CO2 Assistant Oxidative Dehydrogenation of Isobutane to Isobutene Catalyzed by ZnCaZr Solid Solution
-
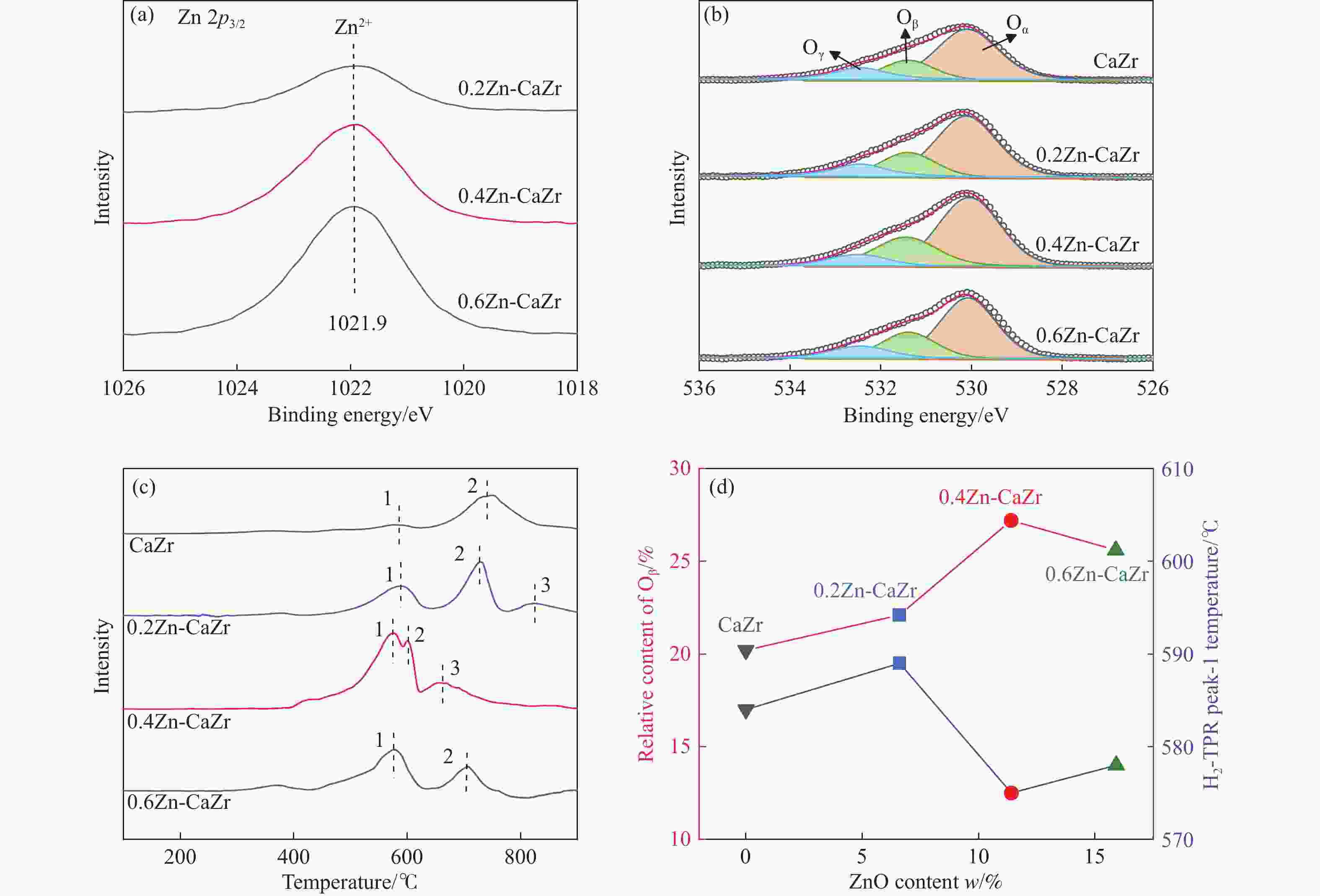
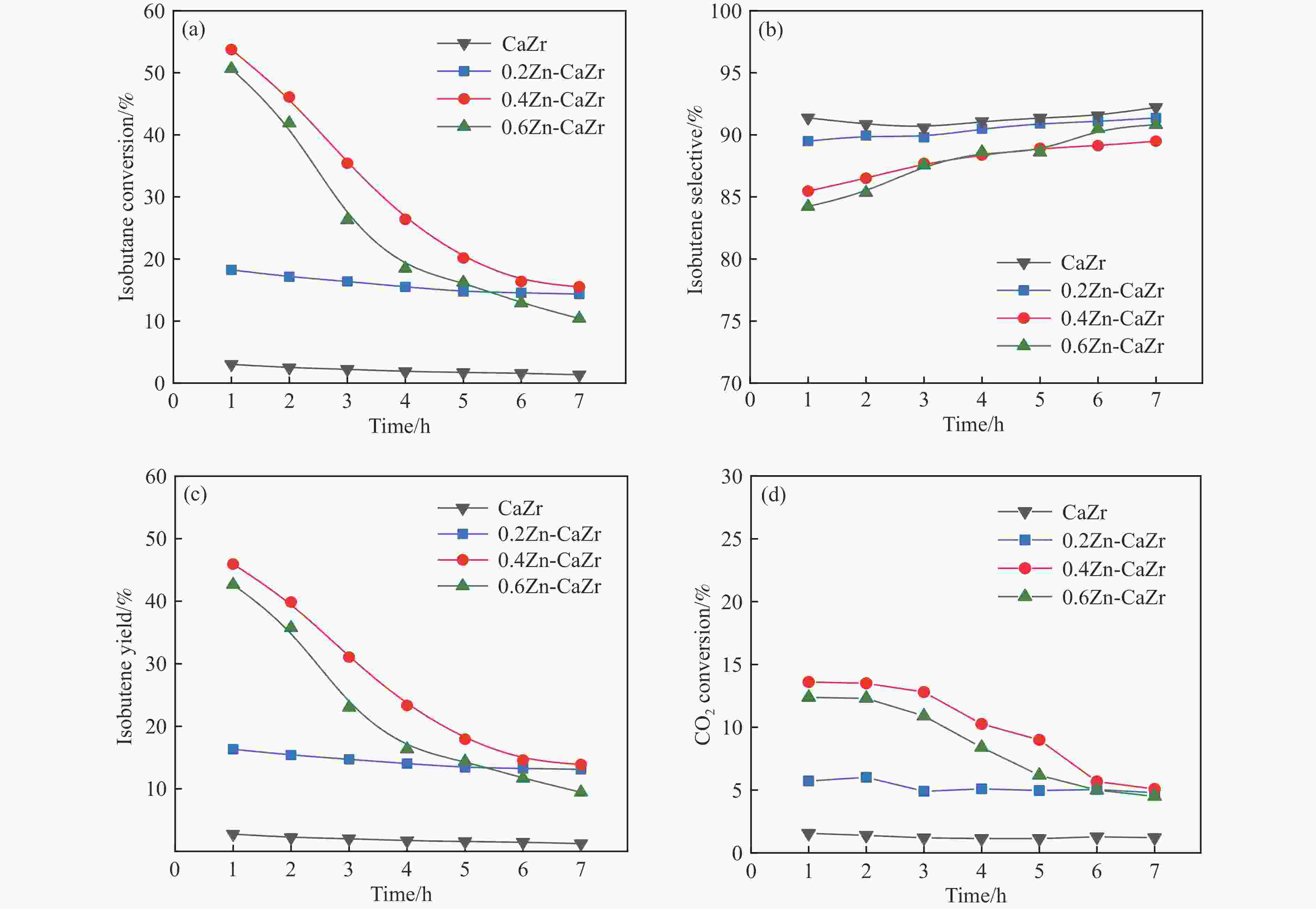
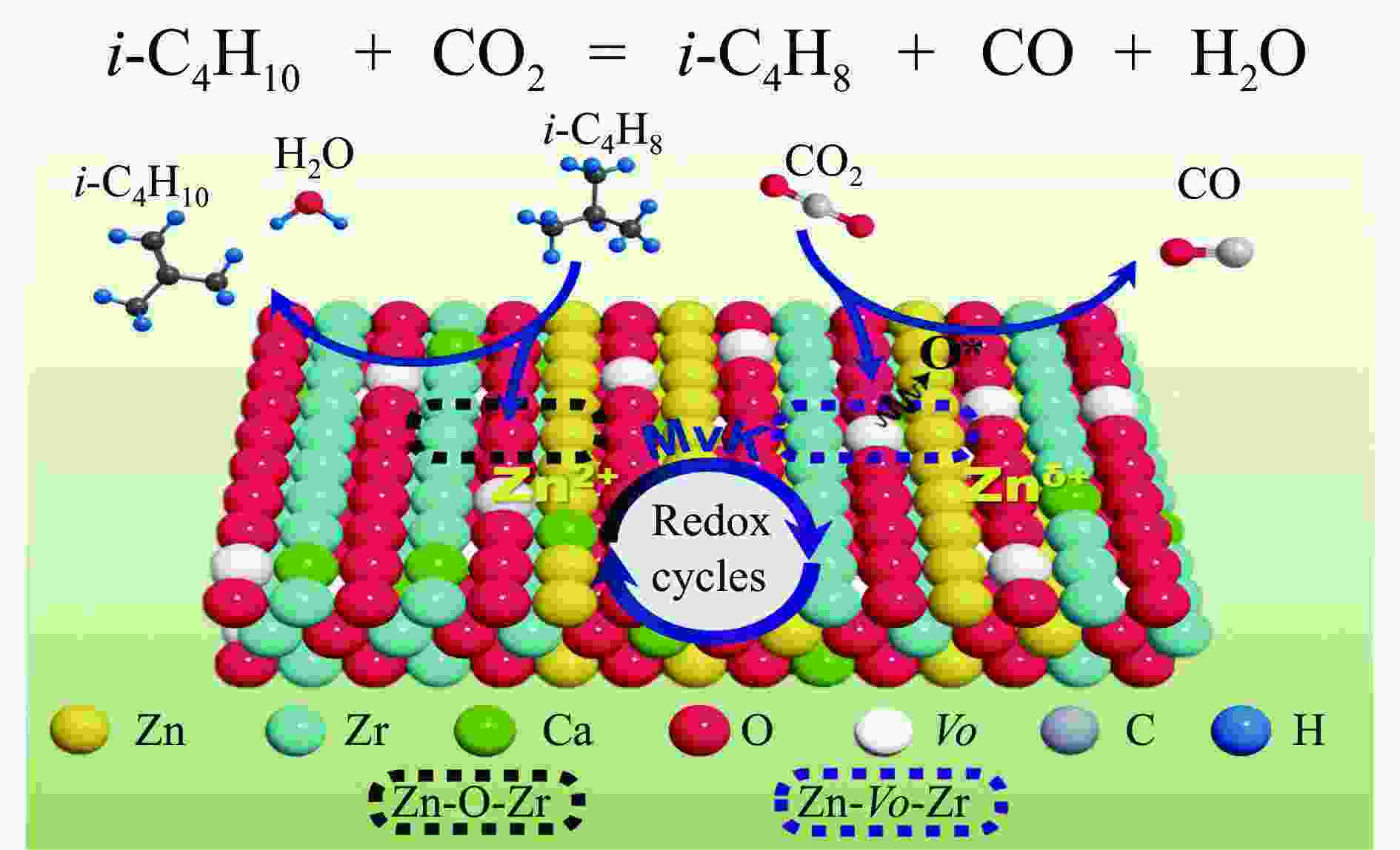
摘要: 本研究采用一锅式共沉淀法制备了xZn-CaZr固溶体催化剂并将其应用于CO2-BDH反应,通过多种手段探明该系列催化剂的理化性质并结合催化性能阐述其构效关系及表面氧化还原机制。研究表明,xZn-CaZr催化剂在Zn含量为6%−12%的情况下形成了Zn物种高度分散的固溶体结构,且氧缺陷的数量与Zn的含量几乎成正比。在xZn-CaZr催化剂上,晶格氧的数量和氧迁移率是决定催化性能的关键因素,其中,0.4Zn-CaZr催化剂展示出最佳的催化活性,而0.2Zn-CaZr催化剂展示出最佳的反应稳定性。该研究为进一步开发绿色高性能的CO2-BDH催化剂提供了参考价值。Abstract: CO2-assisted oxidative dehydrogenation of isobutane to isobutene (CO2-BDH) is an environmentally friendly low-carbon dehydrogenation process, which can effectively utilize greenhouse gas CO2 while producing value-added product isobutylene. Besides, the soft oxidizing property of CO2 can break the thermodynamic limitation of dehydrogenation reaction and avoid the problem of deep oxidation, which makes isobutylene highly selective. However, its industrialization is still challenged by the lack of green and efficient catalysts. In this work, xZn-CaZr solid solution catalysts was prepared by one-pot co-precipitation method and applied to CO2-BDH reaction. The physicochemical properties of all catalysts were investigated by various means, and the structure-activity relationship and surface redox mechanism were described in combination with catalytic performance. The results show that xZn-CaZr catalysts formed solid solution structure with Zn species (6%−12%) existing in highly dispersion state, and the "confinement effect" given by mesoporous skeleton and strong metal-support interactions contributes to the stable distribution of nanoscale sites and generates more Zn-O-Zr interfaces. When excessive Zn species (16%) is added, the ZnO crystal will have obvious phase separation from the solid solution phase. The surface chemical states of different catalysts were analyzed by XPS, and it was found that the relative content of Oβ increased first and then decreased with the increase of Zn content. In addition, the surface reduction characteristics of the catalyst indicated that the promotion of an appropriate amount of Zn species can improve the mobility of lattice oxygen, thus 0.4Zn-CaZr catalyst showing the highest relative content of Oβ and the best oxygen conductivity. In the activity evaluation of different xZn-CaZr catalyst, 0.4Zn-CaZr catalyst shows the best catalytic dehydrogenation activity but its stability is poor, while 0.2Zn-CaZr catalyst has the best reaction stability. Moreover, the catalytic performance of ZnCaZr catalyst under different C4H10-CO2 ratios was also investigated, which indicated that the higher CO2 content in the feed gas was helpful to improve the catalytic stability and isobutene selectivity. Combined with the surface chemical state and carbon deposition information of the spent catalyst, it was found that the relative content of Oβ on the surface of 0.4Zn-CaZr catalyst decreases obviously, but the carbon deposition rate was slow. On the contrary, the relative content of Oβ for 0.2Zn-CaZr catalyst decreased less, but its carbon deposition rate was faster. We believe that the amount and mobility of lattice oxygen over xZn-CaZr catalysts were revealed as key factors in determining the catalytic performance. Notably, higher content and superior mobility of lattice oxygen can enhance the redox function of the solid solution catalyst itself and improve the activation performance of CO2. The strong oxygen supply capacity can ensure the continuous MvK catalytic cycle on the Zn-O-Zr interface, and avoid the deep accumulation of inert carbon deposits while improving the dehydrogenation activity of isobutane and selectivity of isobutene. This study shed lights on the further design and development of green and efficient CO2-BDH catalysts.1) #:共同第一作者
-
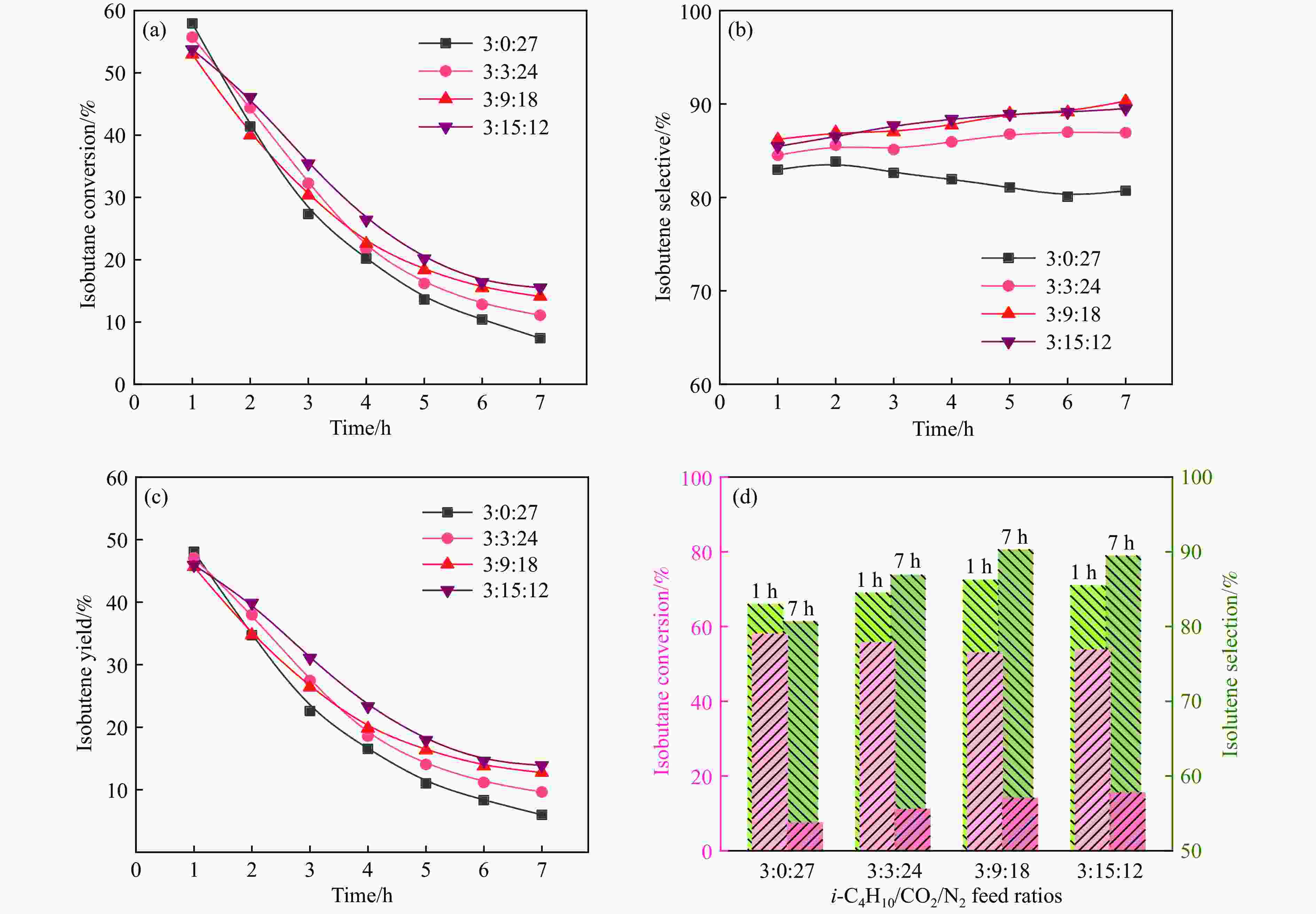
图 4 0.4Zn-CaZr催化剂在不同进气比条件下的CO2-BDH反应性能
Figure 4 CO2-BDH reaction performance of 0.4Zn-CaZr catalyst under different feed ratiosReaction condition: C4H10∶CO2∶N2 = 3∶0:27、3∶3:24、3∶9∶18、3∶15∶12, 570 ℃, atmospheric pressure, GHSV = 6000 mL/(g·h)
(a): Isobutane conversion; (b): isobutene selectivity; (c): isobutene yield; (d): Comparison of activity and selectivity at 1 h and 7 h.
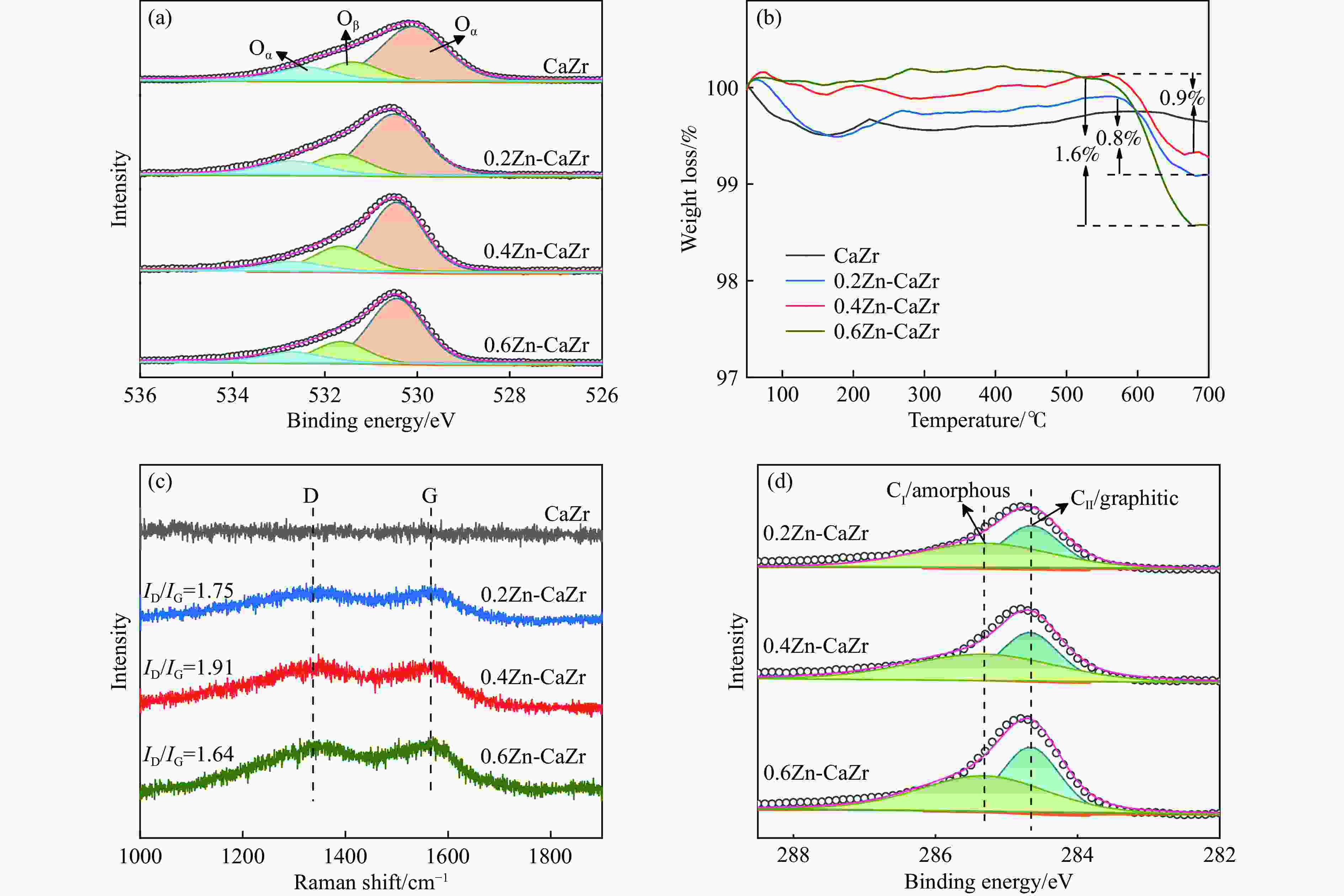
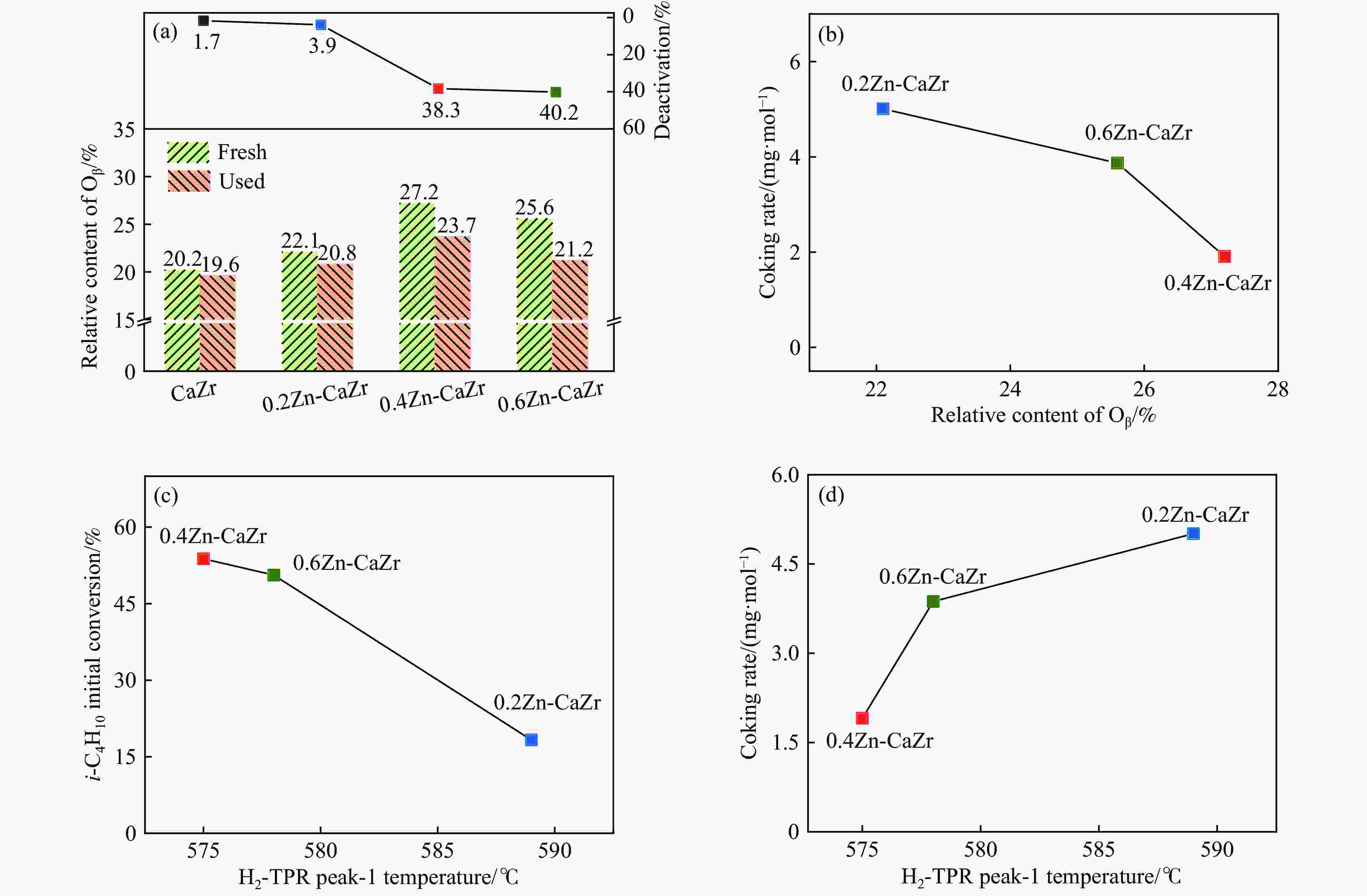
图 6 (a)反应前后催化剂的表面Oβ相对含量及异丁烷转化率损失量; (b) 催化剂的表面Oβ相对含量与积炭速率的关系; H2-TPR中的1类还原峰值温度与催化剂的异丁烷起始转化速率(c)及积炭速率(d)的关系
Figure 6 (a) The relative content of Oβ and the loss of isobutane conversion before and after reaction; (b) the relationship between the relative content of Oβ on the catalyst surface and the coking rate. The relationship between the reduction temperature of peak-1 in H2-TPR and (c) the initial isobutane conversion, (d) coking rate of the catalysts
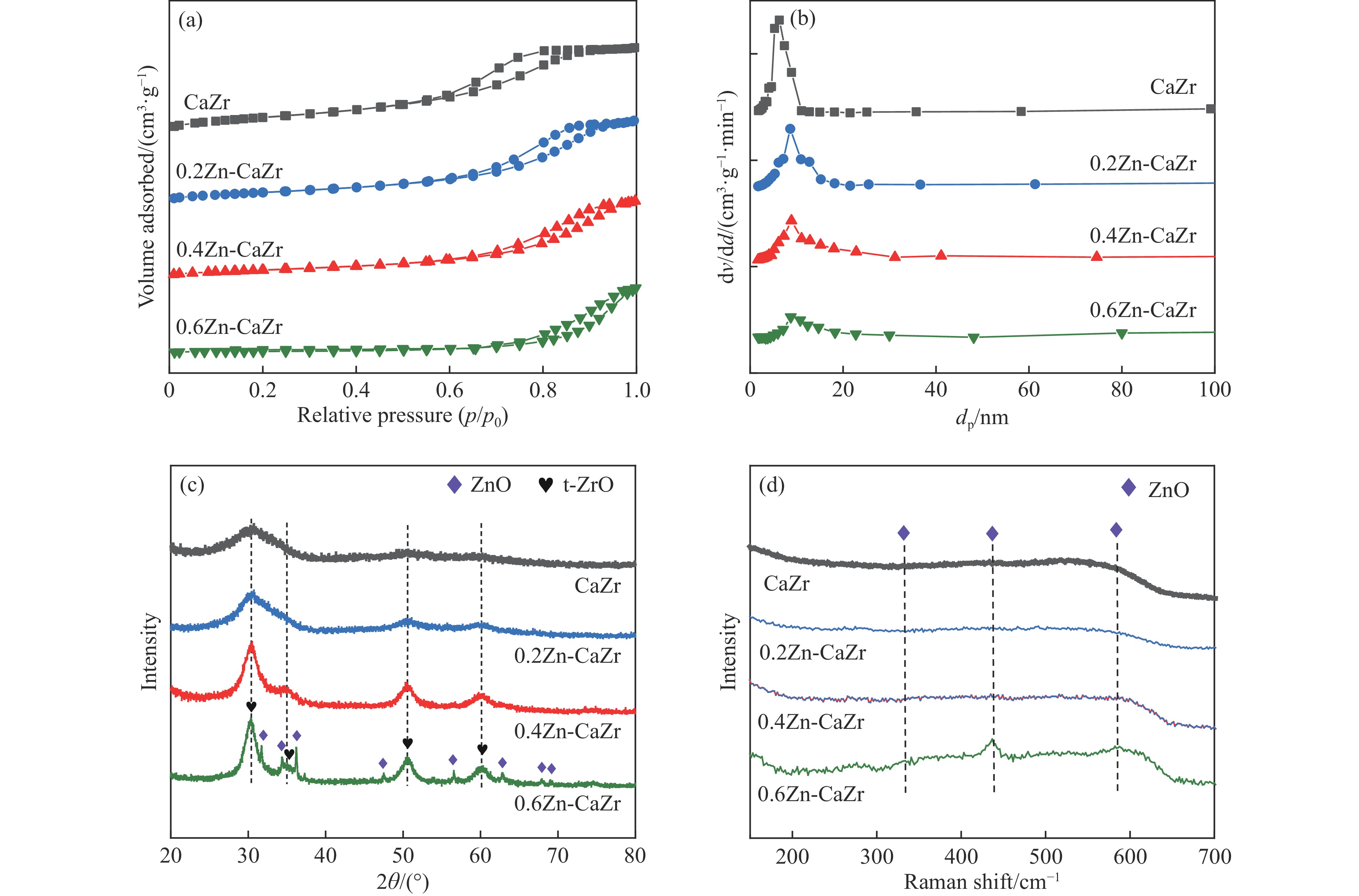
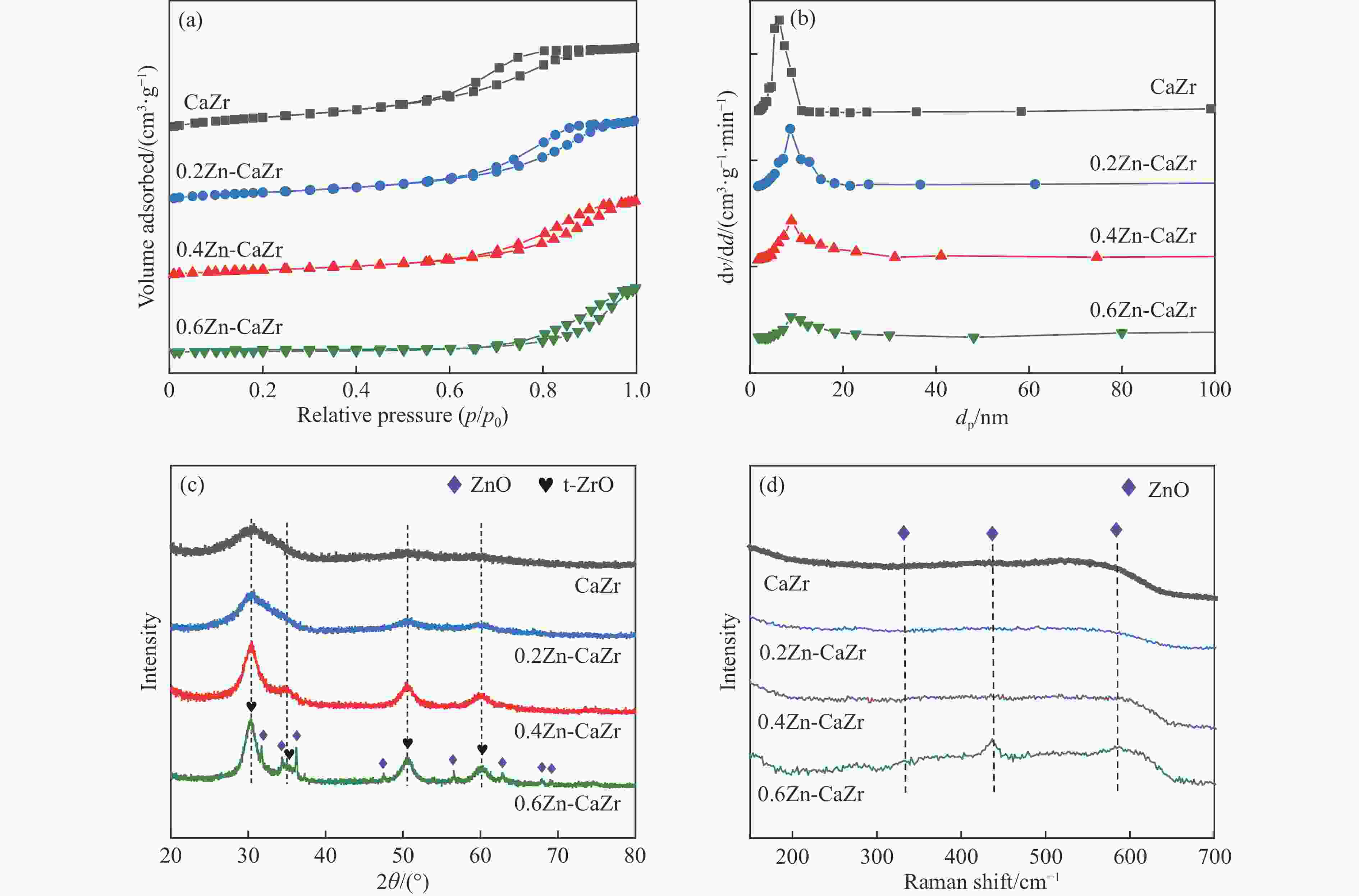
表 1 xZn-CaZr催化剂的元素含量及织构参数
Table 1 Elemental content and texture parameters of xZnO-CaZr catalyst
Catalyst Zn loading/% Ca loading/% Zr loading/% A/(m2 g−1) vp/(cm3 g−1) dp/nm ZrO2 crystal size/nm CaZr — 4.6 52.3 210.7 0.75 5.4 15 0.2Zn-CaZr 6.6 4.2 49.0 159.4 0.43 7.4 19 0.4Zn-CaZr 11.4 3.8 46.9 135.8 0.41 8.8 30 0.6Zn-CaZr 15.9 3.1 40.5 76.9 0.46 8.0 36 表 2 催化剂的表面Oβ相对含量和H2-TPR峰值温度
Table 2 Surface relative content of Oβ and H2-TPR Peak temperature on the fresh catalysts
Catalyst Relative content of Oβ /% H2-TPR peak temperature/℃ 1 2 3 CaZr 20.2 584 738 — 0.2Zn-CaZr 22.1 589 729 824 0.4Zn-CaZr 27.2 575 602 663 0.6Zn-CaZr 25.6 578 708 — 表 3 反应1h时的异丁烷和CO2转化率、主要烃类产物选择性及异丁烯产率
Table 3 Conversion of isobutane and CO2, selectivity of main hydrocarbon products and isobutene yield at 1h reaction
Catalyst Conversion/% Selectivity/% i-C4H8 yield/% i-C4H10 CO2 i-C4H8 C3H8 C3H6 C2H6 C2H4 CH4 CaZr 3.0 1.5 91.3 2.3 1.8 0.9 0.8 2.9 2.7 0.2Zn-CaZr 18.2 5.7 89.5 2.8 2.1 1.3 1.3 3.0 16.2 0.4Zn-CaZr 53.8 13.6 85.5 3.1 2.7 1.9 1.6 5.2 45.9 0.6Zn-CaZr 50.6 12.4 84.2 3.0 2.9 1.8 1.8 6.3 42.6 表 4 反应后催化剂的XPS分析、表面碳信息及积炭速率
Table 4 Surface relative content of Oβ, carbon information and coking rate of used catalysts
Catalyst Relative content of Oβ/% Coke content/% ID/IG CⅠ/CⅡ Coking rate/(mg·mol−1) CaZr 19.6 — — — — 0.2Zn-CaZr 20.8 0.8 1.75 1.42 5.01 0.4Zn-CaZr 23.7 0.9 1.91 1.51 1.91 0.6Zn-CaZr 21.2 1.6 1.64 1.20 3.87 -
[1] LIU J, ZHOU W, JIANG D, et al. Insights into the doping effect of rare-earth metal on ZnAl2O4 supported PtSn catalyzed isobutane dehydrogenation[J]. Catal Today,2021,368:58−65. doi: 10.1016/j.cattod.2020.04.016 [2] WANG J, LIU M, LI J, et al. Elucidating the active-phase evolution of Fe-based catalysts during isobutane dehydrogenation with and without CO2 in feed gas[J]. ACS Catal,2022,12(10):5930−5938. doi: 10.1021/acscatal.1c05907 [3] OTROSHCHENKO T, JIANG G Y, KONDRATENKO V A, et al. Current status and perspectives in oxidative, non-oxidative and CO2-mediated dehydrogenation of propane and isobutane over metal oxide catalysts[J]. Chem Soc Rev,2021,50(1):473−527. doi: 10.1039/D0CS01140A [4] OTROSHCHENKO T, SOKILOV S, STOYANOVA M, et al. ZrO2‐based alternatives to conventional propane dehydrogenation catalysts: active sites, design, and performance[J]. Angew Chem Int Edit,2015,54(52):15880−15883. doi: 10.1002/anie.201508731 [5] LIU Y, XIA C, WANG Q, et al. Direct dehydrogenation of isobutane to isobutene over Zn-doped ZrO2 metal oxide heterogeneous catalysts[J]. Catal Sci Technol,2018,8(19):4916−4924. doi: 10.1039/C8CY01420E [6] GAO Y G, JIE X Y, WANG C Z, et al. One-pot synthesis of ca oxide-promoted Cr catalysts for the dehydrogenation of propane using CO2[J]. Ind Eng Chem Res,2020,59(28):12645−12656. doi: 10.1021/acs.iecr.9b06703 [7] ZHANG Y, ZHAO Y, OTROSHCHENKO T, et al. Control of coordinatively unsaturated Zr sites in ZrO2 for efficient C-H bond activation[J]. Nat Commun,2018,9(1):3794. doi: 10.1038/s41467-018-06174-5 [8] WELLER M, Oxygen mobility in yttria-doped zirconia studied by internal friction, electrical conductivity and tracer diffusion experiments[J]. Solid State Ionics, 2004, 175(1-4): 409-413. [9] OTROSHCHENKO T, KONDRATENKO V A, RODEMERCK U, et al. ZrO2-based unconventional catalysts for non-oxidative propane dehydrogenation: Factors determining catalytic activity[J]. J Catal,2017,348:282−290. doi: 10.1016/j.jcat.2017.02.016 [10] LIAO D, YANG L, SONG G, et al. CO2-assisted oxidative dehydrogenation of ethane to ethylene over the ZnO-ZrO2 catalyst[J]. J Fuel Chem Technol,2023,51(10):1421−1431. doi: 10.1016/S1872-5813(23)60360-3 [11] YANG G Q, HE Y J, SONG Y H, et al. Oxidative dehydrogenation of propane with carbon dioxide catalyzed by Zn xZr1– xO2– x solid solutions[J]. Ind Eng Chem Res,2021,60(49):17850−17861. doi: 10.1021/acs.iecr.1c03476 [12] WANG C Z, SUN N N, ZHAO N, et al. Template-free preparation of bimetallic mesoporous Ni-Co-CaO-ZrO2 catalysts and their synergetic effect in dry reforming of methane[J]. Catal Today,2017,281:268−275. doi: 10.1016/j.cattod.2016.03.026 [13] WANG C Z, SUN N N, ZHAO N. et al. Coking and deactivation of a mesoporous Ni-CaO-ZrO2 catalyst in dry reforming of methane: A study under different feeding compositions[J]. Fuel,2015,143:527−535. doi: 10.1016/j.fuel.2014.11.097 [14] WANG C Z, SUN N N, WEI W, et al. Carbon intermediates during CO2 reforming of methane over NiCaOZrO2 catalysts: A temperature-programmed surface reaction study[J]. Int J Hydrogen Energ,2016,41(42):19014−19024. doi: 10.1016/j.ijhydene.2016.08.128 [15] SUN J, BAYLON R A L, LIU C, et al. Key roles of lewis acid-base pairs on Zn xZr yOz in direct ethanol/acetone to isobutene conversion[J]. J Am Chem Soc,2015,138(2):507−517. [16] WANG C Z, SUN N N, KANG M, et al. The bi-functional mechanism of CH4 dry reforming over a Ni-CaO-ZrO2 catalyst: further evidence via the identification of the active sites and kinetic studies[J]. Catal Sci Technol,2013,3(9):2435−2443. doi: 10.1039/c3cy00153a [17] LUO Y J, WeEI C L, MIAO C X, et al. Isobutane dehydrogenation assisted by CO2 over silicalite-1-supported ZnO catalysts: Influence of support crystallite size[J]. Chinese J Chem,2020,38(7):703−708. doi: 10.1002/cjoc.202000042 [18] DENG Q, DUAN X, NG D H L, et al. Ag nanoparticle decorated nanoporous ZnO microrods and their enhanced photocatalytic activities[J]. ACS Appl Mater Inter,2012,4(11):6030−6037. doi: 10.1021/am301682g [19] BAYLON R A L, SUN J, KOVARIK L, et al. Structural identification of Zn xZr yOz catalysts for Cascade aldolization and self-deoxygenation reactions[J]. Appl Catal B-Environ,2018,234:337−346. doi: 10.1016/j.apcatb.2018.04.051 [20] 杨淑倩, 贺建平, 张娜, 等. 稀土掺杂改性对Cu/ZnAl水滑石衍生催化剂甲醇水蒸气重整制氢性能的影响[J]. 燃料化学学报,2018,46(2):179−188. doi: 10.1016/S1872-5813(18)30010-0YANG Shuqian, HE Jianping, ZHANG Na, et al. Effect of rare-earth element modification on the performance of Cu/ZnAl catalysts derived from hydrotalcite precursor in methanol steam reforming[J]. J Fuel Chem Technol,2018,46(2):179−188. doi: 10.1016/S1872-5813(18)30010-0 [21] GAO P, DANG S, LI S, et al. Direct production of lower olefins from CO2 conversion via bifunctional catalysis[J]. ACS Catal,2017,8(1):571−578. [22] WANG Y, ZHAN W, CHEN Z, et al. Advanced 3D hollow-out ZnZrO@C combined with hierarchical Zeolite for highly active and selective CO hydrogenation to aromatics[J]. ACS Catal,2020,10(13):7177−7187. doi: 10.1021/acscatal.0c01418 [23] ZHENG Y, LI J, ZHANG X, et al. Effect of CO2 co-feeding on the stabilization of atomically dispersed iron species over MgAl2O4 during ethane dehydrogenation reactions[J]. ACS Catal,2023,13(16):11153−11163. doi: 10.1021/acscatal.3c02029 [24] WANG Y, CHEN Y, ZHANG J, et al. High transformation of propane in reaction with CO2 to propylene on ZrO2-combined Fe-based catalysts[J]. Catal Sci Technol,2023,13(19):5734−5744. doi: 10.1039/D3CY00915G [25] SILVA-CALPA L D R, ZONETTI P C, RODRIGUES C P, et al. The Zn xZr1- xO2- y solid solution on m-ZrO2: Creating O vacancies and improving the m-ZrO2 redox properties[J]. J Mol Catal A-Chem,2016,425:166−173. doi: 10.1016/j.molcata.2016.10.008 [26] ATANGA M A, REZAEI F, JAWAD A, et al. Oxidative dehydrogenation of propane to propylene with carbon dioxide[J]. Appl Catal B-Environ,2018,220:429−445. doi: 10.1016/j.apcatb.2017.08.052 [27] XIE Z, REN Y, LI J, et al. Facile in situ synthesis of highly dispersed chromium oxide incorporated into mesoporous ZrO2 for the dehydrogenation of propane with CO2[J]. J Catal,2019,372:206−216. doi: 10.1016/j.jcat.2019.02.026 [28] WANG J, SONG Y H, LIU Z T, et al. Active and selective nature of supported CrO x for the oxidative dehydrogenation of propane with carbon dioxide[J]. Appl Catal B-Environ,2021,297:120400. doi: 10.1016/j.apcatb.2021.120400 [29] AL-AWADI A S, EL-TONI A M, LABIS J P, et al. Mesoporous organo-silica supported chromium oxide catalyst for oxidative dehydrogenation of ethane to ethylene with CO2[J]. Catalysts,2021,11(5):642. doi: 10.3390/catal11050642 [30] 吴昊, 王长真, 仇媛, 等. Ni-SiO2催化剂空间限域维度对CH4-CO2重整反应金属抗积炭能力的影响[J]. 高等学校化学学报, 2020, 41(11: 210-217.WU Hao, WANG Changzhen, QIU Yuan, et al. Effect of steric confinement dimension on metal site anti-carbon deposition ability of Ni-SiO2 catalysts in CH4-CO2 reforming[J]. Chem J Chinese U, 2020, 41(11: 210-217.) [31] ZHANG Y, ZHANG G, LIU J, et al. Dry reforming of methane over Ni/SiO2 catalysts: Role of support structure properties[J]. Fuel,2023,340:127490. doi: 10.1016/j.fuel.2023.127490 [32] YANG Z, LI H, Zhou H, et al. Coking-resistant iron catalyst in ethane dehydrogenation achieved through siliceous zeolite modulation[J]. J Am Chem Soc,2020,142(38):16429−16436. doi: 10.1021/jacs.0c07792 [33] LU Y, KANG L, GUO D, et al. Double-site doping of a V promoter on Ni x-V-MgAl catalysts for the DRM reaction: simultaneous effect on CH4 and CO2 activation[J]. ACS Catal,2021,11(14):8749−8765. doi: 10.1021/acscatal.1c01299 [34] BAHARI M B, SETIABUDI H D, TRINH D N, et al. Insight into the influence of rare-earth promoter (CeO2, La2O3, Y2O3, and Sm2O3) addition toward methane dry reforming over Co/mesoporous alumina catalysts[J]. Chem Eng Sci, 2020, 228. -




 下载:
下载: