888−10896. doi: 10.1021/es5022137 [50] FONTS I, AZUARA M, LÁZARO L, et al. Gas Chromatography study of sewage sludge pyrolysis liquids obtained at different operational conditions in a fluidized bed[J]. Ind Eng Chem Res,2009,48(12):5907−5915. doi: 10.1021/ie900421a [51] REN Q, ZHAO C. NO x and N2O precursors from biomass pyrolysis: Nitrogen transformation from amino acid[J]. Environ Sci Technol,2012,46(7):4236−4240. doi: 10.1021/es204142e [52] WEI Y, TIAN H, LIU L, et al. The effects of alkali metals and alkaline earth metals on the mechanism of N-containing gases production during glutamic acid pyrolysis[J]. J Anal Appl Pyrolysis,2022,168:105787. doi: 10.1016/j.jaap.2022.105787 [53] CHEN H, SHAN R, ZHAO F, et al. A review on the NO x precursors release during biomass pyrolysis[J]. Chem Eng J,2023,451:138979. doi: 10.1016/j.cej.2022.138979 [54] ZHANG J, TIAN Y, CUI Y, et al. Key intermediates in nitrogen transformation during microwave pyrolysis of sewage sludge: A protein model compound study[J]. Bioresour Technol,2013,132:57−63. doi: 10.1016/j.biortech.2013.01.008 [55] CHEN W, YANG H, CHEN Y, et al. Transformation of nitrogen and evolution of n-containing species during algae pyrolysis[J]. Environ Sci Technol,2017,51(11):6570−6579. doi: 10.1021/acs.est.7b00434 [56] CHEN H, SI Y, CHEN Y, et al. NO x precursors from biomass pyrolysis: Distribution of amino acids in biomass and Tar-N during devolatilization using model compounds[J]. Fuel,2017,187:367−375. doi: 10.1016/j.fuel.2016.09.075 [57] LI X, DONG Z, DOU J, et al. Catalytic reduction of NO using iron oxide impregnated biomass and lignite char for flue gas treatment[J]. Fuel Process Technol,2016,148:91−98. doi: 10.1016/j.fuproc.2016.02.030 [58] WU X Y, SONG Q, ZHAO H B, et al. Kinetic modeling of inherent mineral catalyzed NO reduction by biomass char[J]. Environ Sci Technol,2014,48(7):4184−4190. doi: 10.1021/es405521k [59] BAILÓN-GARCÍA E, DRWAL E, GRZYBEK T, et al. Catalysts based on carbon xerogels with high catalytic activity for the reduction of NO x at low temperatures[J]. Catal Today,2020,356:301−311. doi: 10.1016/j.cattod.2020.03.004 [60] LI C-Z, TAN L L. Formation of NO x and SOx precursors during the pyrolysis of coal and biomass. Part III. Further discussion on the formation of HCN and NH3 during pyrolysis[J]. Fuel,2000,79(15):1899−1906. doi: 10.1016/S0016-2361(00)00008-9 [61] TIAN F-J, YU J, MCKENZIE L J, et al. Conversion of Fuel-N into HCN and NH3 during the pyrolysis and gasification in steam: A comparative study of coal and biomass[J]. Energy Fuels,2007,21(2):517−521. doi: 10.1021/ef060415r [62] 詹昊, 张晓鸿, 阴秀丽, 等. 生物质热化学转化过程含N污染物形成研究[J]. 化学进展,2016,28(12):1880−1890.ZHAN Hao, ZHANG Xiaohong, YIN Xiuli, et al. Formation of nitrogenous pollutants during biomass thermo-chemical conversion[J]. Prog Chem,2016,28(12):1880−1890. [63] 洪文鹏, 张钰, 姜海峰, 等. CO_2气氛耦合粉煤灰催化生物质热解生油特性分析[J]. 农业工程学报,2022,38(04):235−241.HONG Wenpeng, ZHANG Yu, JIANG Haifeng, et al. Characteristics of bio-oil generated from biomass pyrolysis catalyzed by coal fly ash under CO2 atmosphere[J]. Trans Chinese Soc Agric Eng,2022,38(04):235−241. [64] LI J, TIAN Y, QIAO Y, et al. Synergistic effect of hydrogen atmosphere and biochar catalyst on tar decomposition and methane-rich gas production during biomass pyrolysis[J]. Fuel,2022,330:125680. doi: 10.1016/j.fuel.2022.125680 [65] 马承荣, 肖波, 杨家宽, 等. 生物质热解影响因素研究[J]. 环境技术,2005,(5):16−18+41.MA chengrong, XIAO bo, YANG jiakuan, et al. Study on the effect of operating conditions of biomass pyrolysis[J]. Environ Technol,2005,(5):16−18+41. [66] 刘啸天, 于洁, 孙路石. 温度与粒径对生物质热解特性影响实验研究[J]. 能源研究与管理,2022,(1):57−64.LIU xiaotian, YU jie, SUN lushi. Experimental study on effects of temperature and particle size on biomass pyrolysis characteristics[J]. Energy Res Manag,2022,(1):57−64. [67] SHU Y, ZHANG F, WANG H, et al. An experimental study of NO reduction by biomass reburning and the characterization of its pyrolysis gases[J]. Fuel,2015,139:321−327. doi: 10.1016/j.fuel.2014.08.071 [68] XIAO R, ZHANG J-F, ZHAO L-K. An ammonia-free denitration method: Direct reduction of NO x over activated carbon promoted by Cu-K bimetals[J]. J Fuel Chem Technol,2022,50(5):628−639. doi: 10.1016/S1872-5813(21)60183-4 [69] SHU Y, ZHANG F, WANG F, et al. Catalytic reduction of NO x by biomass-derived activated carbon supported metals[J]. Chin. J Chem Eng,2018,26(10):2077−2083. doi: 10.1016/j.cjche.2018.04.019 [70] RAMALHO P S F, SOARES O S G P, FIGUEIREDO J L, et al. Catalytic reduction of NO over copper supported on activated carbon[J]. Catal. Today,2023,418:114044. doi: 10.1016/j.cattod.2023.114044 [71] CATALÃO R A, MALDONADO-HÓDAR F J, FERNANDES A, et al. Reduction of NO with metal-doped carbon aerogels[J]. Appl Catal, B,2009,88(1):135−141. [72] LEISHMAN C, MAZZONE S, SUN Y, et al. Manganese-based catalysts supported on carbon xerogels for the selective catalytic reduction of NO x using a hollow fibre-based reactor[J]. Catal Today, 2023: 114019. [73] YANG S, ZHU X, WANG J, et al. Combustion of hazardous biological waste derived from the fermentation of antibiotics using TG-FTIR and Py-GC/MS techniques[J]. Bioresour Technol,2015,193:156−163. doi: 10.1016/j.biortech.2015.06.083 [74] RUAN W, WANG Y, LIU C, et al. One-step fabrication of N-doped activated carbon by NH3 activation coupled with air oxidation for supercapacitor and CO2 capture applications[J]. J Anal Appl Pyrolysis,2022,168:105710. doi: 10.1016/j.jaap.2022.105710 [75] XU C, DONALD J, BYAMBAJAV E, et al. Recent advances in catalysts for hot-gas removal of tar and NH3 from biomass gasification[J]. Fuel,2010,89(8):1784−1795. doi: 10.1016/j.fuel.2010.02.014 [76] XIAO K, GUAN R, YANG J, et al. Effects of red mud on emission control of NO x precursors during sludge pyrolysis: A protein model compound study[J]. Waste Manage,2019,85:452−463. doi: 10.1016/j.wasman.2019.01.014 [77] GUO S, LIU T, HUI J, et al. Effects of calcium oxide on nitrogen oxide precursor formation during sludge protein pyrolysis[J]. Energy,2019,189:116217. doi: 10.1016/j.energy.2019.116217 [78] OHTSUKA Y, XU C, KONG D, et al. Decomposition of ammonia with iron and calcium catalysts supported on coal chars[J]. Fuel,2004,83(6):685−692. doi: 10.1016/j.fuel.2003.05.002 [79] REN Q, ZHAO C, WU X, et al. Effect of mineral matter on the formation of NO x precursors during biomass pyrolysis[J]. J Anal Appl Pyrolysis,2009,85(1):447−453. [80] 成洪达, 孟波. 双层中空纤维膜反应器的构建及氨分解制氢研究[C]. 第三届全国新能源与化工新材料学术会议暨全国能量转换与存储材料学术研讨会. 中国江苏苏州, 2018, 75.CHENG hongda, MENG bo. Construction of a double-layer hollow fibre membrane reactor and hydrogen production by ammonia decomposition[C]. The 3rd National Academic Conference on New Energy and New Chemical Materials and National Symposium on Energy Conversion and Storage Materials. Suzhou, Jiangsu, China, 2018, 75.) [81] LIU J, ZHANG X, LU Q, et al. Mechanism study on the effect of alkali metal ions on the formation of HCN as NO x precursor during coal pyrolysis[J]. J Energy Inst,2019,92(3):604−612. doi: 10.1016/j.joei.2018.03.012 [82] FANG S, DENG Z, LIN Y, et al. Nitrogen migration in sewage sludge chemical looping gasification using copper slag modified by NiO as an oxygen carrier[J]. Energy,2021,228:120448. doi: 10.1016/j.energy.2021.120448 [83] GU B, CAO J-P, SHAN Y-F, et al. Catalytic Fast pyrolysis of sewage sludge over HZSM-5: A study of light aromatics, coke, and nitrogen migration under different atmospheres[J]. Ind Eng Chem Res,2020,59(39):17537−17545. doi: 10.1021/acs.iecr.0c01170 [84] 孙志向. 生物质热解过程中燃料氮转化及碱/碱土金属离子催化转化的实验研究[D]. 北京: 华北电力大学; 2014.SUN zhixiang. Experimental study on the fuel-nitrogen transformation and the alkali/alkali-earth metal ions catalysis during the biomass pyrolysis[D]. Beijing: North China Electric Power University; 2014.) -




 下载:
下载: