Study on pyrolysis mechanism and kinetics of two of spiro [4,5] decane and spiro [5,6] dodecane
-
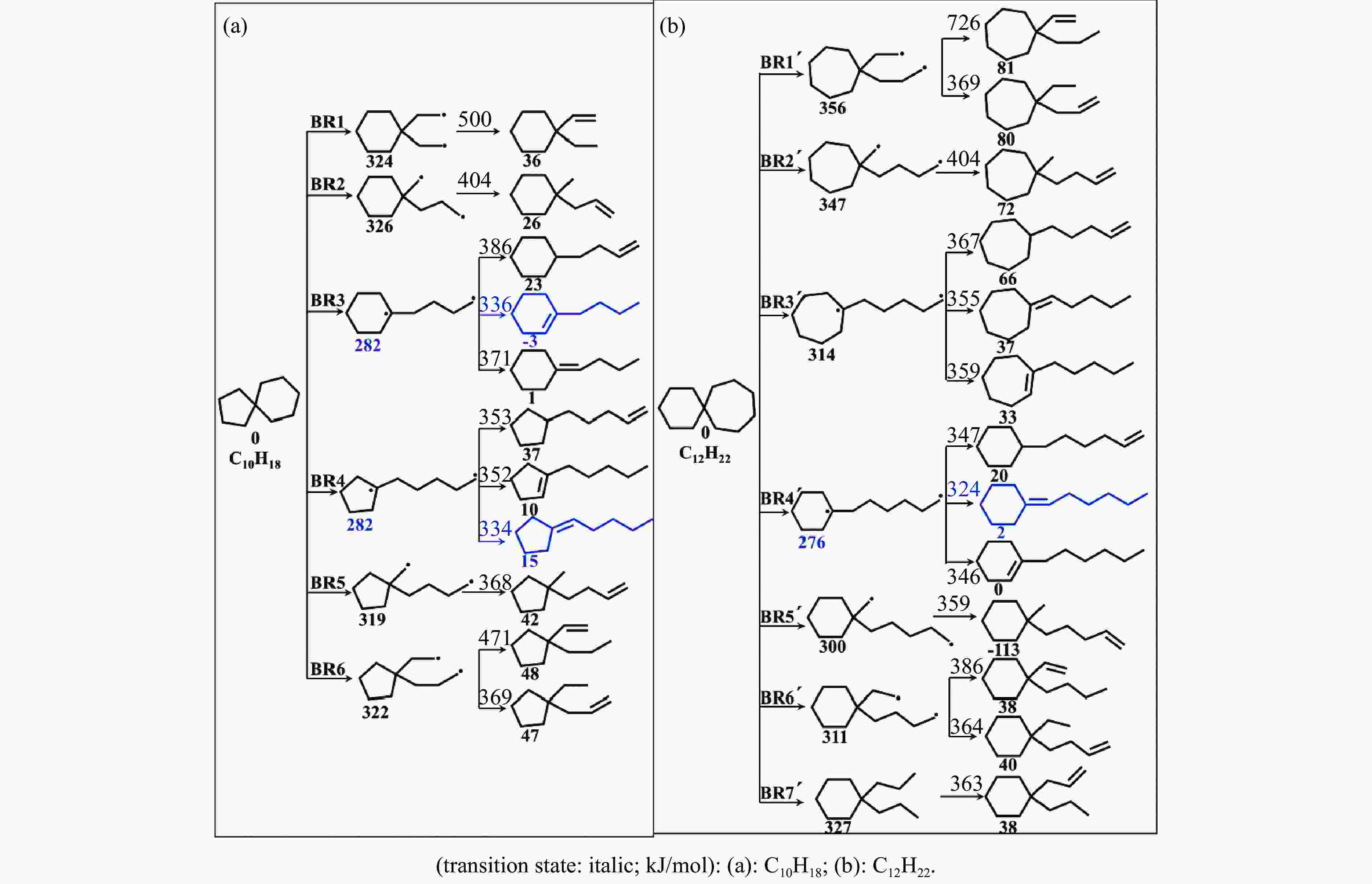
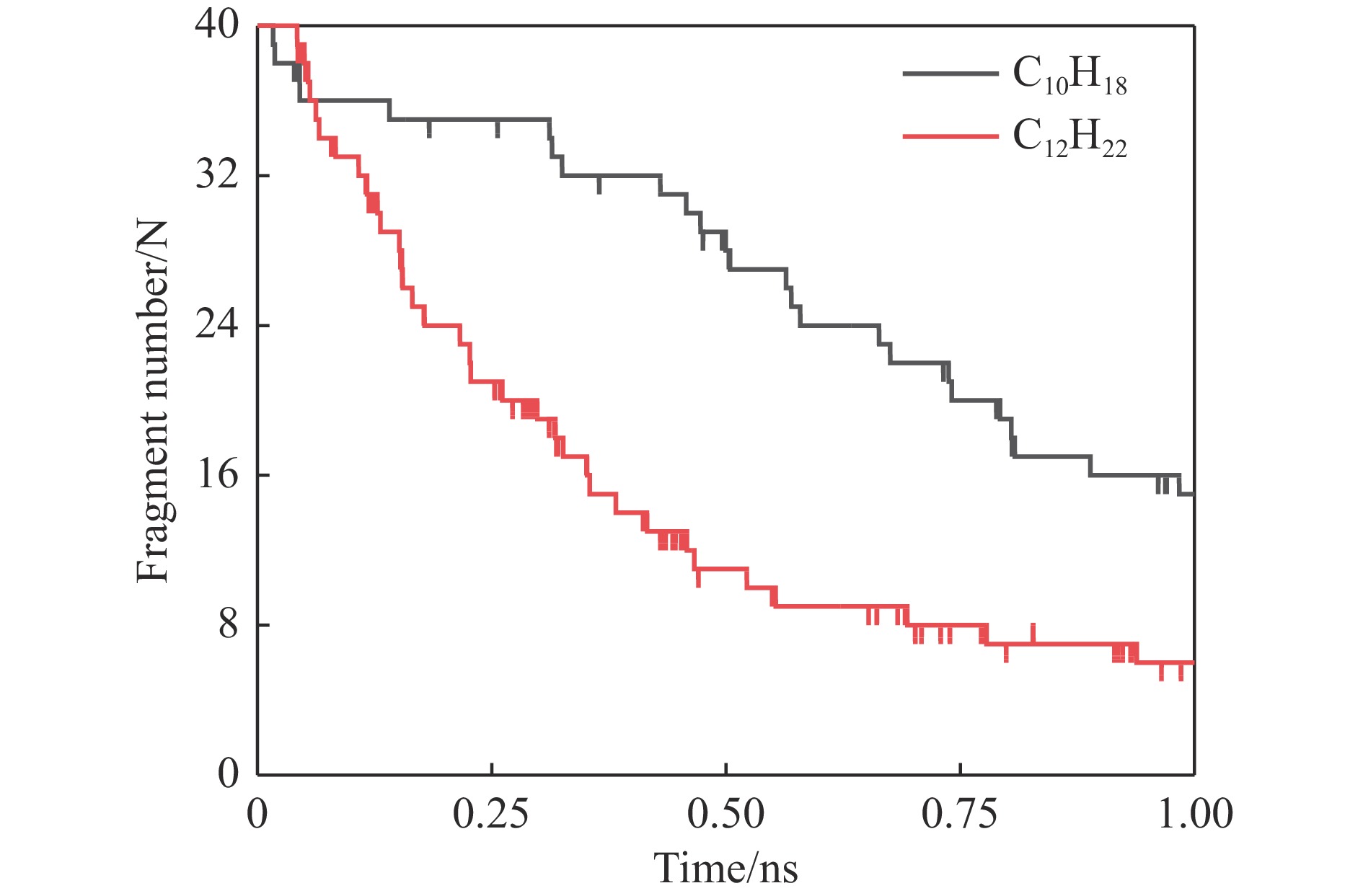
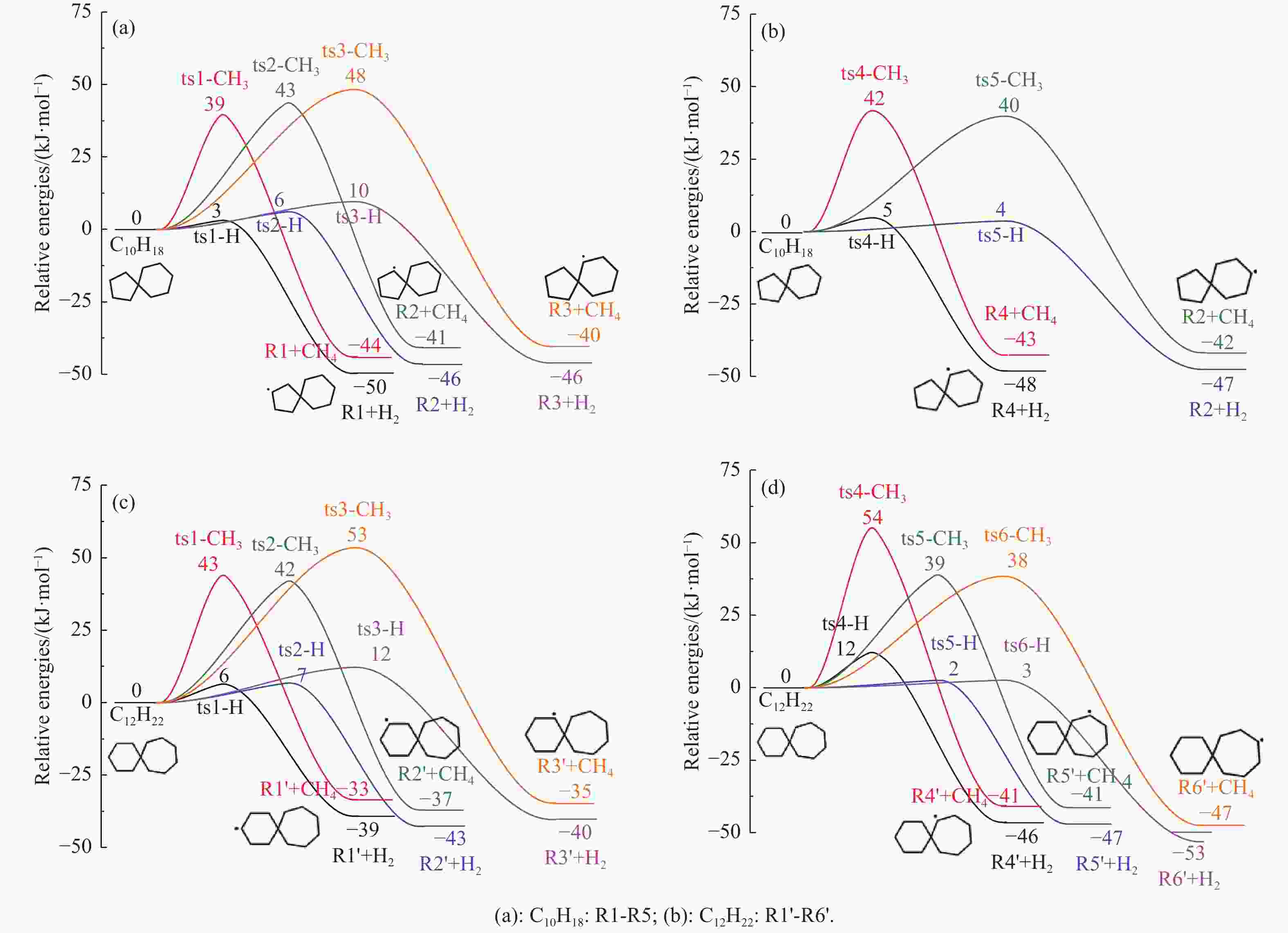
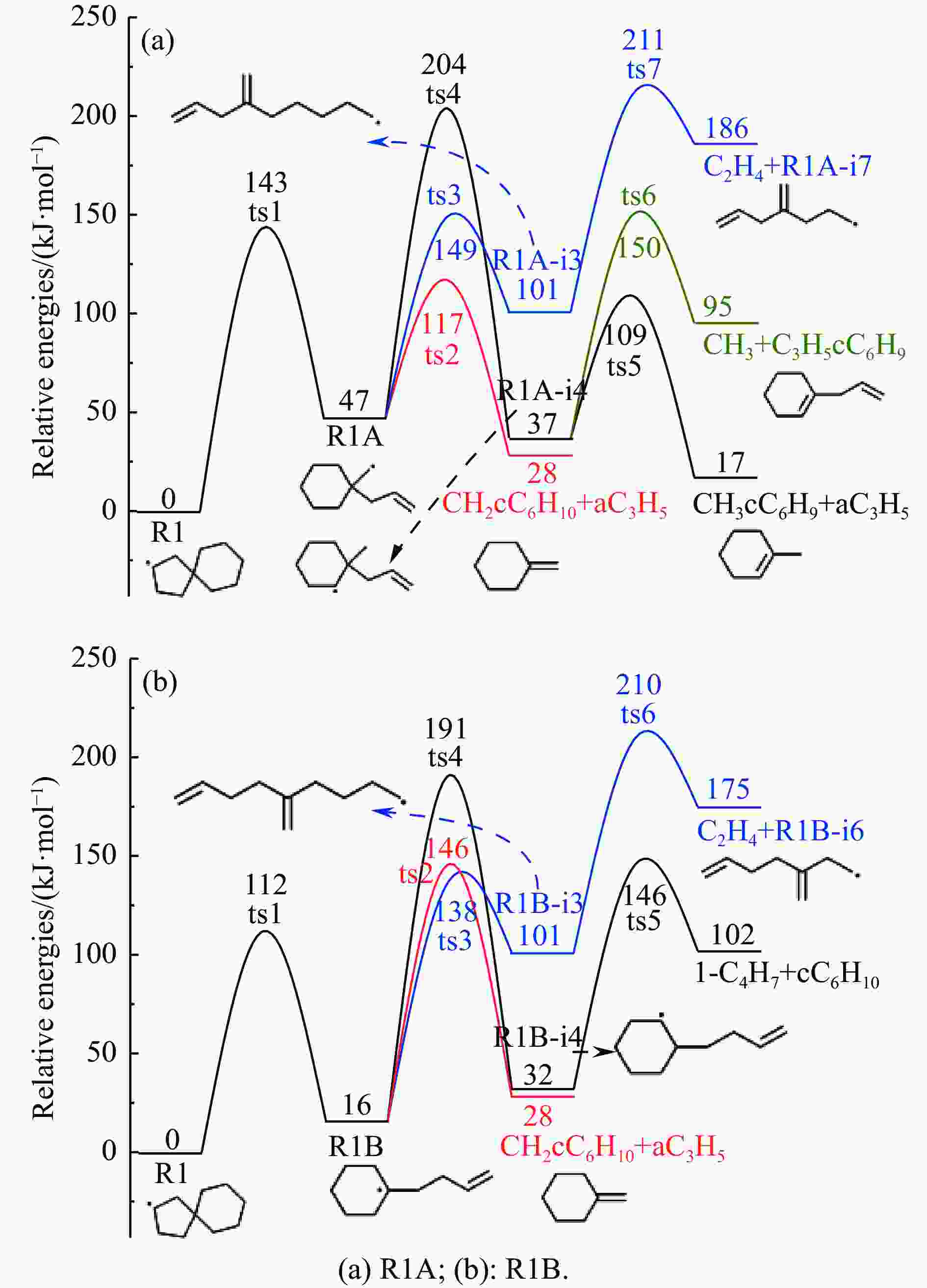
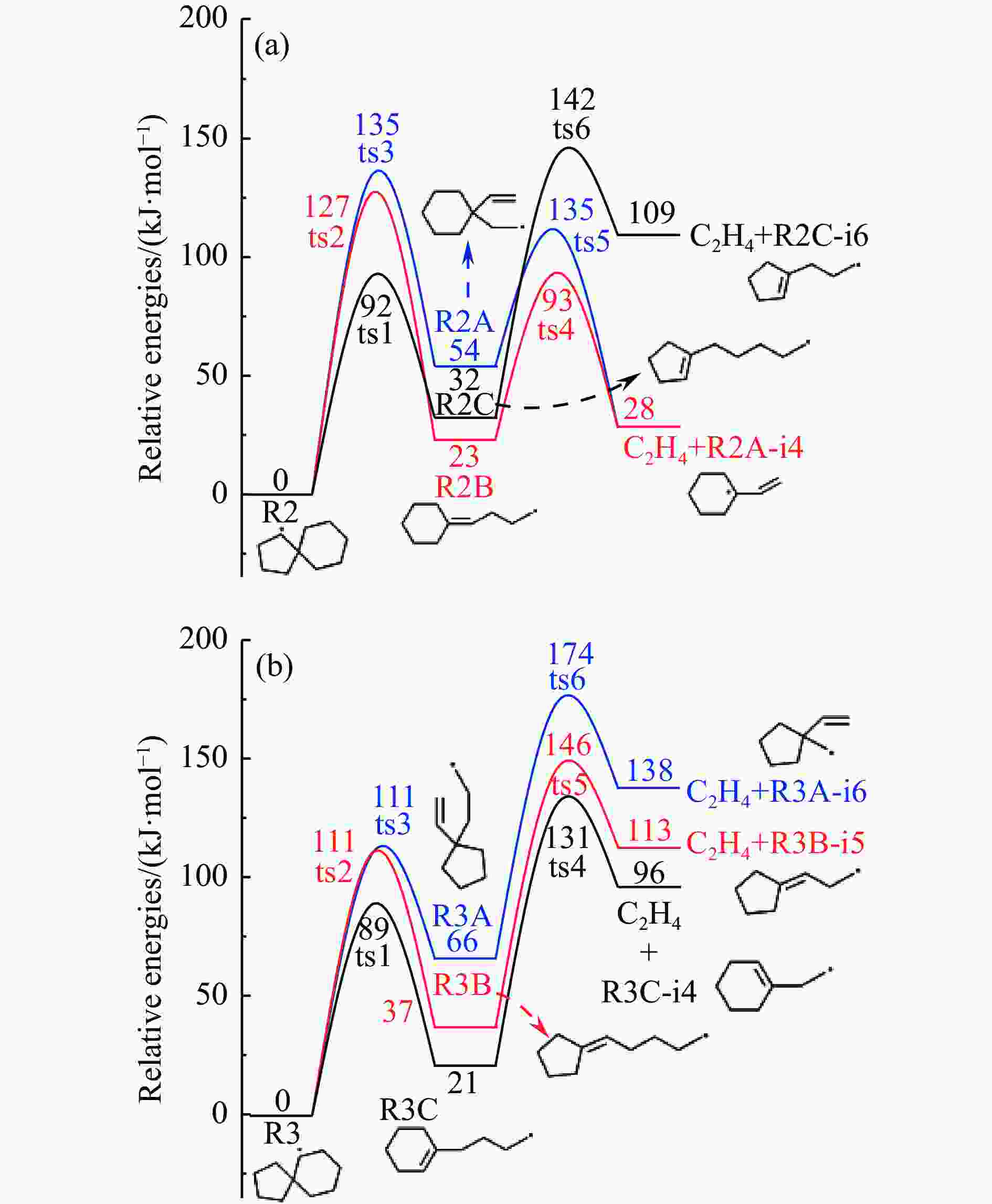
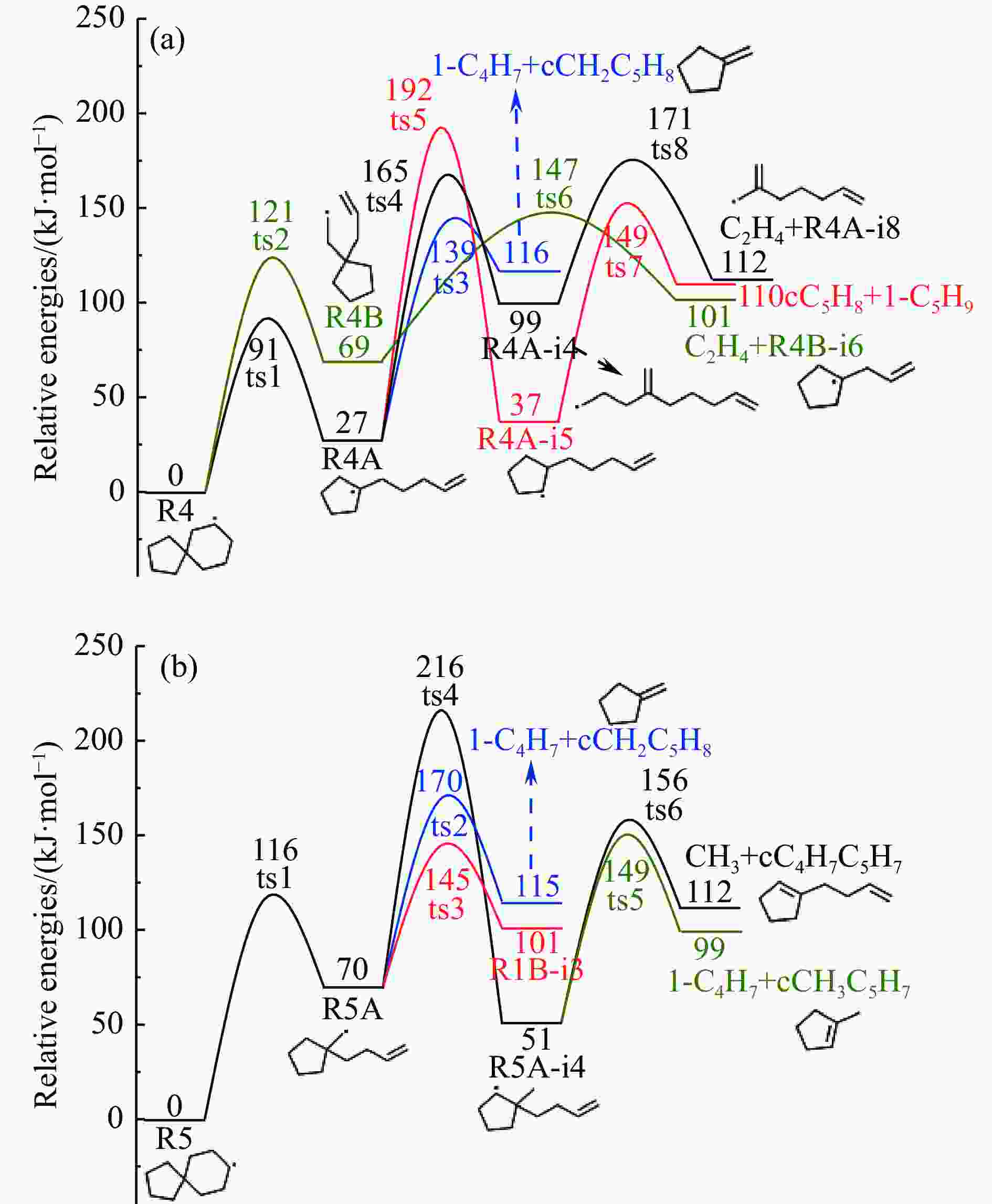
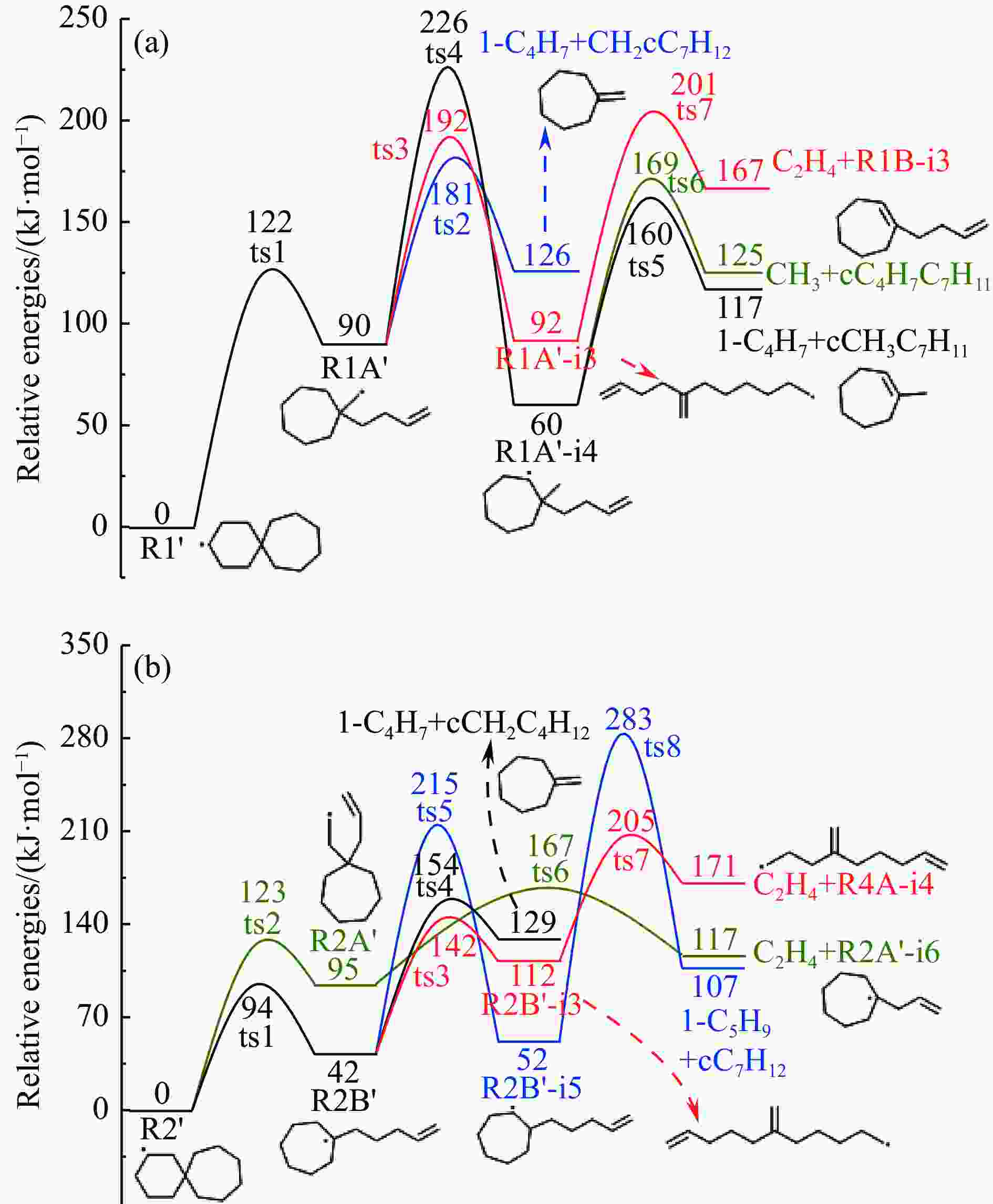
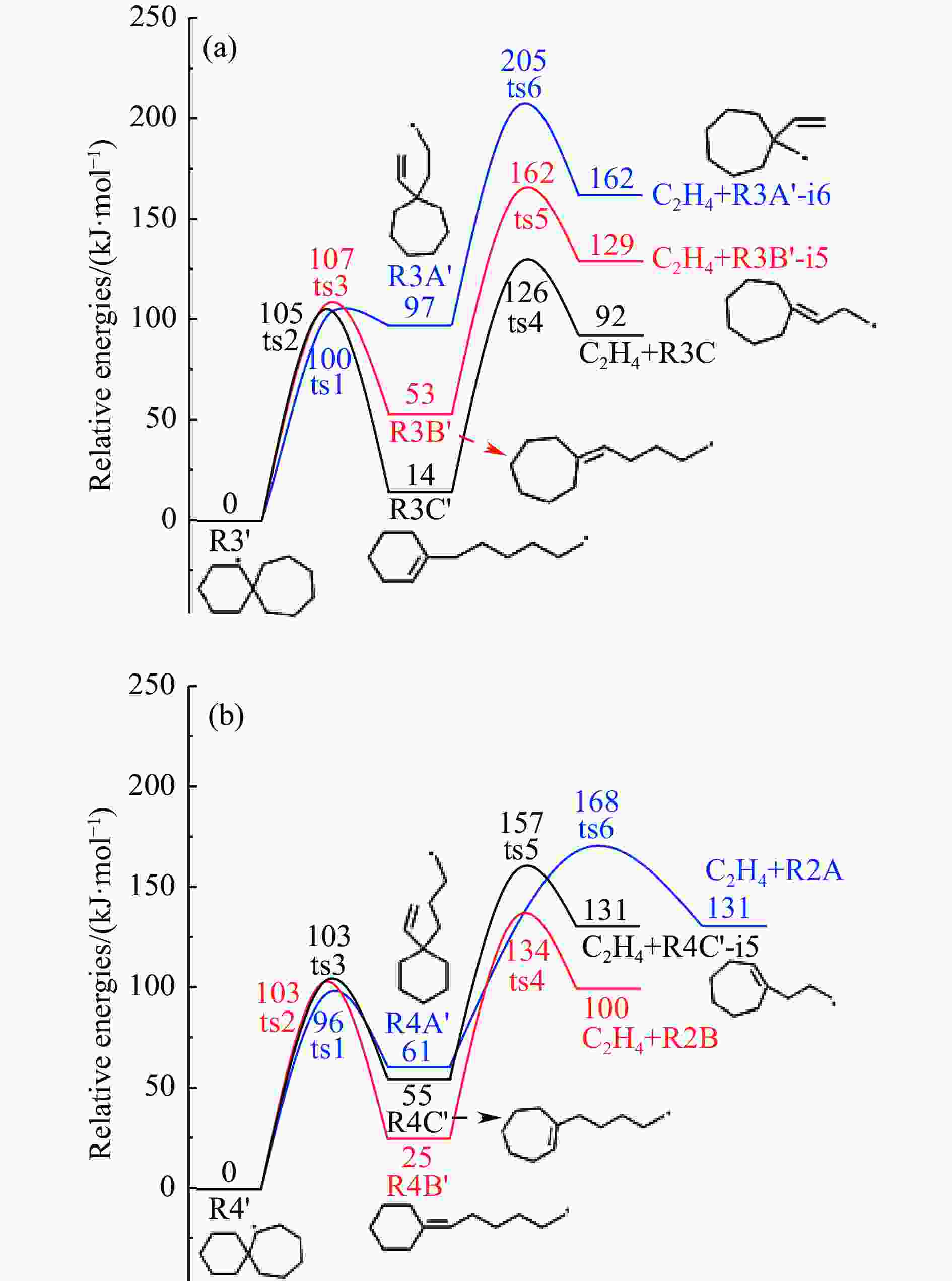
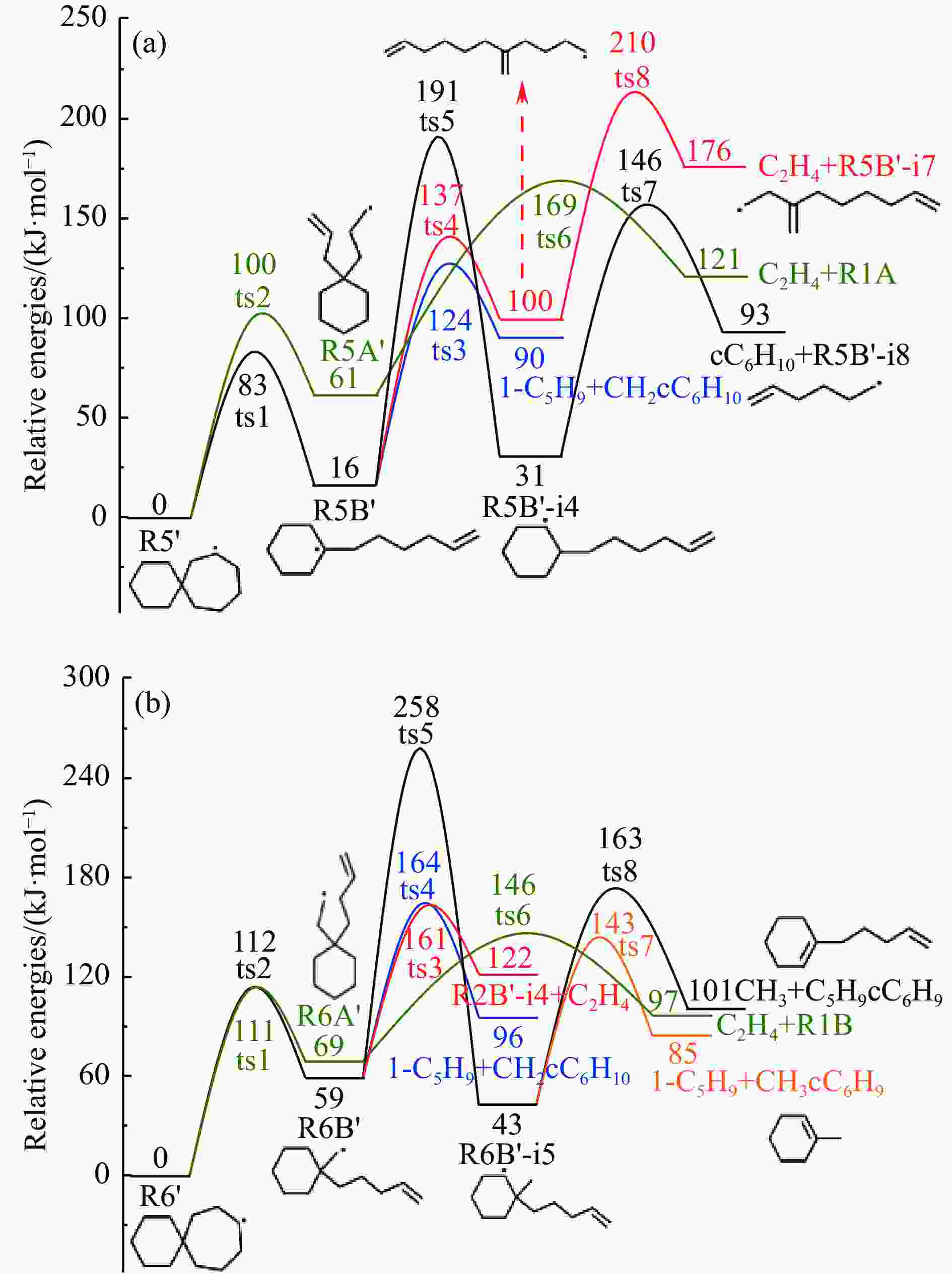
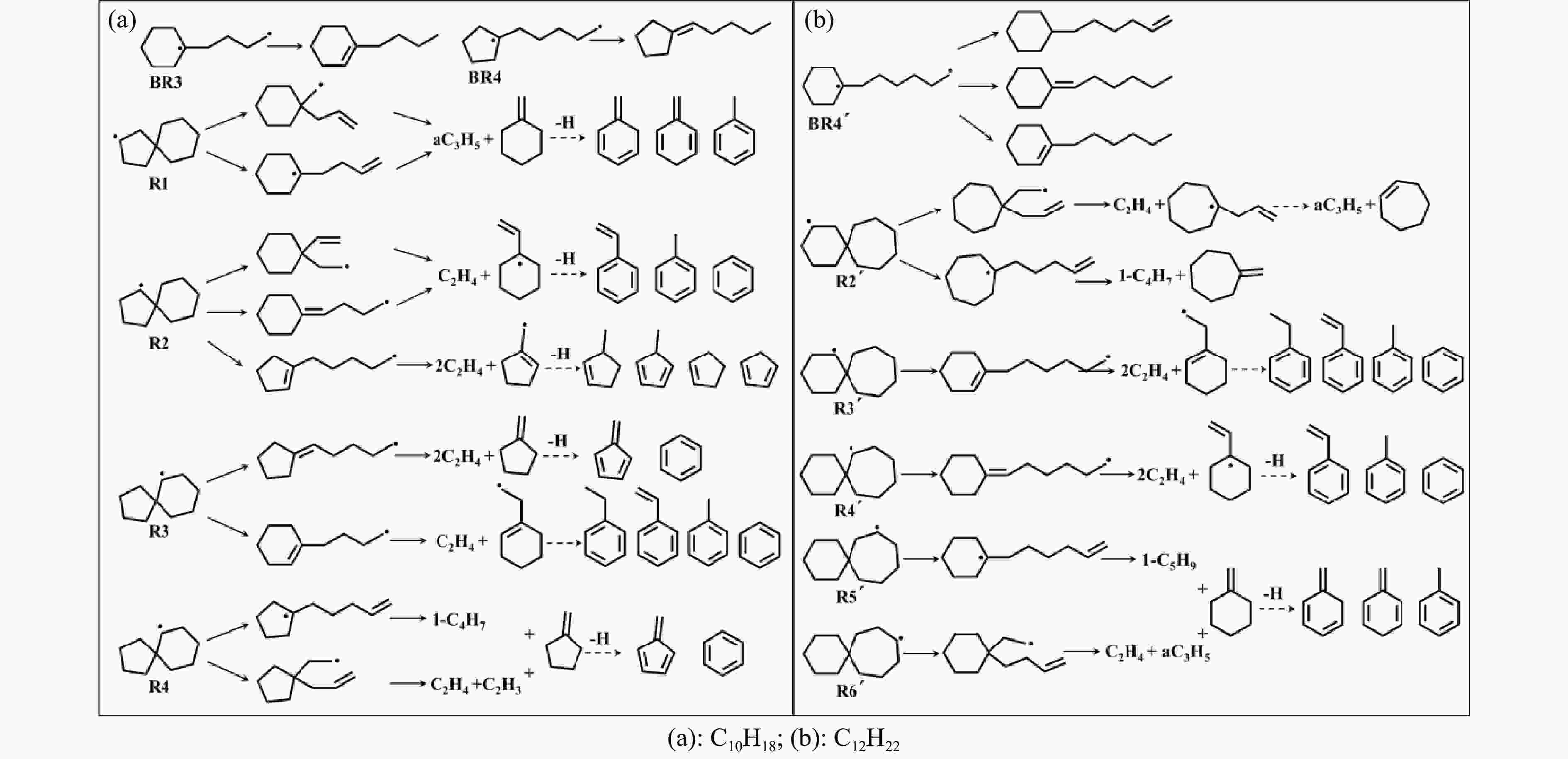
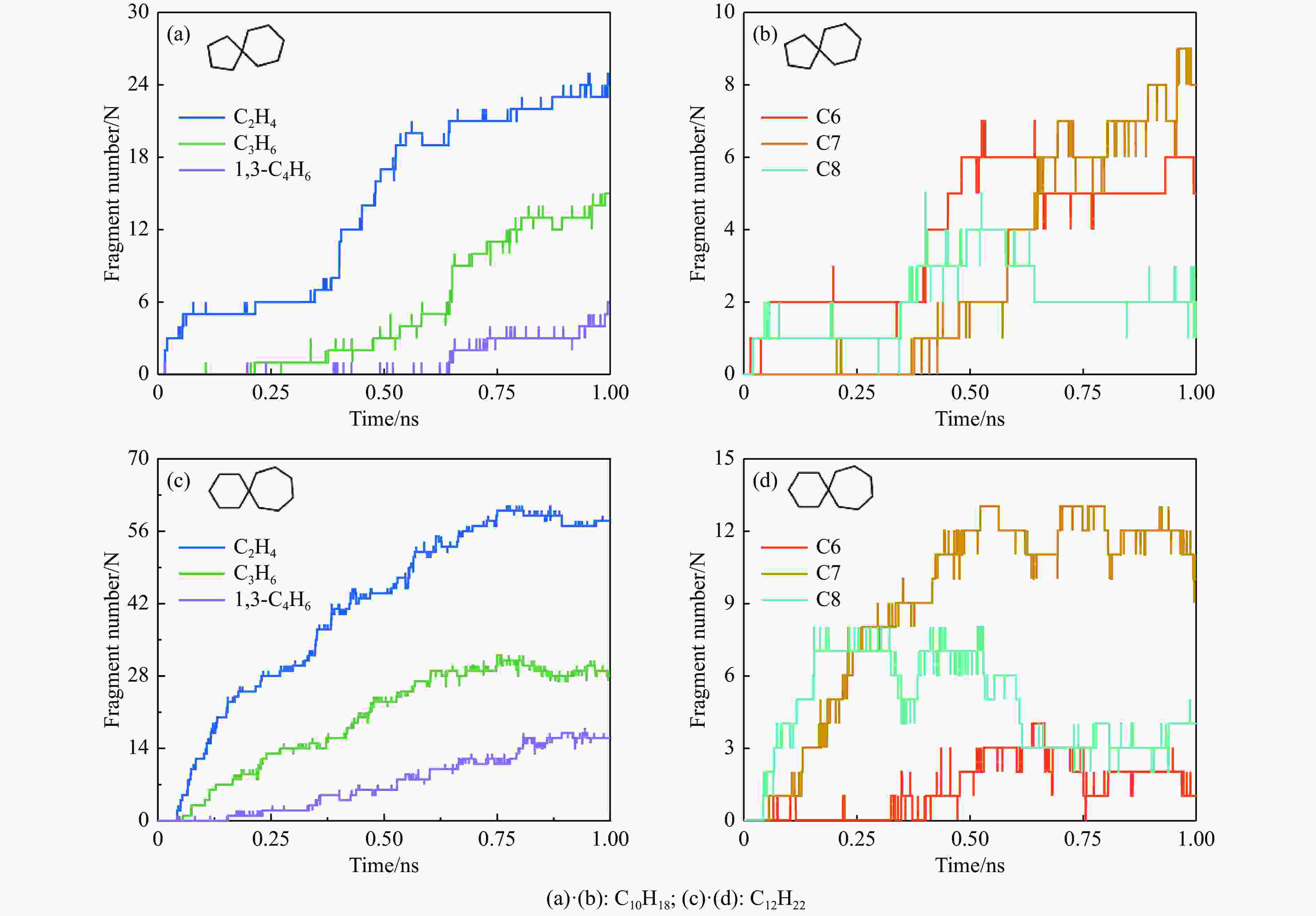
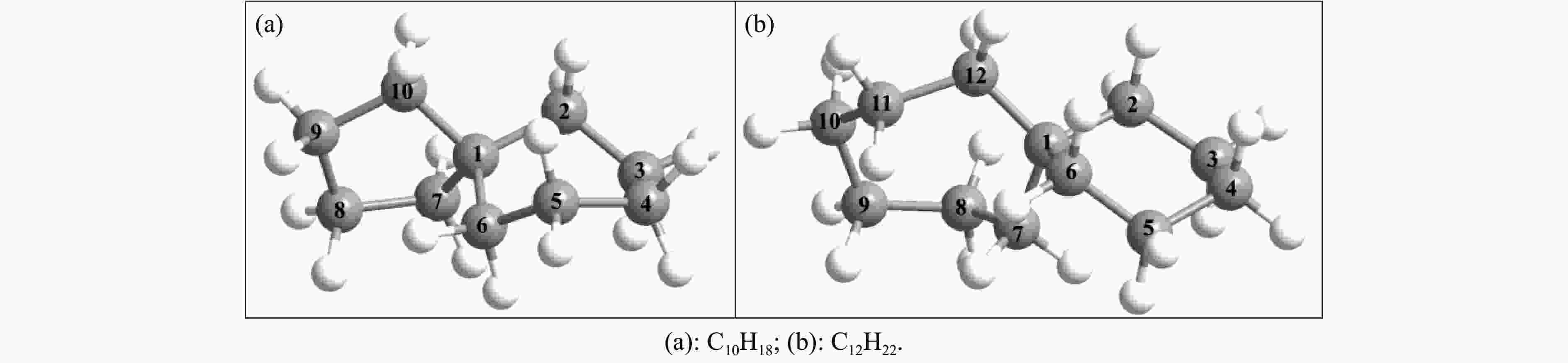
摘要: 采用B3LYP/6-311++G(d,p)和反应性分子动力学方法,对螺[4,5]-癸烷(C10H18)和螺[5,6]-十二烷(C10H22)的热解机理进行研究,揭示不同碳环结构和尺寸效应对燃料初始分解反应活性及小分子和芳烃产物生成行为的影响。结果表明,两种螺环烷烃燃料初始分解路径相似,均通过单分子碳碳键解离发生开环异构反应和小分子自由基进攻燃料母体的氢提取反应而消耗。相较于C10H18,C12H22中分子张力更大的七元环使速控步碳碳键及碳氢键能更低,导致燃料呈现出更低的初始分解温度和更高的反应活性。两种螺环燃料初始分解产生的自由基进一步影响了C1−C7小分子烃类和环烯产物的生成。其中,乙烯的生成始终占据主导地位。由于螺环尺寸效应的影响,链烃和环烯烃的生成表现出明显的结构差异性。对于C10H18分解而言,可能生成大量的五元环烯产物,包括环戊二烯、环戊烯、富烯、甲基环戊二烯和甲基环戊烯;而C12H22中更大的七元环结构,将生成对应的七元环烯产物(环庚烯、亚甲基环庚烷)。Abstract: Active cooling with high-energy-density liquid hydrocarbon fuel is one of promising techniques for the thermal protection of hypersonic aircrafts. To extend the flight range and increase the payload of volume-limited aerospace vehicles, high-energy-density liquid hydrocarbon fuel is directly used as an energetic additive or liquid propellant. Among them, biomass derived spiro-fuels, shown high density, low freezing point and high net heat of combustion due to compact molecular structures, are a kind of significant high-density fuel. The investigation of thermal pyrolysis of these spiro-fuels not only significantly improves the heat sink through the endothermic reaction, but also brings the new challenges of ignition and combustion of the cracked hydrocarbon fuel. The detailed theoretical calculations and molecular dynamics simulations of spiro [4,5] decane (C10H18) and spiro [5,6] dodecane (C12H22) initial pyrolysis were performed to explore the ring-size effect on the consumption of fuels and formation of primary products. The results show that the initial decomposition paths of the two spiro-fuels are very similar, mainly consumed by the open-ring isomerization via the unimolecular C−C dissociations of the six membered ring structure and H-abstractions by small radicals attacking the fuel parents. Due to the lower C−C and C−H bond dissociation energies caused by large tension of the seven membered ring structure, C12H22 may exhibit the lower initial decomposition temperature and higher reaction activity than C10H18. The differences of simultaneously formed fuel radicals during C10H18 and C12H22 initial pyrolysis further affect the formation pathways of C1−C7 small products, cycloalkenes and monocyclic aromatics. Among them, ethylene are the most important products. Due to the presence of inherent six membered rings in two spiro-fuels, monocyclic aromatics mainly originate from multi-step dehydrogenation reactions of fuel radicals, involving benzene, toluene, styrene and ethylbenzene. Notably, the size effect of spiro-ring in two fuels leads the obvious structural differences of the formation of chain hydrocarbons and cycloalkenes. For C10H18, a large number of penta- cycloalkenes may be generated, including cyclopentadiene, cyclopentene, fulvene, methylcyclopentadiene and methylcyclopentene, whereas the seven-membered ring structure of C12H22 may produce corresponding seven-membered products (cyclohexene and methylene cycloheptane). Moreover, the pyrolysis behaviors of these two spiro-fuels at 2000 K based on the ReaxFF molecular dynamics simulation were explored and show well consistent with the main products derived from DFT theoretical calculations. This work performs the DFT theoretical calculations and ReaxFF molecular dynamics simulation on the pyrolysis kinetic mechanisms of two representative high-density biomass fuels of spiro fuels, providing a possible initial pyrolysis path and laying a theoretical foundation for their practical application in engines. However, the complex working environment of the cooling channel poses more challenges to the actual pyrolysis process of the new high-density hydrocarbon fuels. In future research, pyrolysis experiments will be conducted under high temperature and high pressure conditions, and the detailed pyrolysis kinetics models with excellent predictive performance over a wide operating range will be constructed. At the same time, research will be conducted on the subsequent ignition and combustion process of the engine combustion chamber, exploring the impact mechanism of catalysts and fuel additives on this process, assisting in the practical application of fuel, improving fuel combustion efficiency, and effectively controlling pollutant emissions.
-
表 1 联环与螺环烷烃燃料的结构及理化性质[7]
Table 1 Structure and physicochemical properties of bicyclic and spiro fuels
结构 分子式 密度(g/cm3,20 ℃) 冰点/℃ QHHV/(MJ·kg−1) 粘度(mm2/s,25℃) 
C10H18 0.866 −38.0 42.4 1.62 
C12H22 0.886 1.20 42.5 3.72 
C10H18 0.870 −76.0 42.7 2.12 
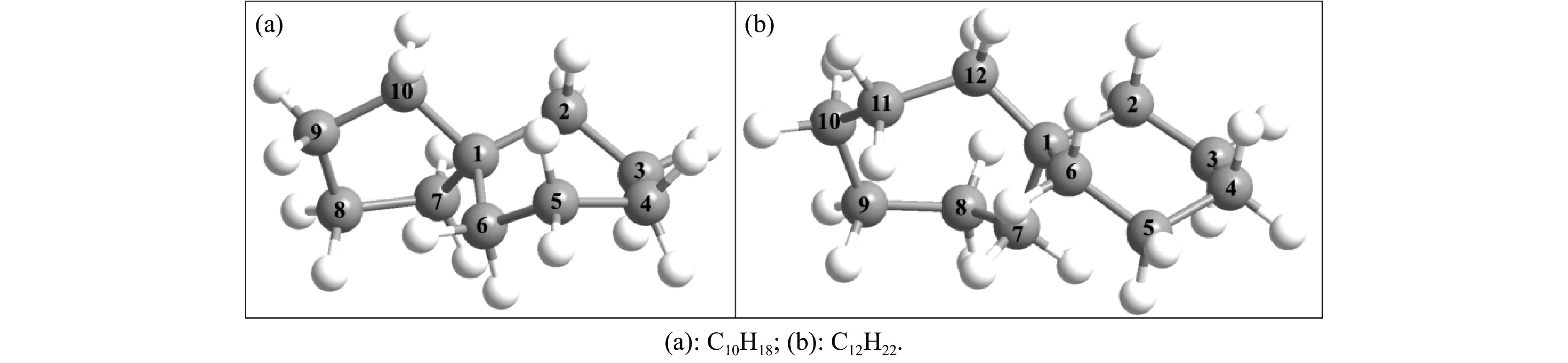
C12H22 0.893 −51.0 43.0 4.37 表 2 利用B3LYP/6-311++G(d, p)和高精度CBS-QB3理论计算获得的C10H18和C12H22的几何结构参数
Table 2 Comparisons of calculated geometries for C10H18 and C12H22 at B3LYP and CBS-QB3 levels
Geometrical
parameterQC NBD B3LYP/6-311++g(d,p) CBS-QB3 B3LYP/6-311++g(d,p) CBS-QB3 r(C1-C2)/Å 1.549 1.550 1.555 1.555 r(C2-C3)/Å 1.533 1.533 1.535 1.535 r(C3-C4)/Å 1.547 1.547 1.534 1.534 r(C4-C5)/Å 1.545 1.545 1.534 1.533 r(C5-C6)/Å 1.533 1.533 1.538 1.538 r(C6-C1)/Å 1.560 1.560 1.554 1.553 r(C1-C7)/Å 1.548 1.547 1.554 1.554 r(C7-C8)/Å 1.541 1.541 1.541 1.541 r(C8-C9)/Å 1.556 1.557 1.540 1.540 r(C9-C10)/Å 1.550 1.550 1.542 1.542 r(C10-C1)/Å 1.549 1.549 − − r(C10-C11)/Å − − 1.538 1.538 r(C11-C12)/Å − − 1.540 1.541 r(C12-C1)/Å − − 1.551 1.551 θ(C2-C1-C6)/(°) 109.9 109.9 107.9 108.0 θ(C10-C1-C7)/(°) 101.0 100.9 − − θ(C12-C1-C7)/(°) − − 111.9 111.9 θ(C7-C1-C2-C6)/(°) 123.6 123.7 118.2 118.2 θ(C10-C1-C2-C6)/(°) 123.8 123.8 − − θ(C12-C1-C2-C6)/(°) − − 120.8 120.9 表 3 利用B3LYP/6-311++G(d, p)和CBS-QB3理论计算获得的C10H18分解的键解离能
Table 3 Dissociation enthalpies of C10H18 to free radicals by C−C and C−H bond scissions at B3LYP and CBS-QB3 levels
Reaction △H of C−C bond disociations (kJ·mol−1) Reaction △H of C−H bond disociations (kJ·mol−1) B3LYP/6-311++g(d,p) CBS-QB3 difference B3LYP/6-311++g(d,p) CBS-QB3 difference C10H18→BR1 324 328 −4 C10H18→R1 388 390 −2 C10H18→BR2 326 329 −3 C10H18→R2 391 393 −2 C10H18→BR3 282 285 −3 C10H18→R3 391 393 −2 C10H18→BR4 282 285 −3 C10H18→R4 389 391 −2 C10H18→BR5 319 322 −3 C10H18→R5 390 392 −2 C10H18→BR6 322 326 −4 -
[1] 刘宁, 史成香, 潘伦, 等. 生物质替代石油原料合成高密度燃料的研究进展[J]. 燃料化学学报,2021,49(12):1780−1790.LIU Ning, Shi Chengxiang, Pan Lun, et al. Progress on using biomass derivatives to replace petroleum for synthesis of high-density fuels[J]. J Fuel Chem Technol,2021,49(12):1780−1790. [2] YANG J, LI N, LI G, et al. Synthesis of renewable high-density fuels using cyclopentanone derived from lignocellulose[J]. Chem Commun,2014,50(20):2572−2574. doi: 10.1039/c3cc46588h [3] WANG W, LI N, LI G, et al. Synthesis of Renewable High-Density Fuel with Cyclopentanone Derived from Hemicellulose[J]. ACS Sustainable Chem Eng,2017,5(2):1812−1817. doi: 10.1021/acssuschemeng.6b02554 [4] XIE J, PAN L, NIE G, et al. Photoinduced cycloaddition of biomass derivatives to obtain high-performance spiro-fuel[J]. Green Chem,2019,21(21):5886−5895. doi: 10.1039/C9GC02790D [5] PAN L, FENG R, PENG H, et al. A solar-energy-derived strained hydrocarbon as an energetic hypergolic fuel[J]. RSC Adv.,2014,4(92):50998−51001. doi: 10.1039/C4RA08868A [6] GUO W, ZHANG X, LIU G, et al. Roles of Hydrogen Donors and Organic Selenides in Inhibiting Solid Deposits from Thermal Stressing of n-Dodecane and Chinese RP-3 Jet Fuel[J]. Ind. Eng. Chem. Res.,2009,48(18):8320−8327. doi: 10.1021/ie900735c [7] XIE J, ZHANG X, PAN L, et al. Renewable high-density spiro-fuels from lignocellulose-derived cyclic ketones[J]. Chem Commun,2017,53(74):10303−10305. doi: 10.1039/C7CC05101H [8] YUE L, QIN X, WU X, et al. Thermal Decomposition Kinetics and Mechanism of 1, 1'-Bicyclohexyl[J]. Energy Fuels,2014,28(7):4523−4531. doi: 10.1021/ef501077n [9] LELE A, KWOM H, GANESHAN K, et al. ReaxFF molecular dynamics study on pyrolysis of bicyclic compounds for aviation fuel[J]. Fuel,2021,297:120724. doi: 10.1016/j.fuel.2021.120724 [10] LIU Y, ZHONG Z, XU S. Pyrolysis mechanism of tetrahydrotricyclopentadiene by ReaxFF reactive molecular dynamics simulations[J]. Comput Theor Chem,2022,1213:113735. doi: 10.1016/j.comptc.2022.113735 [11] LINDGREN E B, MONTEIRO J G S, dos SANTOS A R, et al. Initiation mechanisms and kinetics of the combustion of cyclopentane and cyclopentene from ReaxFF molecular dynamics[J]. Fuel,2021,303:121205. doi: 10.1016/j.fuel.2021.121205 [12] WANG H, ZHOU Y, CAO J, et al. Effect of methyl substituent on adamantane pyrolysis at atmospheric and high pressure: A combined experimental and theoretical study[J]. J Anal Appl Pyrol,2024,177:106299. doi: 10.1016/j.jaap.2023.106299 [13] FU H, WANG Y, LIU B, et al. Experimental and theoretical study of cyclopropanated fuel exo, exo-Tetracyclo[3.3. 1.02, 4.06, 8]nonane pyrolysis in a jet-stirred reactor[J]. Fuel,2024,361:130702. doi: 10.1016/j.fuel.2023.130702 [14] 段君瑞. 综纤维素模型化合物的热解和点火机理研究[D]. 博士, 中国科学技术大学, 2023.DUAN JUNRUI. Mechanism research on pyrolysis and lgnition of holocellulose model compounds [D]. Hefei: University of Science and Technology of China , 2022.) [15] DUAN J, HU H, JI J. Mechanism study on arabinose pyrolysis by combining TG-FTIR-GC–MS and theoretical calculations[J]. Combust Flame,2022,245:112352. doi: 10.1016/j.combustflame.2022.112352 [16] LIU J, ZHANG X, HU B, et al. Formation mechanism of HCN and NH3 during indole pyrolysis: A theoretical DFT study[J]. J Energy Inst,2020,93(2):649−657. doi: 10.1016/j.joei.2019.05.015 [17] LIU L, CHEN S, XU H, et al. Effect of alkyl substituent for cyclohexane on pyrolysis towards sooting tendency from theoretical principle[J]. J Anal Appl Pyrol,2022,161:105386. doi: 10.1016/j.jaap.2021.105386 [18] CHEN S, LI J, ZHU Q, et al. Study on the initial pyrolysis kinetics of strained polycyclic hydrocarbons[J]. Fuel,2023,351:128903. doi: 10.1016/j.fuel.2023.128903 [19] 张婷婷. 不同种类木质素热解的反应分子动力学模拟[D]. 博士, 中国科学院大学(中国科学院过程工程研究所), 2021.ZHANG TINGTING. Pyrolysis simulations of various lignin molecular models by ReaxFF Molecular Dynamics [D]. Beijing: University of Chinese Academy of Science (Institute of Process Engineering, Chinese Academy of Sciences, 2021.) [20] 高明杰. 府谷次烟煤热解的反应分子动力学模拟[D]. 博士, 中国科学院大学(中国科学院过程工程研究所), 2019.GAO MINGJIE. Pyrolysis simulations of Fugu subbituminous coal by ReaxFF Molecular Dynamics [D]. Beijing: University of Chinese Academy of Science (Institute of Process Engineering, Chinese Academy of Sciences, 2019.) [21] 韩嵩. 航空煤油燃烧和碳烟形成初始反应的反应分子动力学模拟[D]. 博士, 中国科学院大学(中国科学院过程工程研究所), 2018.HAN SONG. Initial reaction pathway of aviation kerosene combustion and soot formation revealed by Reactive Molecular Dynamics Simulations [D]. Beijing: University of Chinese Academy of Science (Institute of Process Engineering, Chinese Academy of Sciences, 2018.) [22] 郑默. 基于GPU的煤热解化学反应分子动力学(ReaxFF MD)模拟[D]. 博士, 中国科学院研究生院(过程工程研究所), 2017.ZHENG MO. Coal pyrolysis simulation by GPU-based Reactive Force Field Molecular Dynamics (ReaxFF MD) [D]. Beijing: University of Chinese Academy of Science (Institute of Process Engineering, Chinese Academy of Sciences, 2017.) [23] WANG Y, LIU G. Inhomogeneity Effects on Reactions in Supercritical Fluids: A Computational Study on the Pyrolysis of n-Decane[J]. JACS Au,2022,2(9):2081−2088. doi: 10.1021/jacsau.2c00359 [24] WANG Y, GONG S, LI L, et al. Sub-to-supercritical properties and inhomogeneity of JP-10 using molecular dynamics simulation[J]. Fuel,2021,288:119696. doi: 10.1016/j.fuel.2020.119696 [25] WANG H, ZHANG B, GONG S, et al. Experimental and theoretical study on 2-ethylnorbornane pyrolysis under atmospheric and high pressure[J]. Fuel,2020,272:117724. doi: 10.1016/j.fuel.2020.117724 [26] ZENG M, LI Y, YUAN W, et al. Experimental and kinetic modeling investigation on decalin pyrolysis at low to atmospheric pressures[J]. Combust Flame,2016,167:228−237. doi: 10.1016/j.combustflame.2016.02.009 [27] FRISCH M J, TRUCKS G W, SCHLEGEL H B, et al. Gaussian 09, Revision A. 1, Gaussian, Inc. , [J]. 2009. [28] LEE C, YANG W, PARR R G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density[J]. Phys Rev B,1988,37(2):785−789. doi: 10.1103/PhysRevB.37.785 [29] te VELDE G, BICKELHAUPT F M, BAERENDS E J, et al. Chemistry with ADF[J]. J Comput Chem,2001,22(9):931−967. doi: 10.1002/jcc.1056 [30] ASHRAF C, van DUIN A C T. Extension of the ReaxFF Combustion Force Field toward Syngas Combustion and Initial Oxidation Kinetics[J]. J. Phys. Chem. A,2017,121(5):1051−1068. doi: 10.1021/acs.jpca.6b12429 [31] CHENOWETH K, van DUIN A C T, DASGUPTA S, et al. Initiation Mechanisms and Kinetics of Pyrolysis and Combustion of JP-10 Hydrocarbon Jet Fuel[J]. J. Phys. Chem. A,2009,113(9):1740−1746. doi: 10.1021/jp8081479 [32] ASHRAF C, SHABNAM S, JAIN A, et al. Pyrolysis of binary fuel mixtures at supercritical conditions: A ReaxFF molecular dynamics study[J]. Fuel,2019,235:194−207. doi: 10.1016/j.fuel.2018.07.077 [33] WANG H, ZHONG B, GONG S, et al. Experimental and modeling studies of quadricyclane and 2-ethylnorbornane pyrolysis from atmospheric to high pressure[J]. Combust Flame,2021,226:163−181. doi: 10.1016/j.combustflame.2020.11.042 [34] KUISMA M J, LUNDIN A M, MOTH P K, et al. Comparative Ab-Initio Study of Substituted Norbornadiene-Quadricyclane Compounds for Solar Thermal Storage[J]. J. Phys. Chem. C,2016,120(7):3635−3645. doi: 10.1021/acs.jpcc.5b11489 [35] HERBINET O, SIRJEAN B, BOUNACEUR R, et al. Primary Mechanism of the Thermal Decomposition of Tricyclodecane[J]. J. Phys. Chem. A,2006,110(39):11298−11314. doi: 10.1021/jp0623802 [36] LI H, LIU G, JIANG R, et al. Experimental and kinetic modeling study of exo-TCD pyrolysis under low pressure[J]. Combust Flame,2015,162(5):2177−2190. doi: 10.1016/j.combustflame.2015.01.015 [37] GAO C W, VANDEPUTTE A G, YEE N W, et al. JP-10 combustion studied with shock tube experiments and modeled with automatic reaction mechanism generation[J]. Combust Flame,2015,162(8):3115−3129. doi: 10.1016/j.combustflame.2015.02.010 [38] LU T, LIU Z, CHEN Q. Accurate theoretical evaluation of strain energy of all-carboatomic ring (cyclo [2n] carbon), boron nitride ring, and cyclic polyacetylene[J]. Chinese Phys B,2022,31(12):126101. doi: 10.1088/1674-1056/ac873a [39] ZHONG A, DUAN Y, HUANG Z, et al. An experimental and modeling study on the low-temperature oxidation of methylcyclopentane in a jet-stirred reactor[J]. Fuel,2021,293:120374. doi: 10.1016/j.fuel.2021.120374 [40] WANG Z, CHENG Z, YUAN W, et al. An experimental and kinetic modeling study of cyclohexane pyrolysis at low pressure[J]. Combust Flame,2012,159(7):2243−2253. doi: 10.1016/j.combustflame.2012.02.019 [41] SIKES T, BURDETT K B, SPETH R L, et al. Ring opening in cycloheptane and dissociation of 1-heptene at high temperatures[J]. P Combust Inst,2021,38(1):929−937. doi: 10.1016/j.proci.2020.06.189 [42] WANG H, SUN X, ZHOU Y, et al. Experimental and kinetic modeling study of C5 cyclic hydrocarbons pyrolysis at elevated pressures: Effect of unsaturation on the reactivity and PAH formation[J]. Chem Eng J,2024,480:148342. doi: 10.1016/j.cej.2023.148342 [43] WANG H, ZHANG B, GONG S, et al. Experimental and modeling studies of exo-tetrahydrobicyclopentadiene and tetrahydrotricyclopentadiene pyrolysis at 1 and 30 atm[J]. Combust Flame,2021,232:111536. doi: 10.1016/j.combustflame.2021.111536 [44] GONG S, WANG H, LIU Y, et al. Experimental and kinetic modeling of p-cymene pyrolysis under atmospheric and high pressures[J]. Fuel,2020,260:116407. doi: 10.1016/j.fuel.2019.116407 [45] GONG S, WANG H, LIU Y, et al. Experimental and Kinetic Modeling of Biomass Derived Hydrocarbon p-Menthane Pyrolysis[J]. Energy Fuels,2020,34(10):12634−12645. doi: 10.1021/acs.energyfuels.0c01892 [46] WANG Z, YE L, YUAN W, et al. Experimental and kinetic modeling study on methylcyclohexane pyrolysis and combustion[J]. Combust Flame,2014,161(1):84−100. doi: 10.1016/j.combustflame.2013.08.011 [47] MOLDOVESNU S C. Chapter 2 - Pyrolysis of Hydrocarbons. In Pyrolysis of Organic Molecules (Second Edition)[M], Moldoveanu S. C. , Ed. Elsevier: 2019: 35−161. [48] LISZKA M K, BREZINSKY K. Variable high-pressure and concentration study of cyclohexane pyrolysis at high temperatures[J]. Int J Chem Kinet,2019,51(1):49−73. doi: 10.1002/kin.21229 [49] LISZKA M K, BREZINSKY K. Experimental and modeling study of hex-5-en-1-yl radical pyrolysis at very high pressure and temperature[J]. Combust Flame,2019,201:301−314. doi: 10.1016/j.combustflame.2018.12.029 [50] ZHAO L, YANG T, KAISER R I, et al. A vacuum ultraviolet photoionization study on high-temperature decomposition of JP-10 (exo-tetrahydrodicyclopentadiene)[J]. Phys Chem Chem Phys,2017,19(24):15780−15807. doi: 10.1039/C7CP01571B [51] YUAN W, LI Y, DAGAUT P, et al. Investigation on the pyrolysis and oxidation of toluene over a wide range conditions. I. Flow reactor pyrolysis and jet stirred reactor oxidation[J]. Combust Flame,2015,162(1):3−21. doi: 10.1016/j.combustflame.2014.07.009 [52] YUAN W, LI Y, DAGAUT P, et al. Investigation on the pyrolysis and oxidation of toluene over a wide range conditions. II. A comprehensive kinetic modeling study[J]. Combust Flame,2015,162(1):22−40. doi: 10.1016/j.combustflame.2014.07.011 [53] DJOKIC M R, VAN G K M, CAVALLOTTI C, et al. An experimental and kinetic modeling study of cyclopentadiene pyrolysis: First growth of polycyclic aromatic hydrocarbons[J]. Combust Flame,2014,161(11):2739−2751. doi: 10.1016/j.combustflame.2014.04.013 -




 下载:
下载: